Abstract
Purpose/background
Radiotherapy has been recently reported to boost the therapeutic response of immune checkpoint blockade (ICB); however, few studies have focused on programmed cell death-ligand 1 (PD-L1) expression in locally advanced rectal cancer (LARC) patients who receive preoperative neoadjuvant chemoradiotherapy (neoCRT). The aim of the present study was to investigate the PD-L1 expression status and CD8+ intra-tumoral infiltrating lymphocytes (TILs) before and after neoCRT and its association with clinicopathological characteristics in rectal cancer.
Materials and methods
Immunostainings of PD-L1 and CD8+ TILs were performed in 112 pair-matched LARC patients treated by neoCRT. Tumor PD-L1 expression and CD8+ TILs within the tumor microenvironment before and after neoCRT were evaluated via immunohistochemistry.
Results
High tumor PD-L1 expression was significantly increased from 50 to 63%, and high CD8+ TILs counts were also slightly increased from 32 to 35% after neoCRT treatment. High tumor PD-L1 before and after neoCRT was associated with improved disease-free survival (DFS, pre-neoCRT: p = 0.003 and post-neoCRT: p = 0.003) and overall survival (OS, pre-neoCRT: p = 0.045 and post-neoCRT: p = 0.0001). High CD8+ TILs before neoCRT was associated with improved DFS (p = 0.057), and it was significantly associated with improved DFS after neoCRT (p = 0.039). Patients with high tumor PD-L1 and CD8+ TILs before and after neoCRT were significantly associated with improved DFS (pre-neoCRT: p = 0.004 and post-neoCRT: p = 0.006).
Conclusion
The present results provide evidence that tumor PD-L1 expression and recruitment of CD8+ TILs within the tumor microenvironment were increased by neoCRT treatment. Tumor PD-L1 and CD8+ TILs are prognostic biomarkers for the survival of LARC patients treated with neoCRT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is among the most common cancers worldwide (Jemal et al. 2009), and rectal cancer cases account for ~ 30% of all CRCs (Conde-Muino et al. 2015). However, different therapeutic strategies were required for colon and rectal cancers. Preoperative (neoadjuvant) chemoradiotherapy (neoCRT) has been reported as the most effective therapeutic strategy to control tumor growth and improve clinical outcome in patients with locally advanced rectal cancer (LARC, cT3-4 or cN+ patients) (Sauer et al. 2012; Yoon et al. 2015). After neoCRT treatment, only 15–20% of LARC patients achieve a complete response with no residual tumor, most LARC patients achieve a pathological partial response (Balko and Black 2009). neoCRT treatment not only directly induces cancer cell death but also activates anti-tumor immunity via a process called immunogenic cell death (ICD). This process triggers the release of danger-associated molecular patterns (DAMPs) and cytokines from damaged cancer cells and immune cells to create an inflammatory and immunogenic tumor microenvironment to activate T lymphocytes for anti-tumor immunity (Showalter et al. 2017; Wennerberg et al. 2017).
Programmed cell death 1 ligand 1 (PD-L1/CD274) negatively regulates T lymphocytes to cause lymphocyte “exhaustion” through the programmed cell death 1 receptor (PD-1) (Keir et al. 2008). Upregulation of PD-L1 in malignant cells leads to suppression of cytotoxic CD8+ T lymphocyte activity (Hirano et al. 2005; Topalian et al. 2012). Therefore, PD-1/PD-L1 immunotherapy via immune checkpoint blockade (ICB) has been proposed as a promising therapeutic strategy to re-activate the host immune system to eradicate tumors, which have demonstrated impressive therapeutic responses in patients with melanoma, non-small cell lung cancer (NSCLC), renal cell carcinoma (RCC), and bladder cancer (Wang et al. 2016). However, PD-L1 expression can be induced by immune cell infiltration in several malignancies, and interferon-γ secreted by the infiltrated immune cells is required for PD-L1 induction for adaptive immune resistance (Spranger et al. 2013; Taube et al. 2012). In colorectal cancer, patients with microsatellite instability (MSI) have improved therapeutic response for immune checkpoint blockade (ICB) (Lee et al. 2016; Phipps et al. 2015). But the MSI is rarely noted in rectal cancer.
