Abstract
Purpose
Expression of lysosome-associated membrane protein-1 (LAMP1) on the surface correlates with metastatic potential of B16 melanoma cells. Downregulation of their expression in high metastatic (B16F10) cells reduced their surface expression and metastatic potential. Present investigations explore if overexpression of LAMP1 on the surface of low metastatic (B16F1) cells augment their metastatic ability, and if so, how?
Methods
B16F1 cells were transduced with lentiviral vector carrying mutant-LAMP1 (Y386A) (mutLAMP1). Surface expression of LAMP1 and carbohydrates was analyzed by flow cytometry, immunofluorescence and/or immunoprecipitation and Western blotting. Cell spreading and motility were assessed on components of extracellular matrix (ECM) (fibronectin) and basement membrane (BM) (matrigel), and galectin-3-coated coverslips/plates. Metastatic potential was assessed using experimental metastasis assay.
Results
Pre-incubation with anti-LAMP1 antibodies significantly reduced lung metastasis of B16F10 cells. Overexpression of mutLAMP1 significantly increased its surface expression on B16F1 cells, resulting in increased cellular spreading and motility on fibronectin and matrigel. LAMP1 is the major carrier of poly-N-acetyllactosamine (polyLacNAc) on B16F10 cells. However, significantly higher expression of mutLAMP1 had no effect on galectin-3 binding on cell surface or on spreading or motility of cells on galectin-3-coated coverslips/plates. These cells also failed to show any gain in metastatic ability. This could be because LAMP1 from these cells carried significantly lower levels of polyLacNAc in comparison with B16F10 cells.
Conclusions
PolyLacNAc on B16F10 cells and galectin-3 on lungs are the major participants in melanoma metastasis. Although surface LAMP1 promotes interactions with organ ECM and BM, carbohydrates on LAMP1 play a decisive role in dictating lung metastasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metastasis, the major cause of mortality seen in cancer patients, is a complex multistep process involving detachment from the primary, invasion and intravasation, survival in circulation, extravasation and organ homing (Valastyan and Weinberg 2011). Most of the regional spread of tumors can be explained by anatomical/mechanical mode of metastasis (Weiss 1992). However, the distant metastasis is generally organ-specific (Fidler 2003; Gupta and Massagué 2006; Nguyen et al. 2009). This involves one or all of the following factors, viz, adhesive interactions between the molecules on the surface of tumor cells and the target organ, organ growth microenvironment and, more recently, chemokines and their receptors that have been shown to play a critical role in organ-specific metastasis (Fidler 2003; Irmisch and Huelsken 2013; Poste and Nicolson 1980). Tumors often show several metastasis-associated cell surface modifications (Brooks et al. 2010; McGary et al. 2002). Expression of lysosome-associated membrane protein-1 (LAMP1) on the cell surface is one such modification where a lysosomal protein LAMP1 gets increasingly translocated to the surface of several metastatic tumor cells. LAMP-1 is a highly glycosylated protein which decorates the luminal side of lysosomes. Owing to the presence of highly substituted oligosaccharides, it is thought to protect itself and the lysosomal membranes from intracellular proteolysis (Fukuda 1991; Kundra and Kornfeld 1999). It has been shown to be expressed on surface of human melanoma, human colon carcinoma, human fibrosarcoma, human myelomonocytic leukemia and macrophage–melanoma fusion hybrid cells (Chakraborty et al. 2001; Mane et al. 1989; Sarafian et al. 1998). Its cell surface expression has been shown to correlate with metastatic potential of human colon carcinoma and murine melanoma cell lines (Krishnan et al. 2005; Saitoh et al. 1992). In addition to metastatic tumor cells, increased expression of LAMP1 (also known as CD107a) on the surface has also been observed on cells that are involved in migratory and/or invasive functions such as activated cytotoxic T lymphocytes, natural killer cells, platelets and macrophages as well as embryonic cells (Alter et al. 2004; Betts et al. 2003; Chakraborty et al. 2001; Cohnen et al. 2013; Febbraio and Silverstein 1990; Kannan et al. 1996; McCormick et al. 1998). However, the mechanism by which cell surface LAMP1 may mediate these functions is largely unknown.
Purified LAMP1 has been shown to bind to RGD peptides, ECM components such as fibronectin and collagen type I and BM components such as laminin and collagen type IV (Laferté and Dennis 1988), suggesting that surface LAMP1 might as well interact with organ ECM and BM components. Besides, LAMP1 has also been found to be a major carrier of poly-N-acetyllactosamine (polyLacNAc)-substituted β1,6 branched N-glycans (Dennis et al. 1987; Fukuda 1991; Krishnan et al. 2005). A transformation-related increase in β1,6 branching observed in fibroblasts, metastatic cell line SP1 and macrophage–melanoma fusion hybrids appeared to be associated with increased LAMP1 surface expression (Chakraborty et al. 2001; Heffernan et al. 1989). LAMP1 on cell surface has been shown to provide ligands in the form of sialyl-Lex to E-selectin (Sawada et al. 1993; Tomlinson et al. 2000) and in the form of polyLacNAc to galectin-3 (Inohara and Raz 1994; Krishnan et al. 2005; Sarafian et al. 1998). LAMP1 has also been shown to be present on unique cell surface domains involved in cell locomotion such as membrane ruffles and microspikes (filopodia) (Garrigues et al. 1994). Further, its accumulation at the edges and extensions of A2058 human metastasizing melanoma cells (Sarafian et al. 1998) hints toward its potential role in tumor cell spreading and motility possibly by serving as additional receptors for molecules on ECM, BM and endothelium.
