Abstract
Aim
Our study aims to investigate the association between dietary acrylamide exposure and cancer mortality among Chinese elderly.
Methods
A prospective cohort of 4000 elderly men and women aged 65 years and above (Mr. and Ms. OS Hong Kong study) was recruited from local communities from 2001 to 2003. Dietary exposure to acrylamide was evaluated at baseline based on a validated food frequency questionnaire and an acrylamide database from the 1st Hong Kong Total Diet Study. Data on mortality statistics through March 2014 were obtained from the Death Registry of the Department of Health of Hong Kong with a median follow-up of 11.1 years. Cox proportional hazards models were used to examine the association between the acrylamide exposure and cancer mortality. Sex hormones were assessed in men.
Results
During a median follow-up of 11.1 years (39,271 person-years), we ascertained 330 cancer deaths. Vegetables (43.7%) and cereals (28.9%) products were the major contributors to dietary acrylamide. Compared with the lowest quartile of acrylamide intake (<9.9 µg/day), the multivariable hazard ratios for the highest quartile (>17.1 µg/day) were 1.9 (95% CI 1.3–2.8; P trend < 0.01), 1.9 (95% CI 1.0–3.6; P trend = 0.05), and 2.0 (95% CI 1.0–4.0; P trend = 0.06) for the cancer mortality from overall, digestive and respiratory system, respectively. The associations were attenuated to null after further adjustment for circulating free estradiol in men. No statistically significant interactions were observed between acrylamide exposure and sex, obesity and overall lifestyle pattern scores.
Conclusions
The longitudinal data provided evidence that dietary acrylamide, in amounts that Chinese elderly are typically exposed to, was associated with increased cancer mortality. Circulating free estradiol may mediate the association in men.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer has become the most important cause of death in China and throughout the world (Stewart 2014). Acrylamide was classified as a probable human carcinogen by the International Agency for Research on Cancer (IARC) on the basis of its carcinogenicity in rodents. Animal studies have shown acrylamide is a multisite carcinogen in rodents and causes tumors in skin,mesothelioma, lung, mammary gland, uterus, ovary, testis, thyroid, Harderian gland oral cavity and central nervous system (Bull et al. 1984; Johnson et al. 1986; Friedman 2003). A recent meta-analysis (Pelucchi et al. 2015) indicates that dietary acrylamide has a modest association with kidney cancer and with endometrial and ovarian cancer in never smokers.
Acrylamide is commonly present in carbohydrate-rich foods processed at high temperatures (>120 °C) and formed by Maillard browning reactions, in which amino acids, particularly asparagine, react with reducing sugars (Mottram et al. 2002). High levels of acrylamide have been found in fried and baked potato products and in cereal products such as crisp bread, breakfast cereals and cookies (Mottram et al. 2002). Due to its ubiquitous presence in foods with concentrations at considerably higher levels than other well-known food carcinogens (i.e., polycyclic aromatic hydrocarbons and ethyl carbamate); acrylamide might be responsible for a considerable part of the diet-related cancer incidences and mortality (JECFA 2005). Some studies suggest that acrylamide may affect the cancer risk through hormonal pathways (Hogervorst et al. 2013; Nagata et al. 2015).
Dietary acrylamide can be rapidly and extensively absorbed from the gastrointestinal tract, then metabolized and excreted in urine, mainly as mercapturic acid derivatives of acrylamide and glycidamide (GA). Many observational studies have focused on dietary acrylamide exposure and the incidences of individual cancers (Pelucchi et al. 2011). Few cohort studies have examined the association of acrylamide exposure and cancer mortality, especially among elderly populations. This study aimed to investigate the relationship of dietary acrylamide and cancer mortality, and explore whether endogenous sex hormones may mediate the association based on an 11-year follow-up of an elderly population in Hong Kong.