Recently, classification of tumors microenvironment into subgroups on the basis of PD-L1 status and the density of tumor-infiltrating lymphocytes (TILs) were proposed as a predictive marker for the therapeutic response to ICB (Taube et al. 2012; Teng et al. 2015). Patients with high tumor PD-L1 and TILs are most likely to benefit from PD-1/PD-L1 immunotherapy. Moreover, high PD-L1 and TILs are also associated with improved survival outcome in colorectal cancer and breast cancer (Huang et al. 2018c; Schalper et al. 2014). However, there are only a few studies about PD-L1 expression and CD8+ TILs in rectal cancer treated by neoCRT (Lim et al. 2017; Ogura et al. 2018). In the present study, we aimed to evaluate the relationship between tumor PD-L1 expression and CD8+ TILs in pair-matched locally advanced rectal cancer before and after neoCRT treatment and analyze the survival outcomes according to the immune status focused on PD-L1 expression and CD8+ TILs. These results provide the basis for knowledge of immunologic impact on neoCRT, thus suggesting the potential for a combined strategy of cytotoxic therapy and immune checkpoint inhibitors.
Materials and methods
Patient characteristics, clinical staging, treatment, and pathological evaluation
Two hundred eleven patients with locally advanced rectal cancer were treated at our hospital from 2006 to 2014. Among these patients, 171 received neoCRT followed by surgery. Patients with biopsy-proven locally advanced rectal cancer [cT3-4 or cN+ by endorectal ultrasonography (EUS), computed tomography (CT), or magnetic resonance imaging (MRI)] who were treated with preoperative chemoradiotherapy followed by radical resection at China Medical University Hospital comprised the study cohort. This study was reviewed and approved by the Institutional Review Board (IRB) of China Medical University Hospital [Protocol number: CMUH105-REC2-072]. Tumors were staged based on the American Joint Committee on Cancer (AJCC) staging system. EUS, MRI or CT was used to assess the pretreatment clinical stage, and pretreatment biopsies were reviewed by pathologists as previously described (Huang et al. 2018b).
Patients were treated with chemoradiotherapy with a median radiotherapy dose of 50.4 Gy in 28 fractions and concurrent fluoropyrimidine-based chemotherapy (mainly single-agent orally administration with capecitabine, 500 mg/m2/day b.i.d). Patients were assessed for their clinical response 6–8 weeks after the completion of neoCRT according to rigorous criteria of clinical, endoscopic, and radiologic findings. The three criteria for complete clinical response (cCR) were (a) the absence of a residual ulceration, mass, or mucosal irregularity upon clinical/endoscopic assessment; (b) whitening of the mucosa and the presence of neovasculature; and (c) radiologic imaging, such as CT, RUS, or MRI, without evidence of extrarectal residual disease.
After the chemoradiotherapy regime was completed, surgery was performed 6 to 8 weeks later. Low anterior resection, proctectomy with coloanal reconstruction, abdominoperineal resection, or multivisceral rectal resection were included according to total mesorectal excision (TME) principles. Resected specimen pathologic staging was performed after resection in accordance with the guidelines of the College of American Pathologists. Adjuvant chemotherapy was recommended for patients with metastatic lymph node(s) in surgical specimens and consisted of fluorouracil infusion or capecitabine for a period of 4–6 months. Tumor regression grade (TRG) of a primary tumor after neoCRT was semi-quantitatively evaluated on hematoxylin and eosin-stained slides according to Dworak’s criteria (Dworak et al. 1997): TRG 0, tumor without regression; TRG 1, dominant tumor mass with obvious fibrosis; TRG 2, dominantly fibrotic changes with few tumor cells; TRG 3, very few tumor cells in the fibrotic tissue; and TRG 4, no viable tumor cells.
Construction of tissue microarray (TMA) and immunohistochemistry
Tissue microarrays were constructed from 112 pair-matched pre-neoCRT biopsies and post-neoCRT surgical tissue from rectal cancer patients, and other specimens were not available (material not suitable for IHC) as previously described. Areas of tumor cells were marked on the hematoxylin and eosin (H&E)-stained slides. The corresponding area on the matching paraffin block (donor block) was then identified and marked. We used the AutoTiss 10C system (EverBio Technology Inc., Taipei, Taiwan) to remove the tissue core from these areas of the donor blocks into the recipient block in a precise, arrayed fashion. The punches were 2 mm in diameter, and a maximum of 60 punches were placed on a single block. Sample sections cut on a microtome were then mounted on capillary-gap slides (Dako, Hamburg, Germany).