Using low (B16F1) and high (B16F10) metastatic variants of lung colonizing B16 murine melanoma cells (Hart and Fidler 1980), polyLacNAc-substituted β1,6 branched N-oligosaccharides were shown to promote metastasis of B16F10 cells to the lungs via galectin-3. In addition, LAMP1 was found to be a major carrier of these oligosaccharides in B16 melanoma cells. It was also shown that surface translocation of LAMP1, but not LAMP2, correlated with their metastatic potential (Krishnan et al. 2005). Recently, it was further shown that downregulation of LAMP1 significantly reduced expression of LAMP1 on the surface of B16F10 cells resulting in significant loss of their metastatic potential (Agarwal et al. 2014). LAMP1 has been shown to be a ligand for galectin-3 which is present in highest amounts in mice lungs and expressed constitutively on the surface of lung vascular endothelium (Krishnan et al. 2005).
However, it remains to be elucidated whether expression of LAMP1 protein alone on the cell surface is necessary and sufficient or it also requires glycosylation on LAMP1 especially in the form of polyLacNAc-substituted β1,6 branched N-oligosaccharides for efficient metastasis. The present paper, therefore, aims to investigate the effect of overexpression of LAMP1 on the surface of low metastatic B16F1 cells (deficient in glycosylation machinery) on their spreading and movement on components of ECM and BM together with that on galectin-3, and its bearing on their lung metastasis.
Materials and methods
Cell lines and reagents
B16F1 and B16F10 murine melanoma cell lines were obtained from National Centre for Cell Science, Pune, India. Cell culture reagents were obtained from Invitrogen, USA. Escherichia coli BL 21 with pET3C plasmid containing a full-length human galectin-3 was a kind gift from Dr. Hakon Leffler, Lund University, Sweden. Purified rhgalectin-3 was biotinylated as described in (Bayer and Wilchek 1990). Inbred strains of C57BL/6 mice used for the metastatic assays and other experiments were maintained in the Institute Animal House, and all the animal experiments were approved by the Institutional Animal Ethics Committee.
Cell culture and experimental metastasis assay
Melanoma cells were routinely cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10 % fetal bovine serum (FBS), 0.03 % glutamine, 10 units/ml of Penicillin G-sodium, 10 μg/ml of streptomycin sulfate and 25 μg/ml of amphotericin B. For treatment of cells with LAMP1 antibody, melanoma cells were harvested with 0.25 % trypsin, washed in serum-free DMEM and incubated with 10 μg/ml of LAMP1 antibody for 1 h at 4 °C. For metastasis assays, it was ensured that the cells existed as single cell suspension and had greater than 95 % viability and the assay was performed exactly as described in (Reddy and Kalraiya 2006). Briefly, cells (0.1 million for F10 + anti-LAMP1 experiment and 0.15 million for F1 + mutLAMP1 experiment, contained in 100 μl) were injected intravenously (i.v.) in inbred strains of female C57BL/6 mice via the lateral tail vein. The animals were sacrificed after 21 days, and melanoma colonies on the surface of the lungs were counted using a dissecting microscope.
Generation of mutLAMP1 (Y386A) clones and their transduction in B16F1 cells
Total RNA was prepared from B16F10 cells using TRIzol reagent, and cDNA was synthesized using cDNA synthesis kit (New England Biolabs, USA) as per manufacturer’s protocol. LAMP1 was amplified from cDNA using forward primer having XhoI site (represented in bold italics) followed by Flag tag (represented in bold) and mouse LAMP1 N-terminal sequence (represented in italics): 5′-TATCTCGAGATGGATTACAAGGATGACGATGACAAGGAATTCATGGCGGCCCCCGGCGCC-3′ and reverse primer: 5′-GGATCCCTAGATGGTCTGATAGCCGGCGTGACTCC-3′. The lentiviral vector generated previously, pLV-K18-YFP-IRES-Puro (Sehgal et al. 2012), was digested with XhoI and NotI restriction enzymes to remove K18-YFP and was either self-ligated to obtain empty vector control or ligated to the amplified mouse LAMP1 to generate wild-type LAMP1 (wtLAMP1) vector. The wtLAMP1 vector was further used for site-directed mutagenesis of tyrosine386 of wtLAMP1 to alanine to get mutant-LAMP1 (mutLAMP1) vector using site-directed mutagenesis kit (Stratagene, USA) as per manufacturer’s protocol. The primers used for site-directed mutagenesis included: 5′-AGGAGTCACGCCGGCGCTCAGACCATCTAGGG-3′ and 5′-CCCTAGATGGTCTGAGCGCCGGCGTGACTCCT-3′. The vector control plasmid, the wtLAMP1 plasmid and the mutLAMP1 plasmid were purified and subsequently co-transfected with helper plasmids (pMD2.G and psPAX2) in HEK293FT cells, and infectious viruses containing empty vector control, wtLAMP1 and mutLAMP1 were generated as described previously (Ranjan and Kalraiya 2013) which were used for transduction of B16F1 cells using 8 μg/ml polybrene. The clones were stably selected using puromycin (1 µg/ml). One vector control clone (VC), one wtLAMP1 clone and two mutLAMP1 clones (C1 and C11) growing in the form of isolated colonies were selected. The selected clones were maintained at a concentration of 0.5 μg/ml puromycin.