Methods
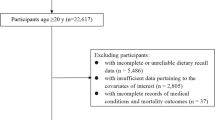
The study of Mr. and Ms. OS Hong Kong was originally designed to investigate the risk factors of osteoporosis. Elderly men and women aged 65 years and above were recruited from 2001 to 2003 through advertisements placed in community centers and housing estates. Those who were unable to walk without assistance of another person, had bilateral hip replacement or were not competent to give informed consent were excluded. The participants were stratified so that approximately 33% were in each of the age groups: 65–69, 70–74, and 75 and above. The study enrolled 4000 participants. Details of the study design have previously been published (Lau et al. 2006). The study was approved by the Clinical Research Ethics Committee of the Chinese University of Hong Kong and complied with the Declaration of Helsinki. The informed consent for this study was obtained before study enrolment and during the data collection.
Eligible participants were invited to the Research Center for baseline clinical assessment, and face-to-face interviews based on structured and standardized questionnaires. Information collected included social demographic data, family and medical history, current use of medications, smoking and drinking of alcohol, tea and coffee. Dietary intakes were assessed by a validated food frequency questionnaire (FFQ) based on the data from the Hong Kong Adult Dietary Survey (Woo et al. 1997). Physical activity was measured by the Physical Activity Scale for the Elderly Questionnaire (PASE) (Washburn et al. 1993). Height was measured by the Holtain Harpenden stadiometer (Holtain Ltd., Crosswell, UK). Body weight was measured by the Physician Beam Balance Scale (Healthometer, IL, USA).
A total of 1489 male subjects were randomly selected to have stored serum analyzed for sex hormones and their precursors (sex hormone-binding globulin (SHBG), estradiol, androstenedione, dehydroepiandrosterone, 5-androstene-3b,17b-diol and dehydroepiandrosterone sulfate). Free fractions of estradiol were calculated as described by Sodergard et al. (1982). All hormone assays were performed by Gas chromatography–mass spectrometry (GC/MS) (Labrie et al. 2009).
Acrylamide intake assessment and the 1st total diet study in Hong Kong
The validated FFQ containing 329 food items was used to estimate dietary nutrients and acrylamide intakes at baseline (Woo et al. 1997). Acrylamide level in individual food items was based on the acrylamide database of the 1st Hong Kong Total Diet Study (HKTDS) (Centre for Food Safety and Department 2011). The food items in FFQ that were used in the acrylamide intake assessment were assigned the mean value of the acrylamide detected in the TDS food items or a value of one half the quantitation limit when concentrations were lower than the quantitation limit (Centre for Food Safety and Department 2011). Dietary acrylamide intake was estimated from the mean acrylamide level (Centre for Food Safety and Department 2011), the frequency of consumption and the portion size of the intake of food items. TDS has been recognized internationally as one of the most cost effective way to estimate dietary exposures to food chemicals for various population groups and to assess their associated health risks. Due to its nature of covering total diet, TDS can identify potentially contaminated foods or food groups that may be present at very low levels. In the 1st HKTDS, a total of 1800 samples for the 150 TDS food items were collected and prepared into table-ready forms on four occasions. On each occasion, three samples of each TDS food item were purchased from various retail outlets in different regions of the territory. The three samples of the same food item were then prepared as for normal consumption, i.e., table-ready, in a manner most representative of and consistent with cultural habits in Hong Kong as far as practicable. They were then homogenized individually and combined into 600 composite samples for the laboratory analysis on food chemicals.
Case ascertainment and follow-up
Data on mortality statistics were obtained from the Death Registry of the Department of Health of Hong Kong till March 2014 with a median follow-up of 11.1 years. Cancer deaths were identified by the cause of death reported on the death certificate, and classified according to the International Classification of Disease (ICD) version 10 codes as those ranging from C00 to D48 for neoplasms. Follow-up of participants continued until death attributable to cancers, with censoring at the time of death for those who died from causes other than cancers.
