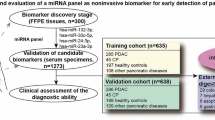
Abstract
Purpose
Recently, we identified the microRNA-99 family as unfavorable prognostic factor in patients with pancreatic ductal adenocarcinoma (PDAC). The aim of this study is to evaluate its value as circulating biomarker for PDAC.
Methods
Tissue and corresponding preoperative blood samples of 181 patients with PDAC UICC Stages I–IV (n = 90), intraductal papillary mucinous neoplasm (IPMN, n = 11), chronic pancreatitis (n = 40), pancreatic cystadenoma (n = 20), and age-matched healthy blood serum controls (n = 20) were collected between 2014 and 2017 prospectively. Expression of microRNA-21 as confirmatory marker and the microRNA-99 family, consisting of microRNA-99a, -99b, and -100, was analyzed by qRT-PCR. Target analysis of insulin-like growth factor 1 receptor (IGF1R) was performed using tissue array immunohistochemistry and Western blotting.
Results
Expression of microRNA-99 family members was significantly increased in macrodissected tumor tissue and corresponding blood serum samples (p < 0.05) of patients with PDAC of all stages. Correspondingly, its target protein IGF1R was upregulated (p < 0.001) in carcinoma tissue. Circulating and tissue-related microRNA-100 could well discriminate PDAC from healthy samples with area under the receiver operating characteristic (ROC) curve (AUC) values of 0.81 and 0.85, respectively. Low expression of circulating microRNA-100 was associated with significantly improved overall survival (p = 0.004) and recurrence-free survival (p = 0.03) in multivariate analyses. Circulating microRNA-21 was overexpressed in PDAC with fair discrimination between PDAC and healthy controls (AUC = 0.71) and decreased overall survival (p = 0.046) and recurrence-free survival (p = 0.03) in PDAC patients.
Conclusions
Multivariate survival and ROC analyses identified circulating microRNA-100 as potential diagnostic and prognostic marker in PDAC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Pancreatic ductal adenocarcinoma (PDAC) moved from the fourth leading cause of cancer-related death in the USA and Europe to the third, surpassing breast cancer. While overall cancer incidence and death rates are declining worldwide, the incidence of more than 330.000 new cases with similar number of deaths from PDAC is estimated to increase (Lucas et al. 2016; Siegel et al. 2017).
Curative surgery still offers the best chance for survival if PDAC is diagnosed in early stage. This is the case in less than 20% with tumor recurrence in approximately 80% within 5 years postoperatively. Furthermore, only a small improvement of survival could be achieved by adjuvant or palliative chemotherapy with gemcitabine since 1997, erlotinib since 2007, FOLFIRINOX combination therapy since 2011, paclitaxel since 2013, and irinotecan since 2015 (Burris et al. 1997; Conroy et al. 2011; Moore et al. 2007; Von Hoff et al. 2013; Wang-Gillam et al. 2016). Rapid disease progression, late diagnosis at advanced unresectable stages, high resistance to current chemotherapy regimens, and lack of prognostic markers are the sad realities in PDAC.
Recently, we could demonstrate in clinical tissue samples that members of the microRNA-99 family have potential as unfavorable prognostic markers for overall survival and chemotherapy response in patients with PDAC Stage II according to the Union Internationale Contre le Cancer (UICC) (Dhayat et al. 2015a). The value of circulating microRNA-99 family as liquid biopsy marker for PDAC remained undefined.
Patients and methods
Patients and samples
A tissue and blood biobank and follow-up database are maintained prospectively by the Department of General, Visceral and Transplantation Surgery and the Comprehensive Cancer Center Muenster, University Hospital Muenster, Germany. From these, 78 consecutive tissue specimens and 137 partly corresponding blood serum samples of patients with PDAC UICC Stages I (n = 5), II (n = 28), III (n = 29) and IV (n = 28), intraductal papillary mucinous neoplasm (IPMN, n = 11), chronic pancreatitis (n = 40), cystadenoma of the pancreas (n = 20), and age-matched healthy blood serum controls (n = 20) were collected between 2014 and 2017. PDAC stages and progression were categorized according to the current 8th edition of the UICC Tumor Lymph Node Metastasis (TNM) classification of malignant tumors (Amin et al. 2017).