Flow cytometric analysis of galectin-3 binding and surface expression of LAMP1
For flow cytometry of LAMP1, melanoma cells were stained with LAMP1 antibody (clone 1D4B, BD Biosciences, USA) as described previously (Krishnan et al. 2005). For determination of galectin-3 binding, melanoma cells were first fixed by overnight incubation with 1 % paraformaldehyde in PBS (pH 7.4) followed by galectin-3 staining as described previously for biotinylated LPHA (Krishnan et al. 2005). Briefly, 0.5 million melanoma cells were incubated with 30 μg of biotinylated rhgalectin-3 in 40 μl of FACS buffer (PBS pH 7.4, containing 1 % FBS) followed by extra-avidin-FITC (Sigma) diluted 1:25 in FACS buffer. Cells treated with extra-avidin-FITC alone served as control. Fluorescent cells were acquired at 488 nm and analyzed on FACSCalibur using CellQuest software (BD Biosciences).
Detection of expression of LAMP1 on the cell surface by immunofluorescence staining
Immunofluorescence staining was done as described in (Ranjan et al. 2014). Briefly, melanoma cells were seeded on coverslips and grown overnight in complete medium up to 70–80 % confluency. Cells were washed thrice with PBS (pH 7.4) and fixed with 2 % paraformaldehyde at RT for 5 min. They were washed again with PBS, blocked with 3 % BSA in PBS for 1 h at RT in humidified chamber and incubated with primary antibody (LAMP1) for 1 h in humidified chamber, followed by three washes with PBS to remove excess or non-specifically bound antibody. Cells were further incubated with fluorescence-tagged secondary antibody (anti-rat FITC) for 1 h followed by three washes with PBS. Those incubated only with fluorescence-tagged secondary antibody served as isotype control. Nuclei were stained with 5 μg/ml of 4′,6′-diamidino-2-phenylindole dihydrochloride (DAPI) in PBS for 1 min, and coverslips were mounted on slides using vectashield mounting medium. Images were acquired using a Carl Zeiss laser confocal microscope at 63× magnification.
Purification of recombinant human galectin-3
Expression and purification of recombinant human (rh) galectin-3 was carried out exactly as described previously (Krishnan et al. 2005).
Cell spreading assays
Cell spreading assays were done as per (Lagana et al. 2006). Briefly, melanoma cells were harvested, washed free of serum and seeded at a cell density of 0.5 million/ml in serum-free DMEM on the coverslips coated overnight with 75 μg/ml galectin-3, 10 μg/ml fibronectin and matrigel (BD Biosciences, USA) in serum-free DMEM at 4 °C. The cells were incubated for 45 min in a CO2 incubator. Coverslips treated with serum-free DMEM only served as control. Bound cells were fixed in 4 % paraformaldehyde, permeabilized with 0.5 % Triton X-100 for 15 min and stained with 2 μg/ml Phalloidin-FITC staining solution made in PBS (containing 1 μg/ml of lysolecithin, 10 % methanol, 0.5 % BSA) (Lagana et al. 2006) for 15 min at 37 °C. Nuclei were stained with 5 μg/ml of DAPI in PBS for 1 min. The stained cells were mounted, and images were acquired using a Carl Zeiss laser confocal microscope at 63× magnification.
Wound-healing assays
For wound-healing assays, six well culture dishes were coated overnight with 75 μg/ml of galectin-3, 10 μg/ml of fibronectin and matrigel in serum-free DMEM at 4 °C, followed by blocking of non-specific sites with 2 % BSA for 1 h. Melanoma cells were harvested, seeded at a density of 0.5 million cells per ml of complete medium and incubated at 37 °C for 24 h in a CO2 incubator. The cells were treated with 40 μg/ml mitomycin C (Sigma) for 3 h for inhibiting cell proliferation. A straight, uniform wound (approx. 400 μm in width) was made using a micropipette tip on the monolayer, and the cells were maintained in serum-free DMEM. Wound closure of cells in response to the immobilized galectin-3, fibronectin and matrigel was measured for 16 h by time lapse video imaging of at least three different positions across the length of the wound using a Carl Zeiss inverted microscope at 10x magnification. Uncoated wells, blocked only with BSA, served as control.
Preparation of total cell lysates, protein estimation, SDS-PAGE and Western blotting
Preparation of total cell lysates, protein estimation, SDS-PAGE and Western blotting was done exactly as described previously (Krishnan et al. 2005).
Immunoprecipitation of LAMP1
Total cell lysate (2 mg) was precleared and later incubated at RT for 2 h with 20 μg of anti-LAMP1 antibody. This was followed by addition of 200 μl of protein G Sepharose beads (50 % suspension) (GE Healthcare, Amersham, UK) and incubation overnight at 4 °C. The beads were pelleted at 2,000 rpm for 10 min and later washed five times with 1 ml of lysis buffer. The bound proteins were eluted by boiling the beads in 1× Laemmli sample buffer for 5 min, separated on SDS-PAGE, Western blotted and probed with LAMP1 antibody and with biotinylated LPHA or LEA (Vector Labs, USA) as described previously (Krishnan et al. 2005).
Statistical analysis
All the data are represented as mean ± SD unless stated. All the statistical analysis was performed using GraphPad Prism 5. For spreading and experimental metastasis assays, comparison within the group was done by performing one-way ANOVA followed by Bonferroni’s multiple comparison test. For wound-healing assays, two-way ANOVA with the Bonferroni posttest was conducted. (p value <0.05 was considered significant).