Statistical analysis
Statistical analysis was conducted using SPSS version 21.0. All P values were two sided with significance level at 0.05. The contributions of 15 TDS food groups to overall dietary acrylamide exposure were calculated as percentages. We categorized participants into quartiles of dietary exposure of acrylamide based on its distribution in the entire cohort. Differences in the participants’ characteristics by quartiles of dietary acrylamide exposure were compared using Chi-square test for categorical variables or analysis of variance (ANOVA) for continuous variables. Participants contributed person-time from baseline until the date of death, death from any cancers, or 31st March 2014, whichever occurred first. Participants with medical history of any cancers at baseline were excluded and 3823 remained for analysis. Cox proportional hazards models were used to estimate hazard risks (HR) with their 95% confidence intervals (CIs) for the association between acrylamide intake and overall cancer mortality, and cancer mortality from digestive tract and respiratory system, respectively. In multivariable analysis, we controlled for age, sex, education, body mass index (BMI) at baseline, physical activities, history of diabetes, cigarette smoking, total energy intake, alcohol, coffee and tea drinking, dietary carbohydrate and fibers. We tested the proportional hazard assumption using the likelihood ratio test and found no departure from the assumption. Tests for trend were conducted by assigning the median value of acrylamide exposure to each quartile and modeling this variable as a continuous variable. Because cigarette smoking is an important source of acrylamide exposure, we conducted a sensitivity analysis excluding the current smokers. To check for the influence of protopathic bias, the analyses were also done with exclusion of cancer deaths occurring in the first 2 years of follow-up.
Effect modification by other variables on the association between acrylamide intake and cancer mortality was tested. These variables in our analysis were sex, BMI, and score of AHA-DLR (the score of compliance of the guideline of American Heart Association Dietary and lifestyle Recommendation). To evaluate whether sex hormones mediated the association of acrylamide exposure with cancer mortality in men, we added sex hormones individually in the regression model. We tested for potential effect modification by including an interaction term of these variables with the quartiles of acrylamide exposure in the regression models. Subgroup analyses were further conducted.
Results
We observed a mean (±SD) daily intake of acrylamide of 14.6 ± 8.2 μg/day in the study population. The main dietary sources of acrylamide (Table 1) were vegetables and their products (43.7%), followed by cereals and their products (28.9%), then fish, seafood and their products (8.93%), legumes, nuts and seeds and their products (6.75%) and mixed dishes (4.64%).
The participants’ characteristics in quartiles of overall acrylamide intake were indicated in Table 2. Compared with the lowest acrylamide group, those in the highest quartile were younger, had higher education, body weight and smoking dosage; more likely to be married and physically active; higher AHA-DLR scores and higher intake of total energy, carbohydrate, protein and dietary fibers; had higher coffee, tea and alcohol intake; higher intake of French fries, fast foods and red/processed meat.
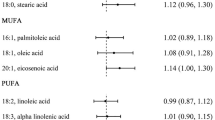
The crude and multivariable-adjusted associations (HR and 95% CI) between acrylamide exposure and cancer mortality are shown in Table 3. We ascertained 330 cancer deaths during total 39,271 person-years of follow-up (mean, 11.1 years). After adjustment for potential covariables, a significant association was observed between acrylamide intake and an overall increased cancer mortality with a HR of 1.9 (95% CI 1.3–2.8; P trend < 0.01). Sensitivity analysis excluding current smokers (n = 274), or cases that died from cancer during the first 2 years of follow-up (n = 61) did not change the results materially. In the current non-smokers, the multivariable-adjusted HR in the highest quartile was 2.0 (95% CI 1.3–3.1; P trend < 0.01) compared with the lowest quartile of acrylamide intake. Subgroup analyses for cancer mortality from digestive tract (HR 1.9; 95% CI 1.0–3.6; P trend = 0.05) and respiratory system (HR 2.0; 95% CI 1.0–4.0; P trend = 0.06) demonstrated similar HRs to those of overall mortality.
Table 4 indicated the results of subgroup analyses between acrylamide intake and potential variables of effect modification. There were no significant interactions between quartiles of acrylamide intake and any of the variables on overall cancer mortality. The associations were largely consistent across sex, BMI (<24 and ≥24 kg/m2) and AHA-DRL scores (above and below median). In men, after adjustment for circulating estradiol but not other sex hormones, the HRs were reduced to around 1, suggesting that the effect of acrylamide exposure on cancer mortality may be mediated through circulating free estradiol. When the analyses were further restricted to the subgroup of elderly who had never smoked, the associations (HRs) were similar but were marginally significant due to reduced power.
