Abstract
Purpose
To compare the efficacy and safety of high dose-intensity combination of methotrexate, vinblastine, adriamycin and cisplatin (HD MVAC) with gemcitabine plus cisplatin (GC) as a neoadjuvant chemotherapy (NAC) in muscle-invasive bladder cancer (MIBC) or locally advanced upper tract urothelial cancer (UTUC).
Patients and methods
A retrospective analysis was conducted for patients with UC (cT2-4aN0-1M0) who received NAC from January 2011 and December 2017 at Asan Medical Center. Pathologic complete response (pCR), down-staging (< ypT2 and no N upstaging), disease-free survival (DFS), OS and safety were compared for each regimen.
Results
Out of a total of 277 patients, 176 patients received GC and 41 patients received HD MVAC. With the exception of age (patients receiving HD MVAC were younger; p = 0.002), other baseline characteristics were well balanced between groups. pCR rates were 27.0% for GC and 22.6% for HD MVAC (p = 0.62), and down-staging rate was 50.8% for GC and 58.1% for HD MVAC (p = 0.47). There were no differences in OS (72.1% vs 73.1% for GC vs HD MVAC; p = 0.58) and DFS (54.9% vs 63.3% for GC vs HD MVAC; p = 0.21) at 3 years. HD MVAC with prophylactic G-CSF was associated with a higher incidence of febrile neutropenia (p < 0.001) than GC. The NAC regimen was not an independent prognostic factor for OS.
Conclusion
Oncologic outcomes were not significantly different between the GC and HD MVAC when used as NAC in MIBC/UTUC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is estimated that 550,000 new cases of bladder cancer occurred worldwide in 2018, with approximately one-third of patients presenting with the muscle-invasive form of the disease (MIBC). More than 20% of patients with non-muscle-invasive bladder cancer progress to MIBC, resulting in 200,000 deaths annually (Bray et al. 2018).
Neoadjuvant chemotherapy (NAC) in MIBC has been established as a standard treatment after SWOG prospective randomized trials demonstrated the efficacy of MVAC as NAC (Grossman et al. 2003). The subsequent meta-analysis of 11 trials encompassing 3005 patients supported the result that NAC led to an absolute improvement of 5-year overall survival (OS) by 5% and disease-free survival (DFS) by 9% (Advanced Bladder Cancer Meta-analysis Collaboration 2003). Despite a high level of evidence, NAC has not been used widely in clinical practice owing to concerns regarding treatment-related toxicity, over-treatment associated with limited accuracy of preoperative staging, and delay to surgery.
In advanced urothelial cancer (UC), the GC (gemcitabine and cisplatin) regimen is preferred to MVAC (methotrexate, vinblastine, adriamycin, and cisplatin) based on comparative efficacy with a better safety profile and tolerability (von der Maase et al. 2000). Besides, high-dose intensity (HD) MVAC with granulocyte colony-stimulating factor (G-CSF) support had statistically significant survival benefit and higher response rate, especially complete response, than MVAC, with comparable tolerance and fewer dose delays in metastatic UC (Sternberg et al. 2001, 2006).
Based on data in the metastatic setting, GC or HD MVAC is preferred to MVAC as NAC treatment in MIBC. Comparative data between GC and HD MVAC in a neoadjuvant setting are limited, and it has not been thoroughly assessed in randomized controlled trials. The National Comprehensive Cancer Network guideline recommends both GC and HD MVAC as preferred regimens without preference between them (National Comprehensive Cancer Network 2020). In comparison, the European Association of Urology and the American Urologic Association do not suggest specific regimens and refer to cisplatin-based chemotherapy (Alfred Witjes et al. 2017; Chang et al. 2017). A few retrospective studies recently compared the clinical outcomes of the HD MVAC regimen and the GC regimen when used as NAC in MIBC, which have shown somewhat contradictory results (Peyton et al. 2018; van de Putte et al. 2016; Zargar et al. 2018).
The evidence of NAC in upper tract urothelial cancer (UTUC) is scarce. The POUT study showed adjuvant platinum-based chemotherapy improved disease-free and metastasis-free survival in UTUC (Birtle et al. 2020). However, due to reduced kidney function after nephroureterectomy, the neoadjuvant setting may be preferred over adjuvant for chemotherapy administration in UTUC, especially for a clinical node-positive disease where the chance of over-treatment is minimal (Chakiryan et al. 2019), and retrospective studies have suggested survival benefit with NAC (Leow et al. 2014). Similar to bladder cancer, the data of comparative efficacy of NAC regimen in UTUC are lacking.