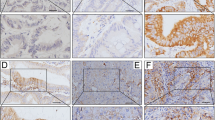
Immunohistochemistry (IHC) was performed using 3-µm thick histological TMA sections as previously described (Huang et al. 2018a, b; Lin et al. 2015; Wang et al. 2018). TMA slides were stained individually with horseradish peroxidase-conjugated avidin–biotin complex (ABC) using the Vectastain Elite ABC Kit (Vector Laboratories, Burlingame, CA, USA) and NovaRed chromogen (Vector Laboratories) and counterstained with hematoxylin. The following antibodies were used in this study: anti-PD-L1 (ab205921, abcam, Cambridge, UK) and CD8 (ab4055, abcam, Cambridge, UK). The stained tissue sections were scored separately by two pathologists blinded to the clinicopathological parameters. Tumor PD-L1 immunostaining was scored in accordance with the intensity and extent of staining on a semiquantitative scale (0–3+) as follows: 0, absent; 1+, weak; 2+, moderate; 3+, strong membrane staining. The percentage of PD-L1 tumor cells was recorded as follows: a score of 0 was assigned when no staining or positive tumor cell proportion was detected in < 5% of the cells; a score of 1 was assigned when membranous staining was present in > 5% of the positive cell proportion. The 5% threshold was based on a previous phase I trial of anti-PD-1 agents and studies of other malignancies (Thompson et al. 2006; Topalian et al. 2012). CD8 staining was positive when detected in the cytoplasm or at the cell membrane of tumor-infiltrating lymphocytes (TILs) and was evaluated using microscopy (OLYMPUS BX53, Tokyo, Japan) according to the intensity of CD8+ TILs. Two pathologists blinded to all sample information evaluated the CD8+ TILs. With respect to the detection of CD8+ TILs, the tissue was reviewed at 40 × magnification, and the area with the highest density of CD8+ TILs adjacent to malignant cells was counted at 400 × magnification (no. of CD8+ TILs/high-power field). The average number of CD8+ TILs in five high-power fields was included in the evaluation. For CD8, a count of zero CD8+ TILs in a high-power field was given a score of 0, a count of 1–3 CD8+ TILs was given a score of 1, a count of 4–10 CD8+ TILs was given a score of 2, and a count of > 10 CD8+ TILs was given a score of 3 (Chiang et al. 2018; Goode et al. 2017; Huang et al. 2018c).
Statistical analysis
SAS statistical software version PC 9.4 (SAS Institute, NC, USA) was used to perform the statistical analysis. All tests reported a two-sided p value with a significance level set at 0.05. Student’s t test, Pearson Chi-square and Fisher’s exact test were used for group comparisons. Cox regression analysis was used to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for univariate and multivariate models. Influential factors that affected the rectal cancer patient survival rate were adjusted in the Cox models, including TRG (3 + 4 vs 1 + 2), clinical response (complete response and partial response vs stable disease and disease progression), and pN stage (positive vs negative). The Kaplan–Meier method was used to estimate the 5-year overall survival and disease-free survival. Survival time was defined as the time from surgery until death. The univariate comparison was performed using the log-rank test.
Results
Patient characteristics
Table 1 presents the clinical pathological characteristics of these pair-matched patients (112 pre-neoCRT biopsies and 97 post-neoCRT surgical tissues). The mean age at diagnosis was 59.4 ± 12.5 years (range 31–90 years). The majority of the patients were men (69%). The median radiation dose was 50.4 Gy administered in 28 fractions (minimum dose: 44.8 Gy; maximum dose: 50.4 Gy). Concurrent chemotherapy was fluorouracil-based for 44% of the LARC patients and capecitabine for 47%. All patients underwent total mesorectal excision (TME) depending on the extent and location of the tumor after neoCRT. Surgical specimens were reviewed and scored based on the Tumor Regression Grade (TRG) system (Rodel et al. 2005). In total, 13% (15/112) of patients exhibited a pathologic complete response (pCR, TRG 4), whereas 87% (97/112) of patients exhibited a pathologic partial response (TRG 1–3). After neoCRT treatment, a pathological tumor regression grade 4 (TRG 4) sample was not included in the TMA because the sample had no residual tumor. Thirty-seven patients (33%) presented with lymph node metastases, and twenty-four patients (21%) presented with distant metastasis (Table 1).
Tumor PD-L1 expression and CD8+ intra-tumoral infiltrating lymphocytes were analyzed by immunohistochemistry (IHC) and examined in tumor tissues and adjacent normal mucosae. PD-L1 was detectable in epithelial cells from normal colonic mucosae and cancer cells (Fig. 1). The clinicopathologic characteristics of patients and the correlation with PD-L1 expression in pre-neoCRT biopsies and post-neoCRT surgical tissues are presented in Table 1.