Discussion
Summary of current findings
This 11-year Chinese elderly cohort study provides the first epidemiologic evidence that dietary acrylamide exposure could potentially increase overall cancer mortality. The association was also present in current non-smokers. Similar increased risks were observed in cancer mortality from digestive tract and respiratory system.
The average acrylamide intakes were 15.9 ± 8.5 µg/day (0.26 µg/kg bw/day) for men and 13.2 ± 7.6 µg/day (0.24 µg/kg bw/day) for women. The intake levels were similar to those reported in a previous survey in Hong Kong adults (Wong et al. 2014) (0.21 µg/kg bw/day) and in the Chinese general population (0.286 µg/kg bw/day) (Zhou et al. 2013); but were in the lower range of the WHO estimate of 0.3–0.8 μg/kg bw/day for developed countries (Wong et al. 2014). This could be due to the lower intake of fried and baked foods among Chinese elderly compared with that of the Western populations (WHO/FAO 2002). Our results indicated the majority of the participants (82%) never consumed acrylamide-rich snack foods.
The major contributors of dietary acrylamide in our data were fried vegetables, baked cereals and their products. The findings were similar to that reported by the 1st Total Diet Study in Hong Kong adults (Wong et al. 2014). Although fried potato or snack foods such as potato chips and biscuits contain the highest acrylamide level, they had only a minor contribution to the overall exposure due to the low consumption in the study participants. Based on our findings, mitigation procedures to minimize the formation of acrylamide in vegetables and cereals by reducing the cooking temperature and time, as well as sugars in foods could probably help to decrease the acrylamide exposure and cancer mortality in the Chinese elderly population.
Studies in rodents have shown positive dose–response relations between acrylamide exposure and cancer in multiple organs (Shipp et al. 2006). However, observational studies on dietary acrylamide intake and its relation with cancer risk have reported inconsistent findings. Some indicated positive association between acrylamide exposure and increased risk of endometrial or ovarian cancer (Hogervorst et al. 2007; Wilson et al. 2010), renal cancer (Hogervorst et al. 2008a, b; Pelucchi et al. 2015), cutaneous malignant melanoma in men (Lipunova 2016), estrogen receptor positive breast cancer (Olesen et al. 2008), lymphatic malignancies (Bongers et al. 2012), colorectal cancer with specific mutations in KRAS and APC (Hogervorst et al. 2014) and lung cancer in smoking men (Hirvonen et al. 2010); but no associations were observed for bladder and prostate cancer (Hogervorst et al. 2008a, b, Larsson et al. 2009), colorectal cancer (Mucci et al. 2006; Hogervorst et al. 2008a, b), lung cancer in men (Hogervorst et al. 2009), gastric, pancreatic and oesophageal cancers (Hogervorst et al. 2008a, b). A recent systematic review and meta-analysis (Pelucchi et al. 2015) of epidemiological studies indicated a borderline association with dietary acrylamide emerged for endometrial (RR = 1.23; 95% CI 1.00–1.51) and ovarian (RR = 1.39; 95% CI 0.97–2.00) cancers in never smokers, and a modest association for kidney cancer.
Studies that examined the relationship between dietary acrylamide and cancer mortality or prognosis were limited. Only one longitudinal study (Olsen et al. 2012) investigated the relationship of dietary acrylamide exposure and breast cancer mortality. The cohort of 420 postmenopausal Danish women of breast cancer indicated that pre-diagnostic acrylamide exposure reduced survival after breast cancer diagnosis (Olsen et al. 2012). Among non-smokers, higher concentrations of GA-Hb were associated with an increased risk of breast cancer specific mortality [HR (95% CI) 1.63 (1.06–2.51)] (Olsen et al. 2012). Our results revealed that dietary acrylamide exposure was associated with increased overall cancer mortality. More research is needed to explore the associations of acrylamide exposure with specific cancer mortality or prognosis.