Results
Blocking cell surface LAMP1 with specific antibodies inhibits lung metastasis of B16F10 cells
Expression of LAMP1 on the cell surface correlates with metastatic potential of B16 melanoma cells, and downregulation of their expression in high metastatic B16F10 cells inhibited their metastasis. To understand how surface LAMP1 possibly participates in metastasis, B16F10 cells were pre-treated with LAMP1-specific antibodies to make it unavailable for interaction, and its effect on metastasis was assessed. Untreated cells or those treated with pre-immune IgG served as controls. Pre-treatment with anti-LAMP1 antibody significantly reduced the metastatic ability of cells as compared to controls (Fig. 1a, b) confirming that surface LAMP1 indeed plays a key role in imparting metastatic phenotype. To explore the possible mechanism by which LAMP1 facilitates interactions with molecules on lungs, B16F1 cells were transduced with mutLAMP1 (Y386A).
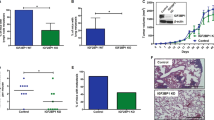
Cell surface LAMP1 plays an important role in lung-specific metastasis. a Melanoma colonies on lungs of C57BL/6 mice injected with untreated B16F10 cells (F10), and those treated with 10 μg/ml of either control rat IgG (F10 + control IgG) or blocking antibodies to LAMP1 (F10 + anti-LAMP1). Four mice were taken in each group. b Graphical representation of mean number of lung colonies. One-way analysis of variance followed by Bonferroni’s multiple comparison test was performed to compare significance (denoted by *** p value <0.001)
Expression of mutLAMP1 (Y386A) in B16F1 cells results in elevated expression of LAMP1 on the cell surface
Mutation in a specific region (Tyr386) of the cytoplasmic tail of LAMP1 has been shown to direct them to cell surface instead of lysosomes (Williams and Fukuda 1990). Stable expression of this mutLAMP1 (Tyr386 to Ala386) by lentiviral infection of B16F1 cells resulted in significantly higher surface expression of LAMP1 in both the clones (C1 and C11) as compared to either uninfected (F1) or those infected with virus with vector alone (vector control—VC). The surface expression of LAMP1 in the clones C1 and C11 was several folds higher even when compared to B16F10 (F10) cells as observed by both flow cytometry (Fig. 2a–c) and immunofluorescence (Fig. 2d). The impact of increased surface expression of LAMP1 on the cellular properties, important from the point of view of metastasis, was explored.
Analysis of LAMP1 expression on the surface of melanoma cells. Comparison of surface expression of LAMP1 by flow cytometry, in a B16F1 cells (gray shade F1), B16F1 cells infected with viruses having empty vector control (pink dotted lines VC) and B16F10 cells (green solid lines F10) and b comparison of the same between vector control (pink dotted lines VC) and B16F1 clones expressing mutLAMP1 (gray shade C1) and (orange solid lines C11). Cells treated with only anti-rat FITC served as control (__). The flow cytometry overlays have been split into two for better understanding of the data. c Graphical representation of the mean fluorescence intensities of surface LAMP1 of the cells shown in a and b. d Immunofluorescence images of the cells stained with anti-LAMP1 antibody and FITC-labelled secondary antibody (green). Nuclei were stained with DAPI (blue). Scale bar 5 μm
Increased surface expression of LAMP1 on B16F1 cells results in significantly increased spreading and motility on fibronectin and matrigel
Purified LAMP1 has been shown to have an affinity for ECM and BM components (Laferté and Dennis 1988). It is possible that the LAMP1 overexpressed on the surface is used as an alternate receptor for these components. The clones overexpressing LAMP1 on the cell surface indeed showed significantly higher spreading on both fibronectin (ECM component) and matrigel (reconstituted BM) as compared to vector control (VC), as seen by laser confocal microscopic images (Fig. 3a, b) and by analyzing ratios of the cytoplasmic to nuclear areas (Fig. 3c, d). The clones also showed much higher motility on these substrates as measured by wound-healing assay (Fig. 4a, c for fibronectin, Fig. 4b, d for matrigel). The results strongly indicate that the increased surface LAMP1 may alter the cellular properties of cells which might eventually be important for metastasis. Wild-type LAMP1 (WT) was also overexpressed in B16F1 cells (Supplementary Fig. S1). But since its expression did neither affect the surface expression of LAMP1 (Supp Fig. S1a and b) nor affect the spreading of melanoma cells on fibronectin (Supp Fig. S1c) to a significant extent as compared to VC, it was not used for further studies.
Effect of increased surface expression of LAMP1 on spreading of melanoma cells on fibronectin and matrigel. Spreading of B16F1 cells infected with viruses having empty vector (VC) or those having mutLAMP1 (C1 and C11) and F10 cells on a fibronectin (FN) and b matrigel (Mat)-coated coverslips as assessed by staining with Phalloidin-FITC (green). DAPI was used to stain the nuclei (blue). Scale bar 5 μm. c Each bar represents ratio of cytoplasmic to nuclear (C/N) area for around 100 cells from two different experiments for spreading on fibronectin (FN) and d for spreading on matrigel (Mat). One-way analysis of variance followed by Bonferroni’s multiple comparison test was performed to compare significance (denoted by *** p value <0.001)
Effect of increased surface expression of LAMP1 on motility of melanoma cells on fibronectin and matrigel. Motility of B16F1 cells infected with viruses having empty vector (VC) or those having mutLAMP1 (C1 and C11) and F10 cells on a fibronectin and b matrigel-coated plates as represented by time lapse video microscopy images at 0 and 16 h of wound closure. c, d Represent mean percent wound closure at 4-h interval on fibronectin and matrigel, respectively. Area of wound closure was measured by Image J software and each image from two different experiments was analyzed at three different positions. Two-way analysis of variance followed by Bonferroni’s multiple comparison test was performed to compare significance from VC (denoted by **** p value <0.0001, *** p value <0.001)
Increased expression of LAMP1 on the surface of B16F1 cells had no effect on their spreading and motility on galectin-3
LAMP1 is a major carrier of polyLacNAc and is a known ligand for galectin-3. Secreted galectin-3 often becomes part of the ECM, BM and even the cell surface (Liu and Rabinovich 2005) and is used as a substratum for cellular adhesion, spreading and movement. Surprisingly, the increased surface expression of LAMP1 had no effect on spreading of these cells (C1 and C11) on galectin-3 as compared to the vector control cells (VC), and the spreading was very similar to that seen on uncoated coverslips, as seen by laser confocal microscopic images (Fig. 5a, b) and quantitated by ratios of cytoplasmic to nuclear areas (Fig. 5c, d). Besides, motility of these cells (C1 and C11) was also almost similar to vector control cells (VC) in the presence of either BSA (Fig. 6a, c) or immobilized galectin-3 (Fig. 6b, d). The lack of any effect is possibly because of low levels of polyLacNAc substitutions as they have been shown to be the major participants in galectin-3-mediated processes.