Tumor PD-L1 expression is associated with 5-year DFS and 5-year OS in LARC patients
We found that 28 LARC patients (25%) died within the 5-year follow-up period, and the estimated 5-year disease-free survival (DFS) and overall survival (OS) rates were 67% and 75%, respectively (Table 2). In the 5-year DFS analysis, patients with negative pN stage (79% vs 59%, p = 0.004), good clinical response (82% vs 61%, p = 0.013), and high tumor PD-L1 expression with the pre-treatment biopsies (86% vs 57%, p = 0.003) exhibited significantly better DFS. Moreover, patients with high CD8+ TILs within tumor microenvironment in the pre-neoCRT biopsies exhibited a tendency for better DFS (83% vs 66%, p = 0.057). After neoCRT regimen treatment, patients with high tumor PD-L1 (82% vs 53%, p = 0.003) and CD8+ TILs (85% vs 63%, p = 0.0039) within the tumor microenvironment in the post-neoCRT surgical tissues exhibit better DFS. In the 5-year OS analysis, patients with high tumor PD-L1 expression in the pre-neoCRT biopsies (91 vs 73%, p = 0.045) and post-neoCRT surgical tissues (93 vs 64%, p = 0.0001) exhibited significantly better OS.
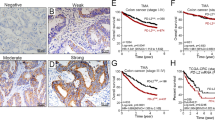
By Kaplan–Meier survival analysis, patients with high tumor PD-L1 expression were associated with a significantly better 5-year DFS in the pre-neoCRT biopsies (Log rank p = 0.003, Fig. 2a) and post-neoCRT surgical tissues (Log rank p = 0.003, Fig. 2b). Patients with high CD8+ TILs exhibit a tendency for better 5-year DFS in pre-neoCRT biopsies (log rank p = 0.0586, Fig. 2c) and are associated with a significantly better 5-year DFS in the post-neoCRT surgical tissues (log rank p = 0.0385, Fig. 2d). These results suggest that upregulated tumor PD-L1 reflects good clinical outcome in LARC patients.
Kaplan–Meier curves of DFS with tumor PD-L1 and intra-tumoral CD8+ TILs in LARC patients. a Kaplan–Meier curves demonstrating that tumor PD-L1 expression is associated with 5-year DFS in pre-neoCRT biopsies (p = 0.003). b Kaplan–Meier curves demonstrating that tumor PD-L1 expression is associated with 5-year DFS in post-neoCRT surgical tissues (p = 0.003). c High density of CD8+ TILs within the tumor microenvironment is with associated improved 5-year DFS in pre-neoCRT biopsies (p = 0.0566). d High density of CD8+ TIL within the tumor microenvironment is associated with improved 5-year DFS in post-neoCRT surgical tissues (p = 0.0385). e Patients with both high tumor PD-L1 and CD8+ TILs within the tumor microenvironment are associated with improved 5-year DFS in pre-neoCRT biopsies (p = 0.0037). f Patients with both high tumor PD-L1 and CD8+ TILs within the tumor microenvironment are associated with improved 5-year DFS in post-neoCRT surgical tissues (p = 0.0061)
Next, we assessed the survival differences between groups classified by these two factors. Within the combined group of CD8+ TIL and tumor PD-L1 subsets, patients with both high CD8+ TILs and tumor PD-L1 levels exhibited significantly better DFS in the pre-neoCRT biopsies (100% vs 65%, p = 0.0037, Fig. 2e) or post-neoCRT surgical tissues (96% vs 63%, p = 0.0061, Fig. 2f). These results suggest that combined CD8+ TILs and tumor PD-L1 expression can be a good prognostic factor for LARC patients who are receiving neoCRT treatment.
Independent risk factor for LARC patients with neoCRT treatment
In the univariate analysis of 5-year DFS, the following parameters were associated with patient survival rate: pN stage and clinical response. Moreover, CD8+ TIL counts and tumor PD-L1 levels were statistically associated with 5-year DFS. Patients with low tumor PD-L1 expression exhibited an increased risk for a poorer 5-year DFS (HR = 3.139, 95% CI 1.410–6.991, p = 0.005), and those with a low density of CD8+ TILs also exhibited a tendency for increased risk of a lower 5-year DFS (HR = 2.308, 95% CI 0.950–5.611, p = 0.065) compared with patients with a high CD8+ TIL count and high tumor PD-L1 in pre-neoCRT biopsies (Table 3). Moreover, patients with both a low CD8+ TIL count and tumor PD-L1 exhibited an increased risk in terms of 5-year DFS in the pre-neoCRT biopsies (HR = 17.01, 95% CI 2.41–2152.60, p = 0.05). Similar results were also observed in the univariate analysis of 5-year OS (Table 3).