About 5 ml of venous blood was collected preoperatively from each participant by a study nurse under standardized conditions from 8 to 10 am as part of the routine ambulatory blood sample collection. The whole blood was separated into serum and cellular fractions by centrifugation at 1200×g for 10 min after a recommended clotting time of minimum 30 min. The supernatant serum was stored at − 80 °C until analysis. Intraoperatively tissue samples were immediately partly cryopreserved in liquid nitrogen and partly fixed in 10% buffered formalin and then processed into a paraffin-embedded block and stored at room temperature. Sections from each of the 78 specimens were examined by a pathologist and graded histologically. All cancerous specimens showed vital tumor tissue.
Ethical approval for postoperative tissue collection was obtained (Ethics committee, University Muenster, Az: 1IXHai v. 11.8.2011 and Az: 2016-074-f-S) and all patients provided informed written consent. All patients with suspicion of resectable PDAC underwent radical resection and were assigned to duodenopancreatectomy or left pancreatic resection with standard lymphadenectomy. Explorative laparotomy with excisional biopsy was performed in patients with locally advanced unresectable and/or metastatic unresectable tumor burden with the presence of arterial infiltration, unresectable liver metastasis, and peritoneal carcinomatosis.
Patients that received immunosuppression, neoadjuvant chemo- or radiotherapy were excluded to avoid potential influences on microRNA expression. Perioperative clinical data, histopathological information and follow-up data were collected for all patients (Tables 1, 2, Supplementary Table 1). The primary endpoints of this study were disease-specific overall survival and recurrence-free survival.
Selection of microRNAs and their target proteins
Previously we could show that high expression of microRNA-21, microRNA-99a, and microRNA-100 in PDAC tissue correlated significantly with chemoresistance and poor overall survival (Dhayat et al. 2015a). Insulin-like growth factor 1 receptor (IGF1R) is a known target protein of the microRNA-99 family consisting of microRNA-99a, -99b, and -100 (Fujino et al. 2017; Ge et al. 2014; Huang et al. 2013; Lerman et al. 2011; Li et al. 2013; Sun et al. 2014). MicroRNA-21 and its target phosphatase and tensin homolog (PTEN) were used as known deregulated molecules in PDAC and served as confirmatory controls.
MicroRNA expression data of tissue specimens were normalized to expression levels of the three housekeeping genes RNU1A, SNORD68 and SNORD96A, selected from a total of ten tested housekeeping genes in PDAC tissue. Circulating microRNA expression data were normalized to the synthetic microRNA-39 from Caenorhabditis elegans (cel-microRNA-39) as spiked-in control.
RNA isolation and quantification of microRNA-99 family and microRNA-21
Tumor macrodissection by an experienced pathologist and RNA purification from each formalin-fixed paraffin-embedded (FFPE) tissue sample through robotic workstation (QIAcube, Qiagen, Hilden, Germany) were realized as described previously (Dhayat et al. 2015a). Total RNA isolation from cryopreserved blood serum samples was done using QIAzol Lysis Reagent (Qiagen) as a part of the miRNeasy Serum/Plasma Kit (Qiagen) according to the manufacturer’s instructions. 3.5 µl synthetic miRNA-39 from Caenorhabditis elegans (cel-microRNA-39) was added as a spike-in control (1.6 × 108 copies/µl working solution).
RNA concentration and purity were assessed by Agilent 2100 Bioanalyzer and RNA 6000 Nano/Pico LabChip (Agilent Tech., Boeblingen, Germany). Samples with RIN > 7 were considered intact and used for analysis. RNA was stored at − 80 °C. Quantitative real-time (qRT) PCR was performed using the miScript PCR system (Qiagen) as described previously (Dhayat et al. 2015a). Quantitative microRNA analysis was performed using CFX Manager Software v2.1 (Bio-Rad Laboratories, Munich, Germany). Expression of microRNA-99a, microRNA-99b, microRNA-100, and microRNA-21 was analyzed quantitatively relative to the housekeeping genes by the ΔΔCt (cycle threshold) method (Livak and Schmittgen 2001).
Tissue array immunohistochemistry
Tissue microarray (TMA) and immunostaining by the Dako Autostainer Plus S3400 and the Dako Real Detection System (Alkaline Phosphatase/RED, Rabbit/Mouse, K5005) were performed as described previously (Dhayat et al. 2015a). Sections from these arrays were stained with the primary antibodies IGF1Rβ (monoclonal antibody, 1:400, clone D406W, Cell Signaling Technology, Danvers, MA, USA) as known target protein of the microRNA-99 family and PTEN (monoclonal antibody, 1:200, clone NCH-38, Dako, Hamburg, Germany) as known target protein of the microRNA-21. Immunohistochemical staining was evaluated semi-quantitatively, according to the percentage of cells showing specific immunoreactivity. Two independent investigators evaluated, in a blinded manner, antibody staining using light microscopy (Eclipse E1000M and NIS-Elements D3.1 imaging software, Nikon, Tokyo, Japan) and a scoring system with a scale ranging from 0 to 4+ as defined previously (Dhayat et al. 2015a).