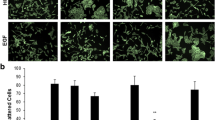
Effect of increased surface expression of LAMP1 on spreading of melanoma cells on galectin-3. Spreading of B16F1 cells infected with viruses having empty vector (VC) or those having mutLAMP1 (C1 and C11) and F10 cells on a uncoated (Un) and b galectin-3 (Gal3)-coated coverslips as assessed by staining with Phalloidin-FITC (green). DAPI was used to stain the nuclei (blue). Scale bar 5 μm. c, d Each bar represents ratio of cytoplasmic to nuclear (C/N) area for around 100 cells from two different experiments for spreading on uncoated c and galectin-3-coated coverslips d. One-way analysis of variance followed by Bonferroni’s multiple comparison test was performed to compare significance
Effect of increased surface expression of LAMP1 on motility of melanoma cells on galectin-3. Motility of B16F1 cells infected with viruses having empty vector (VC) or those having mutLAMP1 (C1 and C11) and F10 cells on a BSA and b galectin-3-coated plates as represented by time lapse video microscopy images at 0 and 16 h of wound closure. c, d Represent mean percent wound closure at 4-h interval on BSA and galectin-3, respectively. Area of wound closure was measured by Image J software and each image from two different experiments was analyzed at three different positions. Two-way analysis of variance followed by Bonferroni’s multiple comparison test was performed to compare significance from VC (denoted by *** p value <0.001)
Increased surface expression of LAMP1 neither increases galectin-3 binding to B16F1 cells nor increases their metastatic potential
LAMP1 is a highly glycosylated molecule and is a major carrier of polyLacNAc. In spite of >20-fold increase in expression of LAMP1 on the surface of clones C1 and C11 as compared to even B16F10 cells (Fig. 2c), it did not result in any gain of their metastatic potential as compared to the parent B16F1 cells (Fig. 7a). Moreover, the binding of galectin-3 to these cells also remained largely unaltered (Fig. 7b, c). Immunoprecipitation experiment revealed that β1,6 branched N-oligosaccharides and polyLacNAc (probed with LPHA and LEA, respectively) on LAMP1 from VC and C1 cells were comparable and were much lower as compared to that present on LAMP1 from F10 cells (Fig. 7d). This clearly suggests that carbohydrates on LAMP1 may play a crucial role in lung metastasis.
Increased surface expression of LAMP1 on B16F1 cells has no effect on lung metastasis. a Melanoma colonies on lungs of C57BL/6 mice injected with F1, VC, C1, C11 and F10 cells. Five mice were taken in each group. b Comparison of galectin-3 binding by flow cytometry using biotinylated galectin-3 in uninfected B16F1 cells (red dotted lines F1) or those infected with viruses having empty vector as control (pink dotted lines VC) with B16F1 clones expressing mutLAMP1 (blue dotted lines C1) and (orange dotted lines C11) and with B16F10 cells (green solid line F10). Cells treated with only extra-avidin FITC (__) served as control. c Graphical representation of the mean fluorescence intensities of galectin-3 binding of all the cells. d Comparison of β1,6 branched N-oligosaccharides (LPHA) and polyLacNAc (LEA) on normalized amounts of immunoprecipitated LAMP1 from B16F10 (F10) cells and B16F1 cells having either empty vector (VC) or mutLAMP1 (C1), by Western blotting
Discussion
Expression of LAMP1 on the cell surface correlates with metastatic potential of B16 melanoma cells, and downregulation of its expression results in its decreased surface expression and concomitantly decreased metastasis (Agarwal et al. 2014; Krishnan et al. 2005). LAMP1 on the cell surface possibly facilitates metastasis by providing high density of high affinity ligands for galectin-3 expressed in highest amounts on the lungs and constitutively on its vascular endothelium, or by interacting with specific ECM/BM molecules on the lungs. Its importance in the metastatic process was further confirmed when B16F10 cells pre-incubated with LAMP1-specific antibodies showed significantly reduced metastatic potential (Fig. 1). Binding of these antibodies possibly prevented LAMP1 from interacting with such molecules on the lungs.
Although our previous results point toward the role of polyLacNAc-substituted N-oligosaccharides on LAMP1 in the metastatic process (Agarwal et al. 2014), purified LAMP1 has also been shown to have an affinity for several ECM and BM components such as fibronectin, laminin, collagen-I and IV and even RGD peptides (Laferté and Dennis 1988). On the cytoplasmic end, LAMP1 has been shown to interact with ezrin (Federici et al. 2009) which functions as a linker between the actin cortical cytoskeleton and various membrane-bound molecules (Bretscher et al. 2002; Neisch and Fehon 2011). This may influence cellular properties important for motility. It is thus possible that both LAMP1 protein and the carbohydrates on it contribute in influencing the metastatic process.
