Abstract
Purpose
Rectal melanoma (RM) is a lethal malignancy which is not well understood. While cases are rising, data concerning effective management are limited. The present paper sought to elucidate the epidemiology and prognosis of RM, while also analyzing the role of adjuvant radiation therapy (RT).
Methods
We used the surveillance, epidemiology, and end results program to find all cases of RM diagnosed between 2004 and 2011. Patients 18 or older with non-metastatic disease who had undergone surgery were included. Data regarding the age, race, sex, marital status, stage, and radiation sequence with surgery were extracted from the database and analyzed. Disease-free (DFS) and overall survival (OS) was studied for the group overall and between subgroups.
Results
Median age at diagnosis was 69 years. RM is significantly more common in whites compared to nonwhites and occurs equally in males and females. Most patients are diagnosed at an early stage. Prognosis is poor with a median DFS of 27 months and median OS of 22 months. There were no differences in outcomes based on age, sex, marital status, or stage; however, OS was improved in nonwhites as compared to whites (P = 0.04). RT did not improve DFS (27 vs 28 months for surgery vs surgery and radiation, P = 0.82) or OS (19 vs 22 months for surgery vs surgery and radiation P=0.80) regardless of stage.
Conclusions
RM is an aggressive disease primarily affecting older, white patients. RT does not improve survival, regardless of stage. Optimal management of this lethal disease remains to be elucidated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Melanoma accounts for less than one percent of all skin cancers, but it accounts for the majority of skin cancer deaths (American Cancer Society 2016). While mucosal melanoma (MM) accounts for less than one percent of all melanomas, it carries a particularly poor prognosis (Chang et al. 1998). The anorecum is the third most common site of melanoma overall, and is the second most common subsite of MM, after the head and neck (Chang et al. 1998; Klas J et al. 1999). While, the incidence of anorectal melanoma (ARM) has been increasing over the last several decades, the mortality rates have remained unchanged, with five-year overall survival (OS) ranging from 10 to 20 % (Cagir et al. 1999; Callahan et al. 2016).
Patients typically present with symptoms such as rectal bleeding or pain secondary to the primary lesion, resulting in the majority of cases being diagnosed before metastatic disease has developed (Callahan et al. 2016; Kiran et al. 2010; Chen et al. 2016). Adequate management of loco-regional disease, therefore, is of critical importance. Surgery has historically been the primary treatment modality for ARMs, with much debate in the literature as to the optimal extent of surgery (Kiran et al. 2010; Iddings et al. 2010). One of the major limitations in the study of ARMs is the small number of patients available for study. Indeed, much of the published literature on the optimal management comes from case reports (Park et al. 2015; Tanaka et al. 2008; Bullard et al. 2003; Malik et al. 2002; van Schaik et al. 2008; Liptrot et al. 2009). While the optimal extent of surgery remains a subject of debate, there is even less published literature regarding adjuvant treatment with either chemotherapy or radiation (Kelly et al. 2011).
Epidemiological studies have shown that melanomas arising in the rectum are more common than those arising in the anus (Callahan et al. 2016). Furthermore, the incidence of rectal melanoma (RM) has increased at a faster rate than that of anal melanoma (Chen et al. 2016). Despite these epidemiological trends, these two entities are frequently studied together (Callahan et al. 2016; Chen et al. 2016; Iddings et al. 2010; Ballo 2002; Ciarrocchi et al. 2016). The goal of the present study, therefore, was to better characterize the epidemiology, treatment, and outcomes of patients with RM utilizing the surveillance, epidemiology, and end results (SEER) program. We further sought to analyze the effect of radiation therapy (RT) on treatment outcomes.
Methods
IRB approval was obtained. The SEER Program (version 8.2.1) was used to find all cases of RM diagnosed between 2004 and 2011 in patients aged 18 or older. Patients were only included in this study if the primary site was labeled as rectum with an International Classification of Disease (ICD-0-3) code corresponding to malignant melanoma (8720/3). All patients with metastatic disease were excluded from the final analysis. After excluding all patients with distant disease, we found that all but three patients had undergone some form of surgery ranging from local tumor excision to partial colectomy. We thus excluded these three patients from our final analysis. Finally, because we were interested in analyzing the effects of RT on patient outcomes, patients for whom the status of RT was unknown were also excluded.