Western blotting
Cryopreserved tissue samples were dissociated in RIPA lysis buffer with freshly added 1× Protease Inhibitor Cocktail (Cell Signaling Technology) using a TissueLyserLT bead mill (Qiagen, Hilden, Germany). The lysate was cleared by centrifugation at 1000×g for 20 min at 4 °C followed by centrifugation at 14000×g for 45 min at 4 °C. Protein quantification was carried out using Pierce™ BCA Protein Assay Kit (ThermoScientific™, Waltham, MA, USA). Proteins were separated by sodium dodecyl sulfate (SDS)-6% polyacrylamide gel electrophoresis and blotted to a PVDF membrane (Merck, Darmstadt, Germany). The membrane was blocked in 5% (w/v) non-fat milk (Sigma-Aldrich, St.Louis, MO, USA) in TBS buffer with 0.05% (v/v) Tween-20 (Applichem, Darmstadt, Germany) for 45 min at room temperature. The primary antibody anti-IGF1Rβ (rabbit monoclonal (D406W) at 1:1000) was diluted in blocking buffer at target-specific concentrations and incubated overnight at 4 °C. Rabbit anti-actin (A2066, Sigma-Aldrich, St. Louis, MO, USA) was used as a loading control at 1:2000. The membrane was washed three times with TBS–Tween and incubated with secondary antibody anti-rabbit IgG-HRP (A6154, Sigma-Aldrich, St.Louis, MO, USA, 1:14,000) for 1 h at room temperature. After three washing, peroxidase was detected using Immobilon™ ECL western blotting substrate (Millipore, Schwalbach, Germany).
Statistical analysis
Statistical analysis was performed with the SPSS® Statistics Version 22 (IBM Corp. Armonk, NY) for Windows®. Data were expressed as medians of mean normalized expression with standard deviation (SD) or standard error of the mean (SEM). Bar charts demonstrating the median (middle quartile) and SEM were used to show normalized microRNA expression data (2−ΔΔCt) in the different study groups. Comparison between gene expression and categorical variables was conducted using the nonparametric Mann–Whitney U test or the Kruskal–Wallis test to test more than two groups. To test the correlation between the clinicopathological data and the expression of the microRNAs, we used the Fisher’s two-tailed exact test and whenever appropriate the χ2 test. All of the variables were dichotomized. For analysis of follow-up data, life table curves were calculated using the Kaplan–Meier method. Log rank test was used for comparison of overall survival rates between the groups with high and low microRNA. The primary end points were disease-specific survival or relapse-free survival, as measured from the date of surgery to the time of the last follow-up or cancer-related death or tumor relapse, respectively. Data of patients who were still alive and without evidence of tumor relapse at the end of the study were censored. A Cox proportional hazards regression model was used to estimate hazard ratios and 95% confidence intervals (CIs) and to perform multivariate survival analysis using a forward stepwise variable selection procedure based on the likelihood ratio. Variables with significant p values in the univariate analysis were included in the multivariate analysis.
The predicted probability of being diagnosed with PDAC or non-cancerous pancreatic tissue was used as a surrogate marker to construct the receiver operating characteristic (ROC) curve. Area under the ROC curve (AUC) with its corresponding 95% confidence interval (CI) was used as an accuracy index for evaluating the diagnostic performance of the selected microRNA. Values for p < 0.05 were considered to be statistically significant.
Results
Clinicopathologic characteristics
A total of 181 patients with PDAC UICC Stage I (n = 5), II (n = 28), III (n = 29) and IV (n = 28), IPMN (n = 11), chronic pancreatitis (n = 40), and healthy controls (n = 40) were analyzed (Table 1). Patients with neuroendocrine carcinoma were excluded. The male to female ratio was approximately 1.2:1. There was no significant correlation of PDAC with age, gender, body mass index, smoking or pre-surgical diabetes mellitus. A correlation of PDAC diagnosis was evident for alcohol consumption, pre-surgical pancreatitis, elevated levels of CA19-9 and CEA.