The low metastatic B16F1 cells that express much lower levels of both surface LAMP1 and polyLacNAc-substituted β1,6 branched N-oligosaccharides provide a perfect system to investigate the role of LAMP1 and its associated oligosaccharides, in the metastatic process. Transduction of mutLAMP1 in B16F1 cells provided an ideal model to explore the mechanism by which LAMP1 may influence metastasis, as it resulted in significantly higher expression on the cell surface. These cells expressed >20-fold higher expression of LAMP1 on the cell surface as compared to even B16F10 cells (Fig. 2). Considerably increased expression of LAMP1 on cell surface resulted in significant increase in their spreading and motility on both fibronectin (ECM) and matrigel (BM) (Figs. 3, 4). Although integrins are the known receptors for such components, this is the first report which shows that LAMP1 expressed in such higher amounts on the surface could influence these cellular properties and the underlying mechanism would be worth investigating.
Alternatively, LAMP1 may also promote interactions with molecules on the target organ via high levels of glycosylated structures on it. LAMP1 is a highly glycosylated protein. More than 60 % of its weight is contributed by carbohydrates. Each LAMP1 molecule carries 17–20 N-glycosylation sites that are often substituted further with structures such as Lewis antigens and polyLacNAc (Fukuda 1991). As major portion of LAMP1 is extracellular, it may provide ligands for endogenous lectins such as selectins and galectin-3 expressed on the organ vascular endothelium, in an easily accessible manner (Häuselmann and Borsig 2014).
Lungs express highest amounts of galectin-3 and express it on all the major compartments of the lungs including constitutive expression on the surface of its vascular endothelium (Dange et al. 2014; Krishnan et al. 2005). Previously, polyLacNAc-substituted β1,6 branched N-oligosaccharides have been shown to facilitate lung metastasis by anchoring on to galectin-3 on organ endothelium (Krishnan et al. 2005). More recently, we showed that this lectin carbohydrate pair may participate in not just anchoring, but in all the subsequent steps of extravasation such as spreading to stabilize adhesion, degradation of ECM/BM and movement into organ parenchyma (Dange et al. 2014). It has also been shown that polyLacNAc-substituted N- and not O-oligosaccharides participate in all these processes and shRNA-mediated inhibition of polyLacNAc synthesis inhibits these processes including lung metastasis (Dange et al. 2014; Srinivasan et al. 2009).
PolyLacNAc is the most preferred ligand for galectin-3, and LAMP1 was shown to be one of the major carriers of polyLacNAc on high metastatic B16F10 cells. Moreover, the levels of polyLacNAc-substituted β1,6 branched N-oligosaccharides on LAMP1 per se have been shown to correlate with the metastatic potential of melanoma cells (Krishnan et al. 2005). Further, glycosylation in these cells has also been shown to modulate the surface expression of LAMP1 (Agarwal and Kalraiya 2014). Downregulation of LAMP1 in B16F10 cells has been shown to affect the galectin-3-mediated cellular processes and their metastatic potential (Agarwal et al. 2014). Overexpression of surface LAMP1 on B16F1 cells thus may also influence galectin-3-mediated metastatic processes.
However, B16F1 clones overexpressing LAMP1 on the cell surface showed neither enhanced spreading nor motility on galectin-3-coated surfaces (Figs. 5, 6). Even the ability to metastasize to lungs remained unaltered (Fig. 7a). In spite of >20-fold higher surface expression of LAMP1, binding of galectin-3 to the clones overexpressing surface LAMP1 remained unaltered and was much lower as compared to B16F10 cells (Fig. 7b, c). Since galectin-3-mediated effects are dependent on galectin-3-polyLacNAc interactions, it was plausible to think that each LAMP1 molecule expressed on cell surface might not have adequate polyLacNAc units. Immunoprecipitation experiments indeed confirmed that there was no increase in β1,6 branched N-glycans and polyLacNAc (Fig. 7d) on LAMP1 molecules from these cells which was significantly lower as compared to that on LAMP1 from B16F10 cells. The low levels of polyLacNAc-substituted β1,6 branched N-oligosaccharides on LAMP1 in these cells could be due to limitation in availability of enzymes that add β1,6 branch and polyLacNAc in B16F1 cells (Dange et al. 2014; Srinivasan et al. 2009). Although increasing expression of such enzymes in B16F1 cells may increase their metastatic potential, it would be difficult to attribute it solely to the carbohydrates on LAMP1, as they would glycosylate several other surface proteins as well. The present study thus clearly demonstrates that although increased surface expression of LAMP1 may aid in mediating interactions with the ECM and BM components, it has no influence on melanoma metastasis to the lungs unless it carries high density of ligands (polyLacNAc) for galectin-3.
Conclusions
Metastasis being a multistep process, only cells proficient in all the steps of metastasis are able to metastasize. Cells deficient in mediating even one of these critical events are unable to metastasize, which is often referred to as metastatic inefficiency (Fidler 2003; Weiss 1990). These studies demonstrate that interaction of polyLacNAc on surface LAMP1 with galectin-3 on organ endothelium may be a critical rate-limiting step in the arrest and metastasis of melanoma cells to the lungs. In spite of gaining additional characteristics of interacting with organ ECM and BM, unless proficient in getting arrested in target organ endothelium, the cells fail to metastasize.