After neoCRT treatment, patients with low tumor PD-L1 exhibited an increased risk for reduced 5-year DFS (HR = 2.982, 95% CI 1.392–6.385, p = 0.005), and those with a low density of CD8+ TILs also exhibited an increased risk for reduced 5-year DFS (HR = 2.663, 95% CI 1.012–7.011, p = 0.047) compared with patients with a high CD8+ TIL count and high tumor PD-L1 in the post-neoCRT surgical tissues (Table 3). Moreover, patients with both a low CD8+ TIL count and tumor PD-L1 exhibited an increased risk in terms of 5-year DFS in the post-neoCRT surgical tissues (HR = 9.654, 95% CI 1.311–71.071, p = 0.026). These results indicate that CD8+ TILs and tumor PD-L1 exhibit significant prognostic value for locally advanced rectal cancer patients.
Subsequently, we examined whether the inclusion of other variables affected the parameter estimate for CD8+ TILs and tumor PD-L1 (Table 4). Patients with a low tumor PD-L1 level within the tumor microenvironment presented an increased risk for poor DFS either in the pre-neoCRT biopsies (HR = 2.765, 95% CI 1.232–6.209, p = 0.01, Table 4) or post-neoCRT surgical tissues (HR = 2.692, 95% CI 1.245–5.820, p = 0.01, Table 4) after adjustment for age, pN stage, clinical response, and TRG. Moreover, patients with both a low density of CD8+ TILs or a low tumor PD-L1 level within the tumor microenvironment exhibited an increased risk of a poor DFS (HR = 8.132, 95% CI 1.103–59.978, p = 0.04, Table 4). These results demonstrate that the combination of CD8+ TILs and tumor PD-L1 levels is an independent prognostic factor (Table 4).
Discussion
This pair-matched analysis of pre-neoCRT biopsies and post-neoCRT surgical rectal cancer specimens demonstrated that both PD-L1 expression and the density of CD8+ TILs markedly increased after preoperative chemoradiotherapy (neoCRT).
Patients with a consistently high level of PD-L1 expression experienced better DFS and OS, and patients with high CD8+ TILs recruitment within tumor microenvironment by neoCRT also exhibited improved DFS. These rectal cancer patients with both high PD-L1 and CD8+ TILs profiles were associated with best survival outcomes compared with other groups as assessed by Kaplan–Meier analysis. Multivariate analysis models demonstrated that patients with low PD-L1 expression and CD8+ TILs are associated with increased risk on DFS after neoCRT treatment, suggesting that tumor PD-L1 and CD8+ TILs are prognostic factors for locally advanced rectal cancer receiving neoCRT.
To date, few studies evaluated PD-L1 expression after neoCRT in all malignancies, including rectal cancer (Hecht et al. 2016; Jomrich et al. 2016; Ogura et al. 2018; Saigusa et al. 2016). Our analysis including 112 pair-matched rectal cancer patients, 112 pre-neoCRT biopsies and 97 post-neoCRT surgical specimens (15 patients with TRG4), and we observed a significant increase in the proportion of tumor PD-L1. This matched pair analysis of rectal cancer highlights the immunologic impact of neoCRT on the level of PD-L1 checkpoint molecules and CD8+ TIL recruitment, which demonstrates their prognostic value on survival outcome in patients with locally advanced rectal cancers. Based on increased PD-L1 expression and CD8+ TIL density after neoCRT, more prominent tumor-specific immune responses after treatment could be expected. Direct irradiation on tumor tissues upregulates and releases tumor-associated antigens (TAAs) (Gameiro et al. 2014), damage-associated pattern molecules (DAMPs) (Huang et al. 2018b), and major histocompatibility complex molecules (MHC), which is an important underlying mechanism of immunogenic cell death (Pol et al. 2015). Consecutive chemoradiotherapy promotes TAA and DAMP release, increasing inflammatory cytokines and cytotoxic T lymphocytes (CTLs) maturation (Showalter et al. 2017). These effects result in the shift of immunologic equilibrium within the tumor microenvironment. Recent reports have demonstrated that tumor PD-L1 expression was strongly correlated with improved survival outcomes in several malignancies, such as breast cancer (Baptista et al. 2016; Sabatier et al. 2015; Schalper et al. 2014), NSCLC (Velcheti et al. 2014), malignant melanomas (Taube et al. 2012), and CRC (Droeser et al. 2013). However, previous studies have demonstrated that high tumor PD-L1 expression was identified in mismatch repair-deficient (MMR) colorectal cancer patients (Lee et al. 2016), who exhibit a better therapeutic response to anti-PD-1 immunotherapy (Dudley et al. 2016). Kim et al. (2016) reported that PD-L1 expression in MSI-high colorectal cancer is associated with higher CD3+ TILs. MSI-high colorectal cancer is characterized by high mutation load, a high level of TILs, and high PD-L1 expression on tumor and stromal immune cells (Xiao and Freeman 2015). However, MSI-high is rare in rectal cancer (Phipps et al. 2015), and the underlying mechanism of high tumor PD-L1 in the present study should be further investigated.