Mechanisms
The biological mechanism by which acrylamide causes cancer development in humans is yet unclear. Both genotoxic and non-genotoxic pathways have been suggested (Besaratinia and Pfeifer 2007). Acrylamide itself and its epoxide metabolite glycidamide, which is generated by cytochrome P4502E1 (CYP2E1), are clastogenic, and glycidamide forms DNA adducts. Acrylamide may also cause cancer through non-genotoxic mechanisms. Oxidative stress, following depletion of glutathione by acrylamide, is one of the proposed mechanisms. Acrylamide reacts with glutathione and may thus alter the redox status of cells and gene transcription and expression, facilitating cancer development (Catalgol et al. 2009), or it may interfere with DNA repair or hormonal balances (Besaratinia and Pfeifer 2007). In some test systems, aneuploidy was observed upon acrylamide incubation, which might be caused by binding of acrylamide to proteins involved in cell division, such as mitotic/meiotic spindle kinesins (Sickles et al. 2007).
Acrylamide may also be carcinogenic through hormonal pathways (Hogervorst et al. 2007). Our results also suggested that additional adjustment of free estradiol level in multivariable regression model reduced the HRs of dietary acrylamide and cancer mortality to around 1. Acrylamide has been shown to alter steroid hormone levels in premenopausal (Nagata et al. 2015) and postmenopausal women (Hogervorst et al. 2013). There could be interactions between acrylamide intake and genes involved in the generation of sex hormones (Hogervorst et al. 2016; Hogervorst et al. 2017). The influence on circulating estrogen levels is the key mechanism behind several of the factors known to be associated with breast cancer incidence and/or prognosis (Kendall et al. 2007; Folkerd and Dowsett 2010).
Strengths
The strengths of this study include the use of a validated FFQ for acrylamide assessment, the prospective nature precluding selection and recall bias, the long duration of follow-up, and the completeness of case ascertainment through linkage to the registries of the Health Authority.
We assessed acrylamide intake based on an analytical acrylamide database with acrylamide values derived from an extensive and elaborate sampling scheme and chemical analysis of all relevant Hong Kong foods. A previous study suggested that using mean acrylamide levels in foods to estimate the total acrylamide intake could be a valid approach (Zhou et al. 2013). For estimating the long-term exposure to the usual acrylamide intake in our study, the mean acrylamide levels for foods are expected to be even more suitable. Finally, protopathic bias was probably not present in our prospective cohort study, as exclusion of death cases in the first 2 years of follow-up did not alter the conclusions.
Limitations
This study has several limitations. First, as in most of epidemiological studies, we used a validated FFQ to estimate dietary acrylamide exposure. Although the use of FFQs has limitations of possible misclassification, they are the only feasible way in large epidemiological studies to assess the intake of the relevant acrylamide-containing foods over a long time period (Wilson et al. 2009). In addition, the non-differential misclassification could only bias the risk estimates toward null. Although acrylamide adducts to hemoglobin are recognized as the internal dose markers of ‘exposure’ to acrylamide (Dybing et al. 2005), they represent the exposure during the preceding 3–4 months only. The biomarker is not specific with regard to the source of acrylamide. The costs of using biomarkers also limit the size of the population that can be used. In multivariable regression models, we further adjusted for dietary carbohydrate and fibers, the nutrients that are correlated to acrylamide, which suggested that the significant associations of acrylamide and cancer mortality could be due to the acrylamide itself, but not dietary carbohydrate and fiber levels. In addition, dietary intakes by FFQ were only assessed at baseline; we did not conduct the same dietary survey during follow-up. The food intakes that were investigated in 2001–2003 may not be completely representative of the foods that were on the market during follow-up. It has been shown that after 2005, acrylamide levels in some products have decreased (Stadler 2005). However, the reduction of acrylamide mostly occurred in fried potatoes, which had a minor contribution to overall acrylamide exposure in our study population since they had low intake of fried or baked food or snacks.
Second, although both industrial contact and tobacco smoking are important sources of environmental acrylamide exposure (Wirfalt et al. 2008), exposure from diet and drinking water was the major route of acrylamide in our elderly participants since they were non-occupationally exposed and had low prevalence of current smoking (6.8%).