The aim of this study was therefore to compare the clinical outcomes of neoadjuvant GC with those of HD MVAC in patients with localized or locally advanced UC.
Patients and methods
Patients
Between January 2011 and December 2017, 290 consecutive patients with urothelial carcinoma who received neoadjuvant chemotherapy at Asan Medical Center, Seoul, Republic of Korea, were reviewed. Eleven patients who had distant metastasis (include M1a) or non-muscle-invasive bladder cancer were excluded. All remaining patients were histologically confirmed and documented to have stage cT2-4 N0 M0 or cTany N1 M0 cancer. Sixty-two patients who received NAC other than GC or HD MVAC were excluded. Overall, 217 patients were included in this analysis. The Institutional Review Board of Asan Medical Center approved this study.
Treatment and evaluation
GC chemotherapy was performed using the following schedule: gemcitabine 1000 mg/m2 on Days 1 and 8 and cisplatin 70 mg/m2 on Day 1, every 3 weeks. HD MVAC chemotherapy involved methotrexate 30 mg/m2 on Day 1, vinblastine 3 mg/m2 on Day 2, doxorubicin 30 mg/m2 and cisplatin 70 mg/m2 on Day 2, and G-CSF 300 µg/m2 from Days 4–10 or long-acting G-CSF(pegfilgrastim) on Day 2, every 2 weeks. In both groups, patients without cN1 received four cycles of chemotherapy, whereas those with cN1 received six cycles if there was no evidence of disease progression and adverse events were tolerable.
Surgery was conducted only if all lesions were resectable, assessed by the urologic surgeon after NAC. Patients received partial or radical cystectomy, radical nephroureterectomy, or segmental ureterectomy, according to the lesions involved. No surgery was performed in case of clinical disease progression (cPD) to NAC. In the case of medically inoperable patients or refusal of surgery, concurrent chemoradiation or radiotherapy was recommended after NAC. Repeated CT scans were obtained immediately after neoadjuvant chemotherapy before surgery for cN0 disease, while we did additional CT scans after the 3rd cycle of NAC for patients with cN1 disease.
We defined the extent of resection as being macroscopically complete with a negative microscopic margin (R0), macroscopically complete with a positive microscopic margin (R1), or macroscopically incomplete (R2). We determined the pathological response based on cystectomy and pelvic lymph node dissection (PLND) for patients who underwent surgery. PLND was performed per a standardized template. We reviewed the rate of the patient's pathologic down-staging and pathologic complete response (pCR). Down-staging was defined as < ypT2 and no N upstaging at operation. pCR was defined as no evidence of residual tumor (ypT0N0). Toxicity during chemotherapy was classified in accordance with the Common Terminology Criteria for Adverse Events (CTCAE) v4.03 (National Cancer Institute 2010).
Statistical analysis
OS was defined as the duration of time from the start date of NAC to the date of death due to any cause. DFS was defined as the duration of time from the start date of neoadjuvant chemotherapy starting to the date of disease recurrence or death due to any cause, whichever occurred first. Survival rates and corresponding standard errors were estimated using the Kaplan–Meier method, and survival curves were compared using the log-rank test. Baseline characteristics, clinical response rates, pathologic down-staging rate, pCR between groups were compared using Pearson’s chi-square test or Fisher’s exact test for categorical variables and Student’s t-test or Mann–Whitney U test for continuous variables, as appropriate. To identify clinical prognostic factors for OS and DFS, univariate and multivariate analyses were performed using Cox proportional hazard regression modeling. The key baseline characteristics and candidate prognostic factors, including age, sex, tumor histology, clinical stage, hydronephrosis at presentation, history of non-muscle-invasive bladder cancer, and neoadjuvant regimen were included in the univariate analysis. Variables exhibiting a potential association with survival (p < 0.25) in the univariate analysis and neoadjuvant regimen were included in the multivariate analysis. All analyses were computed using SPSS Statistics version 24 (IBM SPSS Inc. Armonk, NY, USA). All tests were two-sided, with p values of < 0.05 considered statistically significant.
Results
Patient characteristics
The baseline characteristics of patients in the GC (n = 176) and HD MVAC (n = 41) groups are presented in Table 1. The characteristics did not differ significantly between the two groups, with the exception of age: patients treated with HD MVAC were younger than those treated with GC (p = 0.002).