Clinicopathologic characteristics of all 90 PDAC patients (Supplementary Table 1) and 48 curatively treated PDAC patients (Table 2) were summarized. The median age at diagnosis of curatively treated PDAC patients UICC Stage I (n = 5), UICC Stage IIa (n = 6), UICC Stage IIb (n = 22), and UICC Stage III (n = 15) was 66 years. Tissue specimens were obtained from patients with PDAC UICC Stages II and III. These patients underwent curative pancreatic head resection (81.2%), pancreatic left resection (14.6%) or total pancreatectomy (4.2%). Tissue samples of 16 patients with benign, non-inflammatory pathology of the pancreas were analyzed. The majority of all 90 PDAC patients (51; 57%) had died by the time of final analysis with a median follow-up of 9 months. Five patients from other groups died (2 chronic pancreatitis, 2 benign, non-inflammatory disorder, and 1 IPMN). The median follow-up of all patients was 16.5 months.
Univariate analyses of all PDAC patients (n = 90) indicated that gender (p = 0.046), UICC stage (p = 0.001), tumor grading (p = 0.023), metastasis, nodal invasion, resection margin, and type of surgery (each p < 0.001) correlated with overall survival. Patients who underwent adjuvant gemcitabine chemotherapy had significantly improved overall survival rates (p < 0.001) (Supplementary Table 1).
Univariate analyses of curatively treated PDAC patients (n = 48) indicated that body mass index (p = 0.023), tumor grading (p = 0.037), and adjuvant chemotherapy (p = 0.004) correlate with overall survival, while tumor grading (p = 0.039) and location of primary tumor (p = 0.05) correlate with recurrence-free survival (Table 2).
Expression of the microRNA-99 family
Macrodissected tissue and in part corresponding blood serum samples of 40 healthy and benign, non-inflammatory pancreas, 40 chronic pancreatitis, 11 IPMN, and 90 PDAC were evaluated by qRT-PCR for the expression of microRNA-99 family members and microRNA-21. In blood serum a significant upregulation of expression in PDAC versus healthy was found for microRNA-100 (FC = 2.38, p < 0.001, r = 3.37), microRNA-99a (FC = 1.94, p = 0.011, r = 2.22), and microRNA-99b (FC = 1.75, p < 0.001, r = 3.66). Expression of microRNA-21 and microRNA-99a was significantly upregulated in PDAC versus chronic pancreatitis (FC = 1.65, p = 0.021, r = 2.08 and FC = 1.62, p = 0.044, r = 1.80) and microRNA-99b expression was significantly upregulated in chronic pancreatitis versus healthy (FC = 1.55, p = 0.011, r = 3.93). Differentiated according to the UICC stages I–IV, all circulating microRNAs were significantly upregulated in PDAC UICC Stage IV versus healthy (p < 0.05). Circulating microRNA-100 was significantly upregulated in UICC Stage II (p = 0.007) and together with microRNA-99b in UICC Stage III (p < 0.028 and p = 0.002) (Table 3; Fig. 1a).
Median 2-ΔΔCq expression and SEM of circulating (a) and tissue (b) microRNAs in different study groups. Asterisk (*) indicates a significant difference of p < 0.05 between PDAC patients and healthy controls. Dollar sign ($) and section sign (#) indicate a significant difference of p < 0.05 between PDAC patients and patients with chronic pancreatitis or IPMN, respectively
Tissue expression of microRNA-21 (FC = 5.46, p < 0.001, r = 5.37), microRNA-100 (FC = 2.95, p < 0.001, r = 3.50) and microRNA-99b (FC = 1.83, p = 0.001, r = 3.28) was significantly upregulated in curatively resected PDAC versus healthy. MicroRNA-21 expression could discriminate between chronic pancreatitis and PDAC (FC = 2.50, p < 0.001, r = 3.10) as well as IPMN and PDAC (FC = 8.46, p < 0.001, r = 5.93). MicroRNA-100, microRNA-99a, and microRNA-99b expression were significantly upregulated in chronic pancreatitis versus healthy (FC = 2.72, p = 0.018, r = 2.86; FC = 2.10, p = 0.042, r = 2.46; FC = 1.71, p = 0.042, r = 2.45). Tissue-related microRNA-21 and microRNA-100 were significantly upregulated in UICC Stage II (p < 0.001 and p = 0.001) and UICC Stage III (p = 0.002 and p = 0.016). All microRNAs were significantly upregulated in UICC stages II and III versus IPMN (p < 0.05) (Table 4; Fig. 1b).