Immune system function is a dynamic balance between stimulatory and inhibitory forces and upshifting the antitumor immunity is a well-known trigger for inhibitory immune checkpoints. In our results, a marked neoCRT-induced immunologic response increased the expression of PD-L1 [pre-neoCRT: 50.0% (56/112) and post-neoCRT: 62.9% (61/97)] and density of CD8+ TILs [pre-neoCRT: 32.1% (36/112) and post-neoCRT: 35.1% (34/97)]. Moreover, patients with sustained high PD-L1 activity or high CD8+ TIL density before and after neoCRT exhibited better survival outcomes. In neoCRT-treated rectal cancer, the mechanisms of increased PD-L1 expression after neoCRT remain to be elucidated. However, these high CD8+ TILs after neoCRT, which could be induced by increased TAAs and DAMPs release by neoCRT (Huang et al. 2018b; Smyth et al. 2015), was associated with high PD-L1 expression.
Recent studies implied that high tumor PD-L1 expression is involved with the feedback mechanism caused by the induction of IFN-γ, especially in tumor cells and CD8+ TILs (Chiang et al. 2018; Droeser et al. 2013). Hence, our data demonstrate that PD-L1 may be upregulated by the host immune response through IFN-γ that is released from CD8+ TILs, suggesting that subsequent treatment may reinvigorate the preexisting anti-tumor immune response, leading to better responses. Therefore, these immunologic factors were used to identify patients with more prominent anti-tumor immunity, suggesting potential candidates who can benefit from further enhancement of tumor-specific immune responses. If increased PD-L1 expression after neoCRT is related to an immune-suppressive microenvironment, immune checkpoint blockade might improve the response to neoCRT in PD-L1 high rectal cancer. Future studies are warranted to establish the validity of a therapeutic strategy combining checkpoint inhibitors with conventional cytotoxic treatments or neoCRT to improve the response rate in rectal cancer.
Taken together, this study demonstrates that increased PD-L1 expression in both pre- and post-neoCRT tissues correlate with improved prognosis for patient with LARC. Moreover, combinational PD-L1 expression and CD8+ TILs may be useful biomarkers to predict outcomes in patients receiving neoCRT treatment for LARC.
References
Balko JM, Black EP (2009) A gene expression predictor of response to EGFR-targeted therapy stratifies progression-free survival to cetuximab in KRAS wild-type metastatic colorectal cancer. BMC Cancer 9:145. https://doi.org/10.1186/1471-2407-9-145
Baptista MZ, Sarian LO, Derchain SF, Pinto GA, Vassallo J (2016) Prognostic significance of PD-L1 and PD-L2 in breast cancer. Hum Pathol 47:78–84. https://doi.org/10.1016/j.humpath.2015.09.006
Chiang SF et al (2018) Upregulation of tumor PD-L1 by neoadjuvant chemoradiotherapy (neoCRT) confers improved survival in patients with lymph node metastasis of locally advanced rectal cancers. Cancer Immunol Immunother. https://doi.org/10.1007/s00262-018-2275-0
Conde-Muino R, Cuadros M, Zambudio N, Segura-Jimenez I, Cano C, Palma P (2015) Predictive biomarkers to chemoradiation in locally advanced rectal cancer. Biomed Res Int 2015:921435 https://doi.org/10.1155/2015/921435
Droeser RA et al (2013) Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. Eur J Cancer 49:2233–2242. https://doi.org/10.1016/j.ejca.2013.02.015
Dudley JC, Lin MT, Le DT, Eshleman JR (2016) Microsatellite Instability as a Biomarker for PD-1 Blockade. Clin Cancer Res 22:813–820. https://doi.org/10.1158/1078-0432.CCR-15-1678
Dworak O, Keilholz L, Hoffmann A (1997) Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis 12:19–23
Gameiro SR, Jammeh ML, Wattenberg MM, Tsang KY, Ferrone S, Hodge JW (2014) Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget 5:403–416. https://doi.org/10.18632/oncotarget.1719
Goode EL et al (2017) Dose-response association of CD8+ tumor-infiltrating lymphocytes and survival time in high-grade serous ovarian. Cancer JAMA Oncol 3:e173290. https://doi.org/10.1001/jamaoncol.2017.3290