Data regarding the age (<70 vs ≥70), race (white vs nonwhite), sex (male vs female), marital status (married vs single), stage (tumor and nodal), and radiation sequence with surgery (no radiation vs radiation with surgery) were extracted from the database. We chose not to analyze our data according to type of surgery performed because there would be too few patients in each subgroup (local excision, wedge or partial resection, pull through with sphincter preservation, total proctectomy, or total proctocolectomy).
Statistics
We summarized the extracted data and compared each parameter by type of treatment received using Pearson two-sided Chi-squared tests. The SEER Collaborative Stage (CS) Data Collection System was then used to assign patients to their appropriate AJCC TNM stages. Per the SEER coding manual, RMs are staged according to rectal staging [Primary Site Rectum, NOS (C20.9)] and do not carry a site-specific melanoma staging system. Patients were grouped by tumor stage (T stage) with Tis, T1 and T2 tumors forming one group and T3 and T4 tumors in a separate group. Patients were also staged according to Nodal Group (N stage), N0 versus N1. Each of these stage groupings was then compared by type of treatment given using the Pearson two-sided Chi-squared test.
Univariate analysis was used to evaluate the effect of the various parameters on disease-free survival (DFS) and on overall survival (OS). DFS was defined as the time from diagnosis to disease recurrence, local or distant. OS was defined as time from diagnosis to death from any cause. Survival follow-up was extended to 91 months (median 17, range 0–91 months). Kaplan–Meier estimates were used to study survival rates for the various subgroups and log-rank statistics were used to compare survival between subgroups. Univariate Cox proportional hazard ratios were used to explore the relationship between survival and the parameters tested. The results were considered significant if P < 0.05.
Results
Of the 135 patients with RM identified in the database, a total of 63 patients met study criteria. Patient characteristics are shown in Table 1. Median age at diagnosis was 69 years. Significantly, more whites were diagnosed with the disease compared to nonwhites (whites/nonwhites: 3.85). The number of males and females was approximately equal (male/female: 1.03), and most patients were married (36 vs 25). The majority of the patients extracted from the database had early stage disease. Of the 63 patients included in our study, 18 received RT in addition to surgery, while the remaining 45 were treated by surgery alone.
There was no statistically significant difference between patient age (<70 vs ≥70) and type of treatment administered (P = 0.81). There were also no differences between race, sex, marital status, or stage and type of treatment administered to the patients, as shown in Table 2.
RM is an aggressive disease with a median DFS of only 27 months (Fig. 1). When we compared outcomes for the various parameters, we did not find any statistically significant differences in DFS between any of our subgroups, as shown in Table 3. Specifically, median DFS did not differ between patients younger or older than 70 (28 vs 25 months, P = 0.17), between whites and nonwhites (25 vs 31 months, P = 0.06), between males compared to females (25 vs 27 months, P = 0.71), or between married and single patients (27 vs 22 months, P = 0.73). There were also no differences in DFS according to T stage (32 vs 16 months for Tis, T1, T2 vs T3, T4, respectively, P = 0.62) or according to N stage (27 vs 11 months for N0 vs N1, respectively, P = 0.94).
Prognosis for all patients regardless of subgroup was poor with a median OS of only 22 months (Fig. 1). While there was no statistically significant difference in OS based on age (23 vs 13 months for <70 years vs ≥70 years, respectively, P = 0.15), gender (18 vs 22 months for males vs females, P = 0.50), or marital status (25 vs 13 months for married vs single patients, P = 0.94), there was a statistically significant improvement in OS based on race (16 vs 28 months for whites vs nonwhites, respectively, P = 0.04) (Table 3). There was no difference in OS according to T stage (P = 0.52) or N stage (P = 0.84).