Diagnostic potential of the microRNA-99 family
Diagnostic potential of microRNAs in blood serum and tissue was analyzed by ROC curve analysis (Fig. 2). For discrimination between PDAC and healthy controls a good to fair discriminatory power was detected for circulating microRNA-100 (AUC = 0.81; p < 0.001), microRNA-99b (AUC = 0.76; p < 0.001), microRNA-99a (AUC = 0.72; p = 0.002), and microRNA-21 (AUC = 0.71; p = 0.005). Diagnostic potential between PDAC and chronic pancreatitis was fair for circulating microRNA-21 (AUC = 0.70; p = 0.005) and poor for microRNA-100 (AUC = 0.64; p = 0.061), microRNA-99a (AUC = 0.68; p = 0.011), and microRNA-99b (AUC = 0.55; p = 0.482). Circulating microRNA-100 and microRNA-99b showed a fair potential to distinguish between healthy control and chronic pancreatitis (AUC = 0.73; p =0.014 and AUC = 0.72; p =0.009).
Diagnostic potential of microRNA-21 (a, b), microRNA-100 (c, d), microRNA-99a (e, f) and microRNA-99b (g, h) as tissue (left column) or circulating (right column) markers of PDAC versus healthy controls, PDAC versus chronic pancreatitis or chronic pancreatitis versus healthy, respectively. P < 0.05 indicates significance. AUC area under the curve
Tissue microRNA-100 (AUC = 0.85; p < 0.001) and microRNA-99b (AUC = 0.85; p < 0.001) were good discriminators between PDAC and healthy control, but poorly to fairly distinguished between chronic pancreatitis and PDAC or healthy control. Tissue microRNA-99a was a poor to fair discriminator in all comparisons. Tissue microRNA-21 enabled an excellent discrimination between PDAC and healthy control (AUC = 0.95; p < 0.001) and also a good to fair discrimination between PDAC and chronic pancreatitis (AUC = 0.82; p < 0.001) and between chronic pancreatitis and healthy (AUC = 0.73; 0.017).
Prognostic impact of the microRNA-99 family
Macrodissected carcinoma tissue and serum samples of 48 curatively treated patients with PDAC UICC I, II and III were evaluated by qRT-PCR for the expression of the microRNA-99 family and microRNA-21 with correlation to survival data (Fig. 3). Patients were categorized into low microRNA and high microRNA categories by cut-off dCt-values approximated to the median. Subgroup analysis of the study groups with low and high expression levels revealed mostly homogeneous distribution of number of patients, age, gender, body mass index, pre-surgical diabetes mellitus, UICC stages, tumor grade, nodal invasion, resection margin, location of primary tumor and radiotherapy (p < 0.05). Inhomogeneous distribution was detected for low versus high expression levels for number of patients for circulating microRNA-21, location of primary tumor for circulating microRNA-99a (p =0.050) and adjuvant chemotherapy for circulating microRNA-21, -100 and -99a (p =0.004, p =0.021, p =0.038).
Univariate Kaplan–Meier survival analysis revealed significantly improved overall survival in PDAC patients with low expression of circulating microRNA-21 (ΔCt cut-off 0.17; p = 0.002) and circulating microRNA-100 (cut-off 5.2; p = 0.001). Correspondingly, improved recurrence-free survival correlated with low expression of circulating microRNA-21 (p = 0.003) and circulating microRNA-100 (p = 0.006).
In curatively treated PDAC patients with UICC stages I, II or III, resection status R0, carcinoma of the pancreatic head and adjuvant chemotherapy with Gemcitabine, high expression of microRNA-100 correlated with extended overall survival (p = 0.035) as well as recurrence-free survival (0.040). In the same group of patients high expression of microRNA-21 correlated with extended recurrence-free survival (p =0.020), but not overall survival (p =0.096).
After controlling for UICC stage, tumor grade, lymphatic invasion and resection status in Cox regression survival analysis, the hazard of high circulating microRNA-21 in curatively treated PDAC patients was 7.8 times that of low circulating microRNA-21 for overall survival (95% CI 1.0–58.1, p = 0.046) and 5.3 times higher for recurrence-free survival (95% CI 1.8–24.3, p = 0.03) in multivariate analysis. For high circulating microRNA-100 the hazard in overall survival analysis was 15.4 that of low circulating microRNA-100 (95% CI 2.3–101.1, p = 0.004) and in recurrence-free survival analysis 4.6 times that of low circulating microRNA-100 (95% CI 1.2–17.9, p = 0.03). High circulating microRNA-100 correlated with sight of synchronous metastases (Spearman p = 0.022), but not with recurrences. High tissue microRNA-99a correlated with metachronous metastases (Spearman p = 0.034) in curatively operated PDAC patients.