Hecht M et al (2016) PD-L1 is upregulated by radiochemotherapy in rectal adenocarcinoma patients and associated with a favourable prognosis. Eur J Cancer 65:52–60. https://doi.org/10.1016/j.ejca.2016.06.015
Hirano F et al (2005) Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res 65:1089–1096
Huang CY et al (2018a) HMGB1 promotes ERK-mediated mitochondrial Drp1 phosphorylation for chemoresistance through RAGE in colorectal cancer. Cell Death Dis 9:1004. https://doi.org/10.1038/s41419-018-1019-6
Huang CY et al (2018b) Cytosolic high-mobility group box protein 1 (HMGB1) and/or PD-1 + TILs in the tumor microenvironment may be contributing prognostic biomarkers for patients with locally advanced rectal cancer who have undergone neoadjuvant chemoradiotherapy. Cancer Immunol Immunother 67:551–562. https://doi.org/10.1007/s00262-017-2109-5
Huang CY, Chiang SF, Ke TW, Chen TW, You YS, Chen WT, Chao KSC (2018c) Clinical significance of programmed death 1 ligand-1 (CD274/PD-L1) and intra-tumoral CD8+ T-cell infiltration in stage II–III colorectal cancer. Sci Rep 8:15658. https://doi.org/10.1038/s41598-018-33927-5
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ (2009) Cancer statistics, 2009. CA Cancer J Clin 59:225–249. https://doi.org/10.3322/caac.20006
Jomrich G, Silberhumer GR, Marian B, Beer A, Mullauer L (2016) Programmed death-ligand 1 expression in rectal cancer. Eur Surg 48:352–356. https://doi.org/10.1007/s10353-016-0447-8
Keir ME, Butte MJ, Freeman GJ, Sharpe AH (2008) PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 26:677–704. https://doi.org/10.1146/annurev.immunol.26.021607.090331
Kim JH, Park HE, Cho NY, Lee HS, Kang GH (2016) Characterisation of PD-L1-positive subsets of microsatellite-unstable colorectal cancers. Br J Cancer 115:490–496. https://doi.org/10.1038/bjc.2016.211
Lee LH et al (2016) Patterns and prognostic relevance of PD-1 and PD-L1 expression in colorectal carcinoma. Mod Pathol 29:1433–1442. https://doi.org/10.1038/modpathol.2016.139
Lim YJ et al (2017) Chemoradiation-induced alteration of programmed death-ligand 1 and CD8+ tumor-infiltrating lymphocytes identified patients with poor prognosis in rectal cancer: a matched comparison analysis. Int J Radiat Oncol Biol Phys 99:1216–1224. https://doi.org/10.1016/j.ijrobp.2017.07.004
Lin TY, Fan CW, Maa MC, Leu TH (2015) Lipopolysaccharide-promoted proliferation of Caco-2 cells is mediated by c-Src induction and ERK activation. Biomedicine (Taipei) 5:5 https://doi.org/10.7603/s40681-015-0005-x
Ogura A et al (2018) Pattern of programmed cell death-ligand 1 expression and CD8-positive T-cell infiltration before and after chemoradiotherapy in rectal cancer European. J Cancer 91:11–20. https://doi.org/10.1016/j.ejca.2017.12.005
Phipps AI et al (2015) Association between molecular subtypes of colorectal cancer and patient survival. Gastroenterology 148:77–87.e72. https://doi.org/10.1053/j.gastro.2014.09.038
Pol J et al (2015) Trial Watch: immunogenic cell death inducers for anticancer chemotherapy. Oncoimmunology 4:e1008866. https://doi.org/10.1080/2162402X.2015.1008866
Rodel C et al (2005) Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol 23:8688–8696. https://doi.org/10.1200/JCO.2005.02.1329
Sabatier R et al (2015) Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget 6:5449–5464. https://doi.org/10.18632/oncotarget.3216
Saigusa S et al (2016) Implication of programmed cell death ligand 1 expression in tumor recurrence and prognosis in rectal cancer with neoadjuvant chemoradiotherapy. Int J Clin Oncol 21:946–952. https://doi.org/10.1007/s10147-016-0962-4
Sauer R et al (2012) Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 30:1926–1933. https://doi.org/10.1200/JCO.2011.40.1836