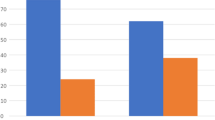
Prognosis was compared between patients receiving surgery alone versus those receiving surgery and radiation. There was no statistically significant difference in DFS based on treatment modality (27 vs 28 months for surgery vs surgery and radiation, respectively, P = 0.82) or in OS (19 vs 22 months for surgery vs surgery and radiation, respectively, P = 0.79), as shown in Fig. 2.
Kaplan–Meier estimates of DFS and OS for patients based on treatment type. Median DFS was 27 versus 28 months for surgery alone versus surgery and radiation (SurgRad), (P = 0.82). Median OS was 19 versus 22 months for surgery alone versus surgery and radiation (SurgRad) (P = 0.80). DFS disease-free survival, OS overall survival, SurgRad surgery and radiation
When we compared outcomes based on treatment and patient stage, no differences in DFS were observed for those receiving surgery versus those receiving a combination of surgery and radiation. Among those with Tis, T1, and T2 tumors, DFS was 28 months for those receiving surgery alone versus 35 months for those receiving surgery and radiation (P = 0.36, Fig. 3). Among those with T3, T4 tumors, DFS was 16 months for those receiving surgery alone versus 9 months for those receiving surgery and radiation (P = 0.39, Fig. 4a). Among those with N0 disease, DFS was 27 months for those receiving surgery alone versus 22 months for those receiving combination therapy (P = 0.52) and, finally, among those with nodal disease, DFS was 16 months for those receiving surgery alone versus 9 months for those receiving surgery and radiation (P = 0.36).
Kaplan–Meier estimates of DFS and OS for patients with Tis, T1, or T2 tumors based on treatment type. Median DFS was 28 versus 35 months for patients treated with surgery alone versus surgery and radiation (SurgRad) (P = 0.36). Median OS was 22 versus 29 months for surgery alone versus SurgRad (P = 0.31). DFS disease-free survival, OS overall survival, SurgRad surgery and radiation
Kaplan–Meier estimates of DFS and OS for patients with T3 or T4 tumor stage based on treatment type. Median DFS was 16 versus 9 months for those treated with surgery alone versus surgery and radiation (SurgRad), respectively (P = 0.39). Median OS was 13 versus 9 months for those treated with surgery alone versus surgery and radiation SurgRad (P = 0.75). DFS disease-free survival, OS overall survival, SurgRad surgery and radiation
Similar to DFS, OS did not differ based on treatment and patient stage. OS among patients with Tis, T1, and T2 tumors was 22 months for those who received surgery alone versus 29 months for those who received combination therapy (P = 0.31, Fig. 3). OS for patients with T3 and T4 tumors was 13 months for surgery alone versus 9 months for surgery plus radiation (P = 0.75, Fig. 4b). OS did not differ among those with N0 disease based on radiation sequence with surgery (P = 0.75) or among those with node positive disease based on the addition of radiation to surgery (P = 0.26).
Discussion
To our knowledge, this is the first paper to look at epidemiological and survival data pertaining exclusively to RM. Furthermore, it is the first study to analyze the effect of radiation on OS in patients with RM using a large population-derived database. Prior studies investigating MM in the distal gastrointestinal tract have either grouped cases of anal and rectal melanoma together or only included cases of anal melanoma (Klas J et al. 1999; Callahan et al. 2016; Chen et al. 2016; Iddings et al. 2010; Ciarrocchi et al. 2016). We chose to focus our analysis on cases of RM alone, not only because it appears to be less well studied, but because the incidence of RM, specifically, appears to be rising (Chen et al. 2016).
We found that, while there were few patients with RM meeting our study criteria, the prognosis for these patients, which excluded those with distant metastatic disease, was nevertheless very poor. RM was found to primarily afflict older adults, with a median age of diagnosis of 69 years. It was significantly more common in whites as compared to nonwhites, in accordance with the findings of Chen et al. (2016) who conducted a similar epidemiological study of anal and rectal melanomas combined. The incidence of RM was equal in men versus women and in married, as compared to single, patients. The type of treatment administered to patients did not vary based on age at diagnosis, race, sex, or marital status, which we expected. However, there was also no difference in treatment regimen offered to patients with early stage disease as compared to those with more locally advanced disease, in whom you might expect a more aggressive treatment approach.