Expression of the microRNA-99 family target protein IGF1R
Expression levels of IGF1Rβ were evaluated in tissue microarray sections of healthy controls, chronic pancreatitis and PDAC (Table 5; Fig. 4a–c). A significant increase in categorical expression of IGF1Rβ was detected in PDAC versus healthy control and in PDAC versus chronic pancreatitis (each p < 0.001). PTEN as a confirmatory target of microRNA-21 showed significant downregulation in PDAC versus healthy control and in PDAC versus chronic pancreatitis (each p < 0.001). Exemplarily, the upregulation of IGF1Rβ in PDAC versus healthy and in chronic pancreatitis versus healthy was shown by Western Blotting in Fig. 4d.
Tissue expression of IGF1R showed no correlation with overall (p = 0.671) or recurrence-free (p =0.370) survival in curatively treated PDAC patients. However, all PDAC patients showed high levels of tissue staining of intensity 3+ or 4+, while healthy controls and chronic pancreatitis were mainly scored as 1+ or 2+ (Table 5). The expression of IGF1R did neither correlate with dichotomized expression levels of circulating or tissue-related microRNAs in curatively treated PDAC patients, nor with sight of synchronous or metachronous metastases.
Discussion
Despite improved diagnostic techniques and treatment strategies, the poor prognosis of PDAC patients has been nearly unchanged over the last decades. There is still a lack of reliable biomarkers to identify PDAC at early surgically manageable tumor stages and to monitor disease recurrence. Mounting evidence suggests that microRNAs are well-preserved molecular targets even in FFPE tissue and liquid biopsy samples with high impact as diagnostic and prognostic markers in different carcinomas. The best known and frequently investigated microRNA-21 is associated with poor survival and chemoresistance in different malignant diseases (Frampton et al. 2015; Giovannetti et al. 2010; Wang et al. 2014). This oncogenic role of microRNA-21 in PDAC with its prognostic impact and repression of its target and tumor suppressor PTEN could be confirmed in this study.
Aberrant expression of the microRNA-99 family with either oncogenic or tumor suppressive function was reported in various human carcinomas, too (Li et al. 2015; Wang et al. 2017). Thereby, microRNA-100 could be revealed as a post-transcriptional regulator of numerous biological processes such as cell cycle, proliferation, epithelial-to-mesenchymal transition, dissemination, and apoptosis associated with poor prognosis for different carcinoma entities. In accordance with other preclinical studies, the microRNA-99 family, especially microRNA-100 may serve as target to enhance sensitivity to chemotherapy and reduce metastatic spread in PDAC (Bera et al. 2014; Dhayat et al. 2015b; Huang et al. 2013; LaConti et al. 2011). In PDAC tissues microRNA-100 was described as an oncogenic microRNA with increased expression in PDAC versus non-neoplastic tissues (Panarelli et al. 2012). Recently, we could demonstrate that overexpression of microRNA-99a and -100 in PDAC tissue correlated significantly with worse outcome and chemoresistance. Furthermore, multivariate survival analyses identified microRNA-100 as unfavorable prognostic factor in resected and adjuvant-treated PDAC UICC Stage II patients (Dhayat et al. 2015a). Following this tissue-based study, we focused now on the impact of the microRNA-99 family as diagnostic and prognostic liquid biopsy marker in a new cohort of patients with PDAC UICC Stages I–IV.
In fact, expression of the microRNA-99 family was increased in macrodissected tumor tissues as well as in corresponding serum samples of PDAC patients. However, chronic pancreatitis was also related to overexpression of the microRNA-99 family without difference to PDAC tissue. Correspondingly, its protein target IGF1R was overexpressed in tissue samples of PDAC or chronic pancreatitis. Recent in vitro studies could reveal that microRNA-100 regulated IGF1R expression was significantly increased in metastatic PDAC cell lines with potential in inflammation mediated carcinogenesis of PDAC (Huang et al. 2013; Subramani et al. 2014). Interestingly, the expression of circulating microRNA-100 was extraordinary high in patients with metastatic Stage IV disease. Additionally, circulating microRNA-100 was able to differentiate between PDAC and healthy pancreas with a good accuracy, suggesting its diagnostic value as non-invasive serum marker. Due to the small number of study patients with PDAC UICC Stage II treated by curative pancreatoduodenectomy and adjuvant chemotherapy, we could not confirm our previous results of tissue-related microRNA-21 and microRNA-100 as unfavorable prognostic factors in this study group (Dhayat et al. 2015a). However, multivariate survival analyses revealed that improved overall and recurrence-free survival correlated significantly with low expression of circulating microRNA-100 and circulating microRNA-21 in patients with curatively resected PDAC of all UICC stages. The value of microRNA-99 family members as independent prognostic circulating biomarkers for each individual PDAC UICC Stage remains undefined because of the limited statistical power. Overall, circulating microRNA-99 family members, especially microRNA-100, are significantly deregulated in PDAC with high diagnostic and prognostic impact.