References
Agarwal AK, Kalraiya RD (2014) Glycosylation regulates the expression of Lysosome Associated Membrane Protein-1 (LAMP1) on the cell surface. J Biosci Technol 5:556–563
Agarwal AK, Gude RP, Kalraiya RD (2014) Regulation of melanoma metastasis to lungs by cell surface Lysosome Associated Membrane Protein-1 (LAMP1) via galectin-3. Biochem Biophys Res Commun 449:332–337
Alter G, Malenfant JM, Altfeld M (2004) CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods 294:15–22
Bayer EA, Wilchek M (1990) Protein biotinylation. Methods Enzymol 184:138–160
Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA (2003) Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods 281:65–78
Bretscher A, Edwards K, Fehon RG (2002) ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol 3:586–599
Brooks SA, Lomax-Browne HJ, Carter TM, Kinch CE, Hall D (2010) Molecular interactions in cancer cell metastasis. Acta Histochem 112:3–25
Chakraborty AK et al (2001) Fusion hybrids with macrophage and melanoma cells up-regulate N-acetylglucosaminyltransferase V, β1-6 branching, and metastasis. Cell Growth Differ 12:623–630
Cohnen A et al (2013) Surface CD107a/LAMP-1 protects natural killer cells from degranulation-associated damage. Blood 122:1411–1418
Dange MC et al (2014) Galectin-3 expressed on different lung compartments promotes organ specific metastasis by facilitating arrest, extravasation and organ colonization via high affinity ligands on melanoma cells. Clin Exp Metastasis 31:661–673
Dennis JW, Laferte S, Waghorne C, Breitman ML, Kerbel RS (1987) Beta 1-6 branching of Asn-linked oligosaccharides is directly associated with metastasis. Science 236:582–586
Febbraio M, Silverstein R (1990) Identification and characterization of LAMP-1 as an activation-dependent platelet surface glycoprotein. J Biol Chem 265:18531–18537
Federici C et al (2009) Pleiotropic function of ezrin in human metastatic melanomas. Int J Cancer 124:2804–2812
Fidler IJ (2003) The pathogenesis of cancer metastasis: the ‘seed and soil’hypothesis revisited. Nat Rev Cancer 3:453–458
Fukuda M (1991) Lysosomal membrane glycoproteins. Structure, biosynthesis, and intracellular trafficking. J Biol Chem 266:21327–21330
Garrigues J, Anderson J, Hellstrom K, Hellstrom I (1994) Anti-tumor antibody BR96 blocks cell migration and binds to a lysosomal membrane glycoprotein on cell surface microspikes and ruffled membranes. J Cell Biol 125:129–142
Gupta GP, Massagué J (2006) Cancer metastasis: building a framework. Cell 127:679–695
Hart IR, Fidler IJ (1980) Role of organ selectivity in the determination of metastatic patterns of B16 melanoma. Cancer Res 40:2281–2287
Häuselmann I, Borsig L (2014) Altered tumor-cell glycosylation promotes metastasis. Front Oncol 4:28
Heffernan M, Yousefi S, Dennis JW (1989) Molecular characterization of P2B/LAMP-1, a major protein target of a metastasis-associated oligosaccharide structure. Cancer Res 49:6077–6084
Inohara H, Raz A (1994) Identification of human melanoma cellular and secreted ligands for galectin-3. Biochem Biophys Res Commun 201:1366–1375
Irmisch A, Huelsken J (2013) Metastasis: new insights into organ-specific extravasation and metastatic niches. Exp Res 319:1604–1610
Kannan K, Stewart RM, Bounds W, Carlsson SR, Fukuda M, Betzing KW, Holcombe RF (1996) Lysosome-associated membrane proteins h-LAMP1 (CD107a) and h-LAMP2 (CD107b) are activation-dependent cell surface glycoproteins in human peripheral blood mononuclear cells which mediate cell adhesion to vascular endothelium. Cell Immunol 171:10–19
Krishnan V, Bane SM, Kawle PD, Naresh KN, Kalraiya RD (2005) Altered melanoma cell surface glycosylation mediates organ specific adhesion and metastasis via lectin receptors on the lung vascular endothelium. Clin Exp Metastasis 22:11–24
Kundra R, Kornfeld S (1999) Asparagine-linked oligosaccharides protect Lamp-1 and Lamp-2 from intracellular proteolysis. J Biol Chem 274:31039–31046
Laferté S, Dennis JW (1988) Glycosylation-dependent collagen-binding activities of two membrane glycoproteins in MDAY-D2 tumor cells. Cancer Res 48:4743–4748
Lagana A, Goetz JG, Cheung P, Raz A, Dennis JW, Nabi IR (2006) Galectin binding to Mgat5-modified N-glycans regulates fibronectin matrix remodeling in tumor cells. Mol Cell Biol 26:3181–3193
Liu F-T, Rabinovich GA (2005) Galectins as modulators of tumour progression. Nat Rev Cancer 5:29–41
Mane SM, Marzella L, Bainton DF, Holt VK, Cha Y, Hildreth JE, August JT (1989) Purification and characterization of human lysosomal membrane glycoproteins. Arch Biochem Biophys 268:360–378
McCormick PJ, Bonventre EJ, Finneran A (1998) LAMP-1/ESG p appears on the cell surface of single celled mouse embryos subsequent to fertilization. In Vitro Cell Dev Biol Anim 34:353–355
McGary EC, Lev DC, Bar-Eli M (2002) Cellular adhesion pathways and metastatic potential of human melanoma. Cancer Biol Ther 1:454–459