DFS outcomes did not vary based on age, sex, race, or marital status. Nor did they vary according to stage, with similarly poor survival for those with limited as compared to more locally advanced disease. OS also did not differ according to age, sex marital status, or stage, but, interestingly, there was a difference in OS based on race, favoring nonwhites by a significant margin. RM not only appears to afflict whites more frequently than nonwhites, but it also seems to follow a more aggressive disease course in this subgroup of patients.
Finally, we were unable to find a significant survival advantage for those treated with radiation in addition to surgery versus those managed by surgery alone. Even among patient with T3 or T4 disease or in those with involved lymph nodes, radiation therapy did not improve disease-free or overall survival. Indeed, in patients with T3 or T4 disease, survival appeared to be nearly twice as long in the absence of radiation; however, this difference was not statistically significant, likely because of the small number of patients in this subgroup.
RM is a highly lethal malignancy which has not been well studied. A major factor precluding adequate study of this disease is the small patient number available for evaluation. Most of the current literature addressing the treatment of ARM has focused on optimal surgical management. In a study performed by Bullard et al. (2003) in which the authors reviewed all cases of ARM referred for surgical resection at their institution, no differences in outcomes were observed based on the type of surgery performed. In 2010, Iddings et al. (2010) analyzed SEER data to determine whether more aggressive surgery with an abdominoperineal resection (APR) improved outcomes compared to transanal excision (TAE) alone. Like Bullard et al. before them, the authors did not find a difference in survival based on the extent of surgery, with overall survival rates of 16 versus 18 months for APR and TAE, respectively.
Other studies have sought to improve on outcomes with surgery alone with the addition of radiation therapy to the management of patients with RM. In a study conducted at MD Anderson, outcomes were analyzed for 23 patients with invasive ARM who had been managed with sphincter-sparing surgical resection and adjuvant radiation (Ballo 2002). The authors concluded that the addition of radiation therapy to surgery was safe and effective with a 5-year overall survival rate of 31 % and a disease-free survival of 37 %. Another study by Moozar et al. (2003) looked at 14 patients treated at their institution with either surgery alone or surgery and radiation. The authors did not find improved outcomes for the seven patients who received radiation therapy following surgery. Another more recent study from MD Anderson sought to build on their previous findings by analyzing outcomes based on extent of radiation, primary site versus primary site plus draining lymphatics (Kelly et al. 2011). The authors did not find superior outcomes in patients treated with a more extensive radiation field, but they did find a higher incidence of lymphedema in these patients.
This study has some limitations which are worth noting. First, the small patient size decreased the statistical power of our analysis and limited our ability to study subgroups of patients based on the type of surgery performed, which may have had an effect on outcomes. We were also forced to group patients with Tis, T1 and T2 tumors and those with T3 and T4 tumors together, to permit analysis with so few patients. Inclusion of patients with Tis lesions may have diluted survival outcomes. Utilization of the SEER database also has its limitations. Data regarding comorbid conditions, treatment-related complications, and systemic therapy were not available. We were also unable to extract data on the site of first failure, which may have been relevant to our analysis on outcomes pertaining to radiation therapy which is a local treatment.
Despite these limitations, this population-based study presents key epidemiological and survival data for RM with a focus on the effect of RT on survival. The addition of radiotherapy does not appear to improve outcomes for patients with RM, even in those with more locally advanced disease. RM is clearly a deadly disease for which optimal management strategies remain to be elucidated.