Conclusions
The circulating microRNA-100 seems to be a suitable diagnostic and independent prognostic marker as well as potential therapeutic target in PDAC patients. Our results emphasize the need for further multi-center prognostic studies on circulating microRNA-100 in the future.
Abbreviations
- AUC:
-
Area under the curve
- CA.19-9:
-
Cancer antigen 19-9
- CI:
-
Confidence interval
- Ct:
-
Cycle threshold
- FFPE:
-
Formalin-fixed paraffin embedded
- IGF1R:
-
Insulin-like growth factor 1 receptor
- IHC:
-
Immunohistochemistry
- IPMN:
-
Intraductal papillary mucinous neoplasm
- miR:
-
microRNA
- NET:
-
Neuroendocrine tumor
- PDAC:
-
Pancreatic ductal adenocarcinoma
- PTEN:
-
Phosphatase and tensin homolog
- qRT-PCR:
-
Quantitative reverse transcriptase polymerase chain reaction
- ROC:
-
Receiver operating characteristic
- SD:
-
Standard deviation
- SEM:
-
Standard error of the mean
- TMA:
-
Tissue microarray
- UICC:
-
Union internationale contre le cancer.
References
Amin MB et al (2017) The Eighth Edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging CA. Cancer J Clin 67:93–99. https://doi.org/10.3322/caac.21388
Bera A, Venkata Subba Rao K, Manoharan MS, Hill P, Freeman JW (2014) A miRNA signature of chemoresistant mesenchymal phenotype identifies novel molecular targets associated with advanced pancreatic cancer. PLoS One 9:e106343. https://doi.org/10.1371/journal.pone.0106343
Burris HA 3rd et al (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15:2403–2413. https://doi.org/10.1200/JCO.1997.15.6.2403
Conroy T et al (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364:1817–1825. https://doi.org/10.1056/NEJMoa1011923
Dhayat SA, Abdeen B, Kohler G, Senninger N, Haier J, Mardin WA (2015a) MicroRNA-100 and microRNA-21 as markers of survival and chemotherapy response in pancreatic ductal adenocarcinoma UICC stage II. Clin Epigenetics 7:132. https://doi.org/10.1186/s13148-015-0166-1
Dhayat SA et al (2015b) MicroRNA profiling implies new markers of gemcitabine chemoresistance in mutant p53 pancreatic ductal adenocarcinoma. PLoS One 10:e0143755. https://doi.org/10.1371/journal.pone.0143755
Frampton AE et al (2015) microRNAs with prognostic significance in pancreatic ductal adenocarcinoma: a meta-analysis. Eur J Cancer 51:1389–1404. https://doi.org/10.1016/j.ejca.2015.04.006
Fujino Y et al (2017) Downregulation of microRNA-100/microRNA-125b is associated with lymph node metastasis in early colorectal cancer with submucosal invasion. Cancer Sci 108:390–397. https://doi.org/10.1111/cas.13152
Ge YY, Shi Q, Zheng ZY, Gong J, Zeng C, Yang J, Zhuang SM (2014) MicroRNA-100 promotes the autophagy of hepatocellular carcinoma cells by inhibiting the expression of mTOR and IGF-IR. Oncotarget 5:6218–6228. https://doi.org/10.18632/oncotarget.2189
Giovannetti E et al (2010) MicroRNA-21 in pancreatic cancer: correlation with clinical outcome and pharmacologic aspects underlying its role in the modulation of gemcitabine activity. Cancer Res 70:4528–4538. https://doi.org/10.1158/0008-5472.CAN-09-4467
Huang JS, Egger ME, Grizzle WE, McNally LR (2013) MicroRNA-100 regulates IGF1-receptor expression in metastatic pancreatic cancer cells. Biotech Histochem 88:397–402. https://doi.org/10.3109/10520295.2012.762460
LaConti JJ et al (2011) Tissue and serum microRNAs in the Kras(G12D) transgenic animal model and in patients with pancreatic cancer. PLoS One 6:e20687. https://doi.org/10.1371/journal.pone.0020687
Lerman G et al (2011) MiRNA expression in psoriatic skin: reciprocal regulation of hsa-miR-99a and IGF-1R. PLoS One 6:e20916. https://doi.org/10.1371/journal.pone.0020916
Li XJ, Luo XQ, Han BW, Duan FT, Wei PP, Chen YQ (2013) MicroRNA-100/99a, deregulated in acute lymphoblastic leukaemia, suppress proliferation and promote apoptosis by regulating the FKBP51 and IGF1R/mTOR signalling pathways. Br J Cancer 109:2189–2198. https://doi.org/10.1038/bjc.2013.562