Third, we have not collected the data on new cancer cases during follow-up but only data on cancer mortality. In addition, we did not analyze the association of acrylamide exposure with each kind of cancer mortality due to insufficient individual cancer deaths. However, animal studies have demonstrated that acrylamide-induced cancers affected various tissues. This is because the molecule of acrylamide is small and hydrophilic, it passively diffuses throughout the body and all tissues are theoretical targets for acrylamide carcinogenesis (Friedman 2003). Our subgroup analyses on mortality in either digestive or respiratory system reported similar findings on the associations of acrylamide exposure and cancer deaths. Another limitation is that we did not collect the treatment information after cancer occurrence. However, the treatment is unlikely to be correlated with acrylamide intake, and thus the association would be reduced towards the null. Future studies on acrylamide and cancer mortality should take treatment into account.
Finally, the study was conducted by non-random sampling in a single center in Hong Kong, so the findings may not be generalizable to ethnic Chinese elsewhere. Additionally, potential residual confounders from unmeasured factors such as past occupational exposure to carcinogens and concomitant carcinogens from foods are possible. However, the increased strength of associations after multivariable adjustment indicates that residual confounding by the covariables is probably not the explanation for the observed acrylamide-associated associations.
Conclusions
Dietary exposure to acrylamide in amounts typically ingested by Chinese elderly was found to be significantly associated with increased overall cancer mortality, and mortality from cancers of the digestive tract and respiratory system. Circulating free estradiol may mediate this association in men. Further prospective studies specifically conducted among new cases of certain cancer are necessary to confirm these findings.
References
Besaratinia A, Pfeifer GP (2007) A review of mechanisms of acrylamide carcinogenicity. Carcinogenesis 28(3):519–528
Bongers ML, Hogervorst JG, Schouten LJ, Goldbohm RA, Schouten HC, van den Brandt PA (2012) Dietary acrylamide intake and the risk of lymphatic malignancies: the Netherlands Cohort Study on diet and cancer. PLoS One 7(6):e38016
Bull RJ, Robinson M, Stober JA (1984) Carcinogenic activity of acrylamide in the skin and lung of Swiss-ICR mice. Cancer Lett 24(2):209–212
Catalgol B, Ozhan G, Alpertunga B (2009) Acrylamide-induced oxidative stress in human erythrocytes. Hum Exp Toxicol 28(10):611–617
Centre for Food Safety, Food and Environmental Hygiene Department (2011) The first Hong Kong Total Diet Study. Methodology 1–34
Dybing E, Farmer PB, Andersen M, Fennell TR, Lalljie SP, Müller DJ, Olin S, Petersen BJ, Schlatter J, Scholz G, Scimeca JA, Slimani N, Törnqvist M, Tuijtelaars S, Verger P (2005) Human exposure and internal dose assessments of acrylamide in food. Food Chem Toxicol 43(3):365–410
Folkerd EJ, Dowsett M (2010) Influence of sex hormones on cancer progression. J Clin Oncol 28(26):4038–4044
Friedman M (2003) Chemistry, biochemistry, and safety of acrylamide. A review. J Agric Food Chem 51(16):4504–4526
Hirvonen T, Kontto J, Jestoi M, Valsta L, Peltonen K, Pietinen P, Virtanen SM, Sinkko H, Kronberg-Kippila C, Albanes D, Virtamo J (2010) Dietary acrylamide intake and the risk of cancer among Finnish male smokers. Cancer Causes Control 21(12):2223–2229
Hogervorst JG, Schouten LJ, Konings EJ, Goldbohm RA, van den Brandt PA (2007) A prospective study of dietary acrylamide intake and the risk of endometrial, ovarian, and breast cancer. Cancer Epidemiol Biomarkers Prev 16(11):2304–2313
Hogervorst JG, Schouten LJ, Konings EJ, Goldbohm RA, van den Brandt PA (2008a) Dietary acrylamide intake and the risk of renal cell, bladder, and prostate cancer. Am J Clin Nutr 87(5):1428–1438