Schalper KA, Velcheti V, Carvajal D, Wimberly H, Brown J, Pusztai L, Rimm DL (2014) In situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clin Cancer Res 20:2773–2782. https://doi.org/10.1158/1078-0432.CCR-13-2702
Showalter A, Limaye A, Oyer JL, Igarashi R, Kittipatarin C, Copik AJ, Khaled AR (2017) Cytokines in immunogenic cell death: applications for cancer immunotherapy. Cytokine 97:123–132. https://doi.org/10.1016/j.cyto.2017.05.024
Smyth MJ, Ngiow SF, Ribas A, Teng MWL (2015) Combination cancer immunotherapies tailored to the tumour microenvironment. Nat Rev Clin Oncol 13:143–158. https://doi.org/10.1038/nrclinonc.2015.209
Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF (2013) Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med 5:200ra116. https://doi.org/10.1126/scitranslmed.3006504
Taube JM et al (2012) Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 4:127ra137. https://doi.org/10.1126/scitranslmed.3003689
Teng MWL, Ngiow SF, Ribas A, Smyth MJ (2015) Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res 75:2139–2145. https://doi.org/10.1158/0008-5472.can-15-0255
Thompson RH et al (2006) Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res 66:3381–3385. https://doi.org/10.1158/0008-5472.CAN-05-4303
Topalian SL et al (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366:2443–2454. https://doi.org/10.1056/NEJMoa1200690
Velcheti V et al (2014) Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest 94:107–116. https://doi.org/10.1038/labinvest.2013.130
Wang X, Teng F, Kong L, Yu J (2016) PD-L1 expression in human cancers and its association with clinical outcomes. Onco Targets Ther 9:5023–5039. https://doi.org/10.2147/OTT.S105862
Wang X et al (2018) RSF-1 overexpression determines cancer progression and drug resistance in cervical cancer. Biomedicine (Taipei) 8:4. https://doi.org/10.1051/bmdcn/2018080104
Wennerberg E, Vanpouille-Box C, Bornstein S, Yamazaki T, Demaria S, Galluzzi L (2017) Immune recognition of irradiated cancer cells. Immunol Rev 280:220–230. https://doi.org/10.1111/imr.12568
Xiao Y, Freeman GJ (2015) The microsatellite instable subset of colorectal cancer is a particularly good candidate for checkpoint blockade. Immunother Cancer Discov 5:16–18. https://doi.org/10.1158/2159-8290.cd-14-1397
Yoon WH, Kim HJ, Kim CH, Joo JK, Kim YJ, Kim HR (2015) Oncologic impact of pathologic response on clinical outcome after preoperative chemoradiotherapy in locally advanced rectal cancer. Ann Surg Treat Res 88:15–20. https://doi.org/10.4174/astr.2015.88.1.15
Acknowledgements
We are grateful for the tissue microarray support from the Translation Research Core, China Medical University Hospital. This study is supported in part by China Medical University Hospital (DMR-107-061), Ministry of Science and Technology Ministry of Science and Technology (MOST107-2314-B-039-027-MY3 and MOST107-2314-B-039-057-MY3, Taiwan), and Ministry of Health and Welfare (MOHW107-TDU-B-212-123004, Taiwan), Health and welfare surcharge of tobacco products, China Medical University Hospital Cancer Research Center of Excellence (MOHW108-TDU-B-212-124024, Taiwan).
Author information
Authors and Affiliations
Contributions
T-WC, S-FC and KC-YH conducted and performed the experiments; WT-LC, T-WK and T-WC enrolled the LARC patients and performed IHC evaluation; S-FC and KSCC supervised this study; S-FC, and KSCC analyzed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was reviewed and approved by the Internal Review Board (IRB) of China Medical University Hospital [Protocol number: CMUH105-REC2-072].
Informed consent
Informed consents were obtained from all participants in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, TW., Huang, K.CY., Chiang, SF. et al. Prognostic relevance of programmed cell death-ligand 1 expression and CD8+ TILs in rectal cancer patients before and after neoadjuvant chemoradiotherapy. J Cancer Res Clin Oncol 145, 1043–1053 (2019). https://doi.org/10.1007/s00432-019-02874-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-019-02874-7