Neoadjuvant chemotherapy, administration, clinical response, and tolerability
The number of median chemotherapy cycles was 4 (IQR 3–4) for the GC group and 4 (IQR 3–5.5) for the HD MVAC group. All patients received at least two cycles of neoadjuvant chemotherapy. The percentage of patients who received fewer than 3 cycles of chemotherapy was 10.2% in the GC group and 2.4% in the HD MVAC group (p = 0.135). The clinical responses to NAC for both groups are listed in Supplement Table 1. There were no differences in clinical response rate between the groups: the cCR rate was 28.4% in the GC group and 17.1% in the HD MVAC group, and the cPD rate was 4.5% in GC group and 7.3% in HD MVAC group (p = 0.337). Grade 3 or worse hematologic adverse events that occurred are presented in Table 2. The incidence of CTCAE Grade 3/4 neutropenia was 46.6% in the GC group and 19.5% in the HD MVAC group; these values are significantly different (p = 0.002). Despite the higher incidence of Grade 3/4 neutropenia in the GC group, the HD MVAC with prophylactic G-CSF group was associated with a higher incidence of febrile neutropenia than GC (0.6% in the GC group vs. 12.2% in the HD MVAC group, p < 0.001). The incidences of severe anemia (5.7% in the GC group vs. 9.8% in the HD MVAC group, p = 0.308) and thrombocytopenia (10.2% in the GC group vs. 12.2% in the HD MVAC group, p = 0.217) were comparable between the two groups. Severe non-hematologic adverse events are detailed in Supplement 2.
Surgery and pathologic outcomes
Overall, 71% of patients underwent surgery after NAC. The proportion of patients who underwent surgery was not different between the two groups (69.3% in the GC group vs. 75.6% in the HD MVAC group, p = 0.426, Table 3).
The rate of incomplete resection was 9% (n = 11) in the GC group and 13% (n = 4) in the HD MVAC group. The down-staging rate was 50.8% in the GC group and 58.1% in the HD MVAC group (p = 0.470). The pCR rate was 27.0% in the GC group and 22.6% in the HD MVAC group (p = 0.613).
Survival outcomes
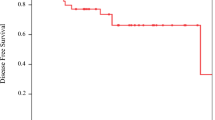
The survival outcome of patients with UC is shown by NAC regimen in Fig. 1. With a median follow-up duration of 37 months, there were no differences in OS and DFS between groups. The 3-year OS was 72.1% in the GC group and 73.1% in the HD MVAC group, the 5-year OS was 63.8% in the GC group and 67.9% in the HD MVAC group (HR = 1.21; 95% CI 0.60–2.43; p = 0.588), the 3-year DFS was 54.9% in the GC group and 63.2% in the HD MVAC group, and the 5-year DFS was 43.5% in the GC group and 63.2% in the HD MVAC group (HR = 1.42; 95% CI 0.81–2.49; p = 0.211).
Subgroup analysis
Our study consisted of patients with bladder cancer, upper tract urothelial cancer (UTUC), and both site involvement. We analyzed two groups of patients, bladder only subgroup and the UTUC subgroup (patients with upper tract lesions); the subgroups contained 180 and 37 patients, respectively.
The baseline characteristics of the bladder subgroup were not significantly difference, except in terms of age (Supplement Table 3). Neither the clinical outcomes (Supplement Table 4) nor the pathologic outcomes differed significantly between the two regimens (Supplement Table 5). The down-staging rate of the bladder-involved subgroup was 55.3% for GC and 55.6% for HD MVAC (p = 0.983), and the pCR rate was 31.9% for GC and 22.2% for HD MVAC (p = 0.510). In the UTUC subgroup, there were no significant differences in pathologic outcomes (Supplement Table 6). But a numerically higher proportion of patients treated with HD MVAC achieved pCR (25% vs. 10.7%) and down-staging (75% vs. 35.7%).
Prognostic factors affecting survival outcomes
The univariate and multivariate analyses of the potential prognostic factors for DFS and OS are summarized in Table 4. In the univariate analysis, the TNM stage, hydronephrosis, anemia, and down-staging to NAC were statistically significant factors associated with OS. Among them, down-staging alone remained as significant factor affecting OS and DFS in multivariate analysis. NAC was not a statistically significant prognostic factor for neither OS nor DFS.
Discussion
Our study showed that the HD MVAC regimen when used as NAC did not show superiority in efficacy and safety in patients with MIBC/UTUC compared with the GC regimen. There were no statistically significant differences in pCR, down-staging rate, OS, and DFS between groups. The proportion of patients who were not operated on owing to clinical progression or a deteriorated condition associated with adverse events was comparable between groups. Even though prophylactic G-CSF was given to all patients receiving HD MVAC, febrile neutropenia occurred more frequently in patients treated with HD MVAC group.