Li C et al (2015) Multiple roles of microRNA-100 in human cancer and its therapeutic potential. Cell Physiol Biochem 37:2143–2159. https://doi.org/10.1159/000438572
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Method Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Lucas AL, Malvezzi M, Carioli G, Negri E, La Vecchia C, Boffetta P, Bosetti C (2016) Global trends in pancreatic cancer mortality from 1980 through 2013 and predictions for 2017. Clin Gastroenterol Hepatol 14:1452–1462 e1454. https://doi.org/10.1016/j.cgh.2016.05.034
Moore MJ et al (2007) Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 25:1960–1966. https://doi.org/10.1200/JCO.2006.07.9525
Panarelli NC, Chen YT, Zhou XK, Kitabayashi N, Yantiss RK (2012) MicroRNA expression aids the preoperative diagnosis of pancreatic ductal adenocarcinoma. Pancreas 41:685–690. https://doi.org/10.1097/MPA.0b013e318243a905
Siegel RL, Miller KD, Jemal A (2017) Cancer Statistics, 2017. CA Cancer J Clin 67:7–30. https://doi.org/10.3322/caac.21387
Subramani R, Lopez-Valdez R, Arumugam A, Nandy S, Boopalan T, Lakshmanaswamy R (2014) Targeting insulin-like growth factor 1 receptor inhibits pancreatic cancer growth and metastasis. PLoS One 9:e97016. https://doi.org/10.1371/journal.pone.0097016
Sun D, Layer R, Mueller AC, Cichewicz MA, Negishi M, Paschal BM, Dutta A (2014) Regulation of several androgen-induced genes through the repression of the miR-99a/let-7c/miR-125b-2 miRNA cluster in prostate cancer cells. Oncogene 33:1448–1457. https://doi.org/10.1038/onc.2013.77
Von Hoff DD et al (2013) Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 369:1691–1703. https://doi.org/10.1056/NEJMoa1304369
Wang W et al (2014) MicroRNA-21 and the clinical outcomes of various carcinomas: a systematic review and meta-analysis. BMC Cancer 14:819. https://doi.org/10.1186/1471-2407-14-819
Wang J, Yu M, Guan S, Zhang G, Cheng Y (2017) Prognostic significance of microRNA-100 in solid tumors: an updated meta-analysis. Onco Targets Ther 10:493–502. https://doi.org/10.2147/OTT.S122774
Wang-Gillam A et al (2016) Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet 387:545–557. https://doi.org/10.1016/S0140-6736(15)00986-1
Acknowledgements
We thank the patients and clinicians contributing to this study. The authors thank particularly Ms Sabine von Rueden, Ms Petra Ahrens, and Ms Anke Peters for their support and expert technical assistance.
Funding
The authors declare that they have no funding to disclose.
Author information
Authors and Affiliations
Contributions
SAD and AJS designed the experiments, performed the statistical analysis and prepared the manuscript. AJS, GK, VR, and SAD performed the immunohistological and molecular experiments. SAD, HU, and NS contributed to the specimens and clinicopathologic data. Critical revision of the manuscript was performed by all participants. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Ethical approval for postoperative tissue and blood collection was obtained (Ethics committee, University Muenster, Az: 1IXHai v. 11.8.2011 and Az: 2016-074-f-S).
Informed consent
All patients provided informed written consent. Written informed consent was obtained from all individual participants included in the study.
Human and animal rights statement
This article does not contain any studies with animals performed by any of the authors.
Availability of data and material
All data generated or analyzed during this study are included in this published article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Stroese, A.J., Ullerich, H., Koehler, G. et al. Circulating microRNA-99 family as liquid biopsy marker in pancreatic adenocarcinoma. J Cancer Res Clin Oncol 144, 2377–2390 (2018). https://doi.org/10.1007/s00432-018-2749-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-018-2749-7