Hogervorst JG, Schouten LJ, Konings EJ, Goldbohm RA, van den Brandt PA (2008b) Dietary acrylamide intake is not associated with gastrointestinal cancer risk. J Nutr 138(11):2229–2236
Hogervorst JG, Schouten LJ, Konings EJ, Goldbohm RA, van den Brandt PA (2009) Lung cancer risk in relation to dietary acrylamide intake. J Natl Cancer Inst 101(9):651–662
Hogervorst JG, Fortner RT, Mucci LA, Tworoger SS, Eliassen AH, Hankinson SE, Wilson KM (2013) Associations between dietary acrylamide intake and plasma sex hormone levels. Cancer Epidemiol Biomarkers Prev 22(11):2024–2036
Hogervorst JG, de Bruijn-Geraets D, Schouten LJ, van Engeland M, de Kok TM, Goldbohm RA, van den Brandt PA, Weijenberg MP (2014) Dietary acrylamide intake and the risk of colorectal cancer with specific mutations in KRAS and APC. Carcinogenesis 35(5):1032–1038
Hogervorst JG, van den Brandt PA, Godschalk RW, van Schooten FJ, Schouten LJ (2016) The influence of single nucleotide polymorphisms on the association between dietary acrylamide intake and endometrial cancer risk. Sci Rep 6:34902
Hogervorst JG, van den Brandt PA, Godschalk RW, van Schooten FJ, Schouten LJ (2017) Interactions between dietary acrylamide intake and genes for ovarian cancer risk. Eur J Epidemiol 32(5):431–441
JECFA (2005) Summary and conclusions of the sixty-fourth meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), 1–47
Johnson KA, Gorzinski SJ, Bodner KM, Campbell RA, Wolf CH, Friedman MA, Mast RW (1986) Chronic toxicity and oncogenicity study on acrylamide incorporated in the drinking water of Fischer 344 rats. Toxicol Appl Pharmacol 85(2):154–168
Kendall A, Folkerd EJ, Dowsett M (2007) Influences on circulating oestrogens in postmenopausal women: relationship with breast cancer. J Steroid Biochem Mol Biol 103(2):99–109
Labrie F, Cusan L, Gomez JL, Martel C, Berube R, Belanger P, Belanger A, Vandenput L, Mellstrom D, Ohlsson C (2009) Comparable amounts of sex steroids are made outside the gonads in men and women: strong lesson for hormone therapy of prostate and breast cancer. J Steroid Biochem Mol Biol 113(1–2):52–56
Larsson SC, Akesson A, Wolk A (2009) Dietary acrylamide intake and prostate cancer risk in a prospective cohort of Swedish men. Cancer Epidemiol Biomarkers Prev 18(6):1939–1941
Lau EM, Leung PC, Kwok T, Woo J, Lynn H, Orwoll E, Cummings S, Cauley J (2006) The determinants of bone mineral density in Chinese men—results from Mr. Os (Hong Kong), the first cohort study on osteoporosis in Asian men. Osteoporos Int 17(2):297–303
Lipunova N, Schouten LJ, van den Brandt PA, Hogervorst JG (2016) A prospective cohort study on dietary acrylamide intake and the risk for cutaneous malignant melanoma. Eur J Cancer Prev doi:10.1097/CEJ.0000000000000268
Mottram DS, Wedzicha BL, Dodson AT (2002) Acrylamide is formed in the Maillard reaction. Nature 419(6906):448–449
Mucci LA, Adami HO, Wolk A (2006) Prospective study of dietary acrylamide and risk of colorectal cancer among women. Int J Cancer 118(1):169–173
Nagata C, Konishi K, Tamura T, Wada K, Tsuji M, Hayashi M, Takeda N, Yasuda K (2015) Associations of acrylamide intake with circulating levels of sex hormones and prolactin in premenopausal Japanese women. Cancer Epidemiol Biomarkers Prev 24(1):249–254
Olesen PT, Olsen A, Frandsen H, Frederiksen K, Overvad K, Tjonneland A (2008) Acrylamide exposure and incidence of breast cancer among postmenopausal women in the Danish Diet, Cancer and Health Study. Int J Cancer 122(9):2094–2100
Olsen A, Christensen J, Outzen M, Olesen PT, Frandsen H, Overvad K, Halkjaer J (2012) Pre-diagnostic acrylamide exposure and survival after breast cancer among postmenopausal Danish women. Toxicology 296(1–3):67–72