Neisch AL, Fehon RG (2011) Ezrin, radixin and moesin: key regulators of membrane–cortex interactions and signaling. Curr Opin Cell Biol 23:377–382
Nguyen DX, Bos PD, Massagué J (2009) Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer 9:274–284
Poste G, Nicolson GL (1980) Arrest and metastasis of blood-borne tumor cells are modified by fusion of plasma membrane vesicles from highly metastatic cells. Proc Natl Acad Sci 77:399–403
Ranjan A, Kalraiya RD (2013) α2, 6 Sialylation associated with increased β1, 6-branched N-oligosaccharides influences cellular adhesion and invasion. J Biosci 38:867–876
Ranjan A, Bane SM, Kalraiya RD (2014) Glycosylation of the laminin receptor (α3β1) regulates its association with tetraspanin CD151: impact on cell spreading, motility, degradation and invasion of basement membrane by tumor cells. Exp Cell Res 322:249–264
Reddy B, Kalraiya RD (2006) Sialilated β1, 6 branched N-oligosaccharides modulate adhesion, chemotaxis and motility of melanoma cells: effect on invasion and spontaneous metastasis properties. Biochim Biophys Acta 1760:1393–1402
Saitoh O, Wang W, Lotan R, Fukuda M (1992) Differential glycosylation and cell surface expression of lysosomal membrane glycoproteins in sublines of a human colon cancer exhibiting distinct metastatic potentials. J Biol Chem 267:5700–5711
Sarafian V et al (1998) Expression of Lamp-1 and Lamp-2 and their interactions with galectin-3 in human tumor cells. Int J Cancer 75:105–111
Sawada R, Lowe J, Fukuda M (1993) E-selectin-dependent adhesion efficiency of colonic carcinoma cells is increased by genetic manipulation of their cell surface lysosomal membrane glycoprotein-1 expression levels. J Biol Chem 268:12675–12681
Sehgal L, Budnar S, Bhatt K, Sansare S, Mukhopadhaya A, Kalraiya RD, Dalal SN (2012) Generation of HIV-1 based bi-cistronic lentiviral vectors for stable gene expression and live cell imaging. Indian J Exp Biol 50:669–676
Srinivasan N, Bane SM, Ahire SD, Ingle AD, Kalraiya RD (2009) Poly N-acetyllactosamine substitutions on N-and not O-oligosaccharides or Thomsen-Friedenreich antigen facilitate lung specific metastasis of melanoma cells via galectin-3. Glycoconj J 26:445–456
Tomlinson J, Wang JL, Barsky SH, Lee MC, Bischoff J, Nguyen M (2000) Human colon cancer cells express multiple glycoprotein ligands for E-selectin. Int J Oncol 16:347–353
Valastyan S, Weinberg RA (2011) Tumor metastasis: molecular insights and evolving paradigms. Cell 147:275–292
Weiss L (1990) Metastatic inefficiency. Adv Cancer Res 54:159–211
Weiss L (1992) Comments on hematogenous metastatic patterns in humans as revealed by autopsy. Clin Exp Metastasis 10:191–199
Williams MA, Fukuda M (1990) Accumulation of membrane glycoproteins in lysosomes requires a tyrosine residue at a particular position in the cytoplasmic tail. J Cell Biol 111:955–966
Acknowledgments
We thank Dr. Hakon Leffler, Lund University, Sweden, for the expression vector for rhgalectin-3 and National Centre for Cell Science, Pune, for the melanoma cell lines. We acknowledge the help extended by Mrs. Vaishali Kailaje, Mrs. Tanuja Durve, Mrs. Mansi Samarth and Mr. Jayraj Kasale for laser confocal and inverted microscopy, Mrs. Rekha Gour and Ms. Shamal Vetale for flow cytometry, Mr. D. S. Chavan and Mr. A. M. Pawar for technical help and Mr. Sanjay Bane for the help in experimental metastasis and immunoprecipitation experiments. We acknowledge the financial assistance in the form of Senior Research Fellowship to Mr. Akhil Kumar Agarwal, Ms. Nithya Srinivasan and Mr. Shyam K, and more from Council for Scientific and Industrial Research (CSIR), Government of India and Department of Biotechnology (DBT), Government of India for funding the project.
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
432_2015_1917_MOESM1_ESM.tif
Overexpression of wtLAMP1 in B16F1 cells has no effect on surface expression of LAMP1 as well as spreading of melanoma cells on fibronectin. a Comparison of surface expression of LAMP1 by flow cytometry, in B16F1 cells infected with viruses having empty vector (VC) or those having either wtLAMP1 (WT) or mutLAMP1 (C1 and C11) and F10 cells. b Immunofluorescence images of the B16F1 cells infected with viruses having empty vector (VC) or those having either wtLAMP1 (WT) or mutLAMP1 (C1) stained with anti-LAMP1 antibody and FITC labelled secondary antibody (green). c Spreading of the same cells on fibronectin (FN) coated coverslips as assessed by staining with Phalloidin-FITC (green). DAPI was used to stain the nuclei (blue). Scale bar = 5 μm (TIFF 2924 kb)
Rights and permissions
About this article
Cite this article
Agarwal, A.K., Srinivasan, N., Godbole, R. et al. Role of tumor cell surface lysosome-associated membrane protein-1 (LAMP1) and its associated carbohydrates in lung metastasis. J Cancer Res Clin Oncol 141, 1563–1574 (2015). https://doi.org/10.1007/s00432-015-1917-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-015-1917-2