References
American Cancer Society (2016) Cancer facts and figures 2016. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf. Accessed 26 July 2016
Ballo MT (2002) Sphincter-sparing local excision and adjuvant radiation for anal-rectal melanoma. J Clin Oncol 20(23):4555–4558. doi:10.1200/JCO.2002.03.002
Bullard KM, Tuttle TM, Rothenberger DA et al (2003) Surgical therapy for anorectal melanoma. J Am Coll Surg 196(2):206–211. doi:10.1016/S1072-7515(02)01538-7
Cagir B, Whiteford MH, Topham A, Rakinic J, Fry RD (1999) Changing epidemiology of anorectal melanoma. Dis Colon Rectum 42(9):1203–1208. http://www.ncbi.nlm.nih.gov/pubmed/10496563. Accessed 26 July 2016
Callahan A, Anderson WF, Patel S et al (2016) Epidemiology of Anorectal Melanoma in the United States. Dermatol Surg 42(1):94–99. doi:10.1097/DSS.0000000000000579
Chang AE, Karnell LH, Menck HR (1998) The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer 83(8):1664–1678. http://www.ncbi.nlm.nih.gov/pubmed/9781962. Accessed 26 July 2016
Chen H, Cai Y, Liu Y et al (2016) Incidence, surgical treatment, and prognosis of anorectal melanoma from 1973 to 2011. Medicine 95(7):e2770. doi:10.1097/MD.0000000000002770
Ciarrocchi A, Pietroletti R, Carlei F, Amicucci G (2016) Extensive surgery and lymphadenectomy do not improve survival in primary melanoma of the anorectum: results from analysis of a large database (SEER). Colorectal Dis. doi:10.1111/codi.13412
Iddings DM, Fleisig AJ, Chen SL, Faries MB, Morton DL (2010) Practice patterns and outcomes for anorectal melanoma in the USA, reviewing three decades of treatment: is more extensive surgical resection beneficial in all patients? Ann Surg Oncol 17(1):40–44. doi:10.1245/s10434-009-0705-0
Kelly P, Zagars GK, Cormier JN, Ross MI, Guadagnolo BA (2011) Sphincter-sparing local excision and hypofractionated radiation therapy for anorectal melanoma: a 20-year experience. Cancer 117(20):4747–4755. doi:10.1002/cncr.26088
Kiran RP, Rottoli M, Pokala N, Fazio VW (2010) Long-term outcomes after local excision and radical surgery for anal melanoma: data from a population database. Dis Colon Rectum 53(4):402–408. doi:10.1007/DCR.0b013e3181b71228
Klas JV, Rothenberger DA, Wong WD, Madoff RD (1999) Malignant tumors of the anal canal: the spectrum of disease, treatment, and outcomes. Cancer. 85(8):1686–1693. http://www.ncbi.nlm.nih.gov/pubmed/10223561. Accessed 26 July 2016
Liptrot S, Semeraro D, Ferguson A et al (2009) Malignant melanoma of the rectum: a case report. J Med Case Rep 3(1):9318. doi:10.1186/1752-1947-3-9318
Malik A, Hull TL, Milsom J (2002) Long-term survivor of anorectal melanoma: report of a case. Dis Colon Rectum 45(10):1412–1415. doi:10.1097/01.DCR.0000029710.49617.7E (discussion 1415–1417)
Moozar KL, Wong CS, Couture J (2003) Anorectal malignant melanoma: treatment with surgery or radiation therapy, or both. Can J Surg. 46(5):345–349. http://www.ncbi.nlm.nih.gov/pubmed/14577706. Accessed 3 Aug 2016
Park JH, Lee JR, Yoon HS et al (2015) Primary anorectal malignant melanoma treated with endoscopic mucosal resection. Intest Res 13(2):170–174. doi:10.5217/ir.2015.13.2.170
Tanaka S, Ohta T, Fujimoto T, Makino Y, Murakami I (2008) Endoscopic mucosal resection of primary anorectal malignant melanoma: a case report. Acta Med Okayama 62(6):421–424. http://www.ncbi.nlm.nih.gov/pubmed/19122689. Accessed 26 July 2016
van Schaik P-M, Ernst M-F, Meijer H-A, Bosscha K (2008) Melanoma of the rectum: a rare entity. World J Gastroenterol 14(10):1633–1635. http://www.ncbi.nlm.nih.gov/pubmed/18330962. Accessed 26 July 2016
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the listed authors have a conflict of interest to disclose.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Tchelebi, L., Guirguis, A. & Ashamalla, H. Rectal melanoma: epidemiology, prognosis, and role of adjuvant radiation therapy. J Cancer Res Clin Oncol 142, 2569–2575 (2016). https://doi.org/10.1007/s00432-016-2245-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-016-2245-x