There are five published studies that compared GC and HD MVAC regimens used as NAC for bladder cancer (Table 5). Three of the studies were retrospective observational analyses, and two were prospective randomized studies (Flaig et al. 2019; Peyton et al. 2018; Pfister et al. 2021; van de Putte et al. 2016; Zargar et al. 2018). However, one is a randomized phase II trial with a primary endpoint of regimen-specific COXEN score (Flaig et al. 2019). The other is a multicenter randomized phase III trial but only reported secondary endpoints of pathology response and toxicity (Pfister et al. 2021). In contrast to our study, Peyton et al. and Zargar et al. showed that the HD MVAC regimen led to higher pCR and longer OS than the GC regimen (Peyton et al. 2018; Zargar et al. 2018). Similar to our analysis, Van de Putte et al. showed no differences in pCR rates between GC and HD MVAC (van de Putte et al. 2016). In the SWOG S1314 trial (COXEN Trial), even though the comparison of the efficacy between the two regimens was not the primary objective, both regimen showed no difference in pCR and pPR (< pT2) between the two regimens (Flaig et al. 2019). In the GETUG/AFU V05 VESPER trial, although the primary endpoint was not reported, there was no difference in pCR between regimens, while more patients in the HD MVAC arm achieved pPR (p = 0.07) (Pfister et al. 2021). The results of our analysis were mostly consistent with those of the latter three studies. Given the fact that the proportions of patients with incomplete NAC cycles (< 3) and those who did not undergo surgery were higher in the GC group in the current study, no statistical difference in pCR rate and OS suggests that it would be difficult to achieve better clinical outcome with just change of regimen from GC to HD MVAC.
The pCR rate in the HD MVAC group was 27.0% and 22.7% for the GC group in the current study. Peyton et al., the SWOG S1314 (COXEN) study, and the VESPER study showed a higher pCR rate than others (Flaig et al. 2019; Peyton et al. 2018; Pfister et al. 2021). The reason for this is probably higher proportions of patients with cT2 disease in these studies, 68.7%, 87%, and 90.3%, respectively. The pCR rate in the HD MVAC group in the current study was comparable with that reported by Choueiri et al., which enrolled patients with similar baseline clinical characteristics with the current study (Choueiri et al. 2014).
Although Grade 3 or higher neutropenia was more frequent in the GC group, the incidence of febrile neutropenia was significantly higher in the HD MVAC group, which were in line with the study reported by Van de Putte et al., and the VESPER trial (van de Putte et al. 2016). The majority of neutropenia encountered in the GC group was found on laboratory exam on day 8. It did not lead to clinically significant neutropenic fever if dose reduction or dose delay of gemcitabine is adequately employed. Besides, grade 3 or worse mucositis developed only in patients in the HD MVAC group, which is in line with higher grade 3 or worse gastrointestinal toxicities in the HD MVAC arm in the VESPER trial. From the perspective of adverse events, the GC regimen seems better and more tolerable.
The present study has several limitations. As anticipated from retrospective study design, selection bias may have occurred. Indeed, there was a statistically significant difference in the age between the HD MVAC and GC groups. The inclusion of patients with UTUC may have influenced the results of the analysis. However, in subgroup analysis involving only patients with MIBC, there were no significant differences in clinical and pathological outcomes. The difference in cohort size between the two groups and the small sample size in total may also have reduced the statistical power to assess the benefit of HD MVAC. A possible underestimation of toxicity may also have occurred because of different sampling points. Also, patients’ co-morbidity and performance status data were not captured and may act as a confounding factor for the statistical analysis.
The ongoing clinical trial results, the GETUG/AFU V05 VESPER trial, are eagerly needed to draw a firm conclusion on the benefit of HD MVAC over GC in terms of survival. However, as the VESPER trial compares six cycles of HD MVAC with four cycles of GC, a new question on an adequate number of NAC would be incurred.
Conclusion
Our findings did not show the superiority of the neoadjuvant HD MVAC regimen to the GC regimen in terms of efficacy and safety in patients with localized or locally advanced urothelial cancer.