Pelucchi C, La Vecchia C, Bosetti C, Boyle P, Boffetta P (2011) Exposure to acrylamide and human cancer—a review and meta-analysis of epidemiologic studies. Ann Oncol 22(7):1487–1499
Pelucchi C, Bosetti C, Galeone C, La Vecchia C (2015) Dietary acrylamide and cancer risk: an updated meta-analysis. Int J Cancer 136(12):2912–2922
Shipp A, Lawrence G, Gentry R, McDonald T, Bartow H, Bounds J, Macdonald N, Clewell H, Allen B, Van Landingham C (2006) Acrylamide: review of toxicity data and dose-response analyses for cancer and noncancer effects. Crit Rev Toxicol 36(6–7):481–608
Sickles DW, Sperry AO, Testino A, Friedman M (2007) Acrylamide effects on kinesin-related proteins of the mitotic/meiotic spindle. Toxicol Appl Pharmacol 222(1):111–121
Södergard R, Bäckström T, Shanbhag V, Carstensen H (1982) Calculation of free and bound fractions of testosterone and estradiol-17β to human plasma proteins at body temperature. J Steroid Biochem 16(6):801–810
Stadler RH (2005) Acrylamide formation in different foods and potential strategies for reduction. Adv Exp Med Biol 561:157–169
Stewart BW, Wild CP (2014) World Cancer Report 2014
Wilson KM, Vesper HW, Tocco P, Sampson L, Rosén J, Hellenäs KE, Törnqvist M, Willett WC (2009) Validation of a food frequency questionnaire measurement of dietary acrylamide intake using hemoglobin adducts of acrylamide and glycidamide. Cancer Causes Control 20(3):269–278
Washburn RA, Smith KW, Jette AM, Janney CA (1993) The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol 46(2):153–162
WHO/FAO (2002) Health implications of acrylamide in food. Report of a Joint FAO/WHO Consultation. WHO. http://apps.who.int/iris/bitstream/10665/42563/1/9241562188.pdf. Accessed 19 July 2017
Wilson KM, Mucci LA, Rosner BA, Willett WC (2010) A prospective study on dietary acrylamide intake and the risk for breast, endometrial, and ovarian cancers. Cancer Epidemiol Biomarkers Prev 19(10):2503–2515
Wirfalt E, Paulsson B, Tornqvist M, Axmon A, Hagmar L (2008) Associations between estimated acrylamide intakes, and hemoglobin AA adducts in a sample from the Malmo Diet and Cancer cohort. Eur J Clin Nutr 62(3):314–323
Wong WW, Chung SW, Lam CH, Ho YY, Xiao Y (2014) Dietary exposure of Hong Kong adults to acrylamide: results of the first Hong Kong Total Diet Study. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 31(5):799–805
Woo J, Leung SSF, Ho SC, Lam TH, Janus ED (1997) A food frequency questionnaire for use in the Chinese population in Hong Kong: description and examination of validity. Nutr Res 17(11):1633–1641
Zhou PP, Zhao YF, Liu HL, Ma YJ, Li XW, Yang X, Wu YN (2013) Dietary exposure of the Chinese population to acrylamide. Biomed Environ Sci 26(6):421–429
Acknowledgements
We wish to thank all participants for their participation and Dr. Edith Lau for her contribution in setting up the cohort.
Author information
Authors and Affiliations
Contributions
ZML conceptualized the study, analyzed the data, interpreted the results, and drafted the manuscript. Suyang Wu helped in the calculation of dietary acrylamide exposure. All the coauthors critically commented on and revised the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
None of the authors have competing interests to report.
Funding
The study was supported by the National Institutes of Health R01 Grant AR049439–01A1 and the Research Grants Council Earmarked Grant CUHK4101/02 M. The funders played no role in the study design, data collection and analysis, interpretation of the data, as well as in writing the manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, Zm., Tse, L.A., Ho, S.C. et al. Dietary acrylamide exposure was associated with increased cancer mortality in Chinese elderly men and women: a 11-year prospective study of Mr. and Ms. OS Hong Kong. J Cancer Res Clin Oncol 143, 2317–2326 (2017). https://doi.org/10.1007/s00432-017-2477-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-017-2477-4