References
Advanced Bladder Cancer Meta-analysis Collaboration (2003) Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis. Lancet 361:1927–1934. https://doi.org/10.1016/s0140-6736(03)13580-5
Alfred Witjes J et al (2017) Updated EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol 71:462–475. https://doi.org/10.1016/j.eururo.2016.06.020
Birtle A et al (2020) Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): a phase 3, open-label, randomised controlled trial. Lancet 395:1268–1277. https://doi.org/10.1016/S0140-6736(20)30415-3
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. https://doi.org/10.3322/caac.21492
Chakiryan N, Martinez A, Gao L, Liu JJ, Amling C, Garzotto M, Kopp RP (2019) Optimizing the sequence of chemotherapy for upper tract urothelial carcinoma with clinically positive regional lymph nodes. J Urol 202:76–82. https://doi.org/10.1097/JU.0000000000000172
Chang SS et al (2017) Treatment of non-metastatic muscle-invasive bladder cancer: AUA/ASCO/ASTRO/SUO guideline. J Urol 198:552–559. https://doi.org/10.1016/j.juro.2017.04.086
Choueiri TK et al (2014) Neoadjuvant dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin with pegfilgrastim support in muscle-invasive urothelial cancer: pathologic, radiologic, and biomarker correlates. J Clin Oncol 32:1889–1894. https://doi.org/10.1200/jco.2013.52.4785
Flaig TW et al (2019) SWOG S1314: A randomized phase II study of co-expression extrapolation (COXEN) with neoadjuvant chemotherapy for localized, muscle-invasive bladder cancer. J Clin Oncol 37:4506–4506. https://doi.org/10.1200/JCO.2019.37.15_suppl.4506
Grossman HB et al (2003) Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 349:859–866. https://doi.org/10.1056/NEJMoa022148
Leow JJ, Martin-Doyle W, Fay AP, Choueiri TK, Chang SL, Bellmunt J (2014) A systematic review and meta-analysis of adjuvant and neoadjuvant chemotherapy for upper tract urothelial carcinoma. Eur Urol 66:529–541. https://doi.org/10.1016/j.eururo.2014.03.003
National Cancer Institute (2010) Common Terminology Criteria for Adverse Events (CTCAE):Version 4.03; 2020. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_4.03.xlsx. Accessed 1 July 2020
National Comprehensive Cancer Network (2020) Bladder Cancer (version 6.2020); 2021. https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf. Accessed 15 Jan 2021
Peyton CC et al (2018) Downstaging and survival outcomes associated with neoadjuvant chemotherapy regimens among patients treated with cystectomy for muscle-invasive bladder cancer. JAMA Oncol 4:1535–1542. https://doi.org/10.1001/jamaoncol.2018.3542
Pfister C et al (2021) Randomized phase III trial of dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin, or gemcitabine and cisplatin as perioperative chemotherapy for patients with muscle-invasive bladder cancer. Analysis of the GETUG/AFU V05 VESPER trial secondary endpoints: chemotherapy toxicity and pathological responses. Eur Urol 79:214–221. https://doi.org/10.1016/j.eururo.2020.08.024
Sternberg CN et al (2001) Randomized phase III trial of high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol no. 30924. J Clin Oncol 19:2638–2646. https://doi.org/10.1200/jco.2001.19.10.2638
Sternberg CN et al (2006) Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur J Cancer 42:50–54. https://doi.org/10.1016/j.ejca.2005.08.032
van de Putte EE et al (2016) Neoadjuvant induction dose-dense MVAC for muscle invasive bladder cancer: efficacy and safety compared with classic MVAC and gemcitabine/cisplatin. World J Urol 34:157–162. https://doi.org/10.1007/s00345-015-1636-y
von der Maase H et al (2000) Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 18:3068–3077
Zargar H et al (2018) Neoadjuvant dose dense MVAC versus gemcitabine and cisplatin in patients with cT3–4aN0M0 bladder cancer treated with radical cystectomy. J Urol 199:1452–1458. https://doi.org/10.1016/j.juro.2017.12.062
Funding
Nothing to be declared.
Author information
Authors and Affiliations
Contributions
Dr. YJ Lee and JL Lee had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: YJ Lee, JL Lee. Acquisition, analysis, or interpretation of data: YJ Lee, JL Lee, YS Kim, BS Hong, YM Cho, JL Lee. Drafting of the manuscript: YJ Lee, JL Lee. Critical revision of the manuscript for important intellectual content: YJ Lee, JL Lee, YS Kim, BS Hong, YM Cho, JL Lee. Statistical analysis: YJ Lee, JL Lee. Administrative, technical, or material support: YJ Lee, JL Lee, YS Kim, BS Hong, YM Cho, JL Lee. Data analysis: YJ Lee, JL Lee.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no relevant conflict of interests.
Consent for publication
All authors consent to publish this study in J Cancer Res Clin Oncol.
Ethics approval
IRB of Asan Medical Center reviewed and approved this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, Y., Kim, Y.S., Hong, B. et al. Comparison of clinical outcomes in patients with localized or locally advanced urothelial carcinoma treated with neoadjuvant chemotherapy involving gemcitabine–cisplatin and high dose-intensity MVAC. J Cancer Res Clin Oncol 147, 3421–3429 (2021). https://doi.org/10.1007/s00432-021-03582-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-021-03582-x