Abstract
Purpose
The literature on primary malignant cardiac tumors is relatively limited because of their rare incidence. This study aimed to provide a proposed treatment strategy for primary malignant cardiac tumors.
Methods
The follow-up outcomes of 29 patients with primary malignant cardiac tumors operated, and 8 primary malignant cardiac tumors considered not operable from 1985 to 2013 in the First Affiliated Hospital of China Medical University were retrospectively analyzed.
Results
Of operation receivers, ten patients had positive surgical margins and nineteen patients had negative surgical margins. Eleven patients received a post-operative neoadjuvant chemotherapy. Patients rejected to surgery had a lower survival compared with operation receivers (15 vs 23 months, P = 0.011). However, there were no significant differences in survival in patients rejected to surgery than in patients who had positive surgical margins (15 vs 16 months, P = 0.874). Patients who had positive surgical margins had a median overall survival duration of only 16 months, whereas patients with negative surgical margins had a median overall survival duration of 27 months (P = 0.002). There were no significant differences in survival in patients with receiving a post-operative adjuvant chemotherapy than in the rest of the population (20 vs 25 months, P = 0.150).
Conclusions
The prognosis for patients with primary malignant cardiac tumors remains very poor. Each patient should be managed on an individual basis, and variety of treatment strategy should be performed. Maximizing the possibility of obtaining negative surgical margins may prolong survival.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Primary cardiac tumors are rare, with an incidence of approximately 0.001–0.030 % in unselected patients at autopsy. Three-quarters of primary cardiac tumors are benign with atrial myxomas comprising 75 % of those. Primary sarcomas of the heart are rare and constitute <25 % of primary cardiac tumors (Silverman 1980; Miralles et al. 1991; Burke et al. 1992). Primary malignant cardiac tumors can affect all age groups but more often occur in adults, 75 % of whom were under 49 years old. The incidence of cardiac tumors in infancy is <10 % (Butany et al. 2005). Most articles are restricted to case report or few examples of primary malignant cardiac tumors (Iwa et al. 2009; Fujita et al. 2009; Messias et al. 2008).
We retrospectively analyzed the techniques for diagnosis, the clinical experience and late results of surgical treatment of 29 cases of primary malignant cardiac tumors and 8 primary malignant cardiac tumors considered not operable at the First Affiliated Hospital of China Medical University over the past 28 years.
Materials and methods
Patients
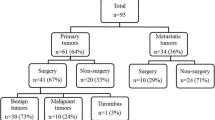
The patient database of the First Affiliated Hospital of China Medical University between 1985 and 2013 was searched. The patients who had metastatic disease at the time of diagnosis were excluded. Finally twenty-nine patients with primary malignant cardiac tumors received operation, and eight primary malignant cardiac tumors considered not operable were identified. Overall, 422 primary cardiac tumors were operated at our institution during the study period.
Of the 37 patients, 17 were males and 20 were females. The mean age was 44.69 ± 13.45 years old (range 12–73). The mean duration of symptoms at the time of diagnosis was 6.88 ± 11.21 months (range 1–48). Of the 37 patients for whom information about presenting symptoms was available, 21 patients (56.8 %) presented with exertional dyspnea, 12 patients (32.4 %) presented with chest pain, and 8 patients (21.6 %) presented with palpitation (Table 1). Six patients had pericardial fluid, and 5 patients had late systolic murmurs. Early diastolic tumor plop was heard in 4 patients, accompanied by increased erythrocyte sedimentation rate and anemia. Pericardiocentesis was performed in 1 patient, and the quality of effusion was described as grossly bloody. All patients had no evidence of remote metastasis at the time of the initial diagnosis and staging.
Electrocardiogram was normal in 8 patients (21.6 %). The abnormal changes included ST-T wave change in 11 (29.7 %), nodal tachycardia in 7 (18.9 %), right ventricular hypertrophy in 5 (13.5 %), left bundle-branch block in 3 (8.1 %) and atrial fibrillation in 3 (8.1 %). Chest X-ray was normal in 12 patients (32.4 %). The abnormal changes included cardiomegaly in 15 (40.5 %), pulmonary congestion in 7 (18.9 %) and pleural effusion in 5 (13.5 %). The echocardiogram was abnormal in all patients (Fig. 1). Transesophageal echocardiography was examined in 6 patients. Computed tomography (CT) scan and magnetic resonance imaging (MRI) demonstrated the presence of the cardiac mass in 16 cases. Multidetector computed tomography (MDCT) demonstrated the presence of the cardiac mass in 13 cases (Fig. 2). The diagnosis for operation receivers was confirmed histologically. The most common histologic type was angiosarcoma (34.5 %), followed by malignant fibrous histiocytoma (13.8 %), and the most common location was the right atrium (37.9 %), followed by the left atrium (Table 2).
a Four-chamber view transthoracic echocardiogram focusing on color flow across medial wall of the main pulmonary artery (MPA) and a mass (arrow) in the pulmonary artery. Color Doppler study showed near obstruction of the left pulmonary artery (LPA), the right pulmonary artery (RPA) and MPA (pulmonary artery endothelia sarcoma). b Transthoracic echocardiography (TTE) showed a 2.4 × 3 cm-sized tumor (arrows) in right atrium (RA) and a 3.8 × 1.8 cm-sized tumor with an irregular echogenicity (arrows) in the right ventricle (RV) invaded the adjacent myocardial tissue (synovial sarcoma). c Echocardiogram, four-chamber view. A large echogenic mass (arrow) in RA invaded the atrioventricular ring. And the post-operative histological examination was an angiosarcoma. d TTE showed a large echogenic mass (arrow) in RA invaded the tricuspid valve (angiosarcoma)
a Contrast-enhanced CT scan showed the tumor (arrows) in the right atrium (RA) came from superior vena cava (SVC) (myxosarcoma). b Sagittal CT scan showed a low attenuation, giant mass (arrow) in RA (angiosarcoma). c Contrast-enhanced MRI showing uniform low signal (arrow) compared with the adjacent blood pool (angiosarcoma). d Preoperative 3D CT scan reconstruction found the noteworthy lack (arrow) of the main pulmonary artery, part of the left pulmonary artery and the right pulmonary artery (pulmonary artery endothelia sarcoma)
Surgical procedure
Median sternotomy allows access for cannulation of the ascending aorta and venae cavae. In order to minimize the risk of embolisation, manipulation of the heart should be minimized until the heart is arrested. The venae cavae are cannulated either through the right atrial wall or directly. Caval snares are used if an opening of the right atrium is planned. Body temperature may be cooled down slightly. Cardiopulmonary bypass is started, the aorta is clamped before manipulation of the heart, and cardioplegia is given. Optimal exposure of the left atrium requires mobilization of both cavae. In some instances, a biatrial incision may be necessary to expose the tumor at the site of its attachment and easy patch closure of the atrial septum if necessary. For right atrial tumors, both venae cavae may be cannulated directly. Cannulation of the jugular or femoral veins for venous drainage can be carried out as an alternative approach. The right atrium is opened widely, and the tumor including its attachment to the cardiac wall is resected. Ventricular tumors were resected by ventriculotomy. Other techniques such as extracardiac resection and pulmonary arteriotomy were also performed. Atrial septal or free wall of the right atrial reconstruction was performed with prosthetic material or autologous pericardial after tumor resection when needed. Valve repair or replacement was also enrolled when the tumor invaded the valve. Cardiac autotransplantation (cardiac explantation, ex vivo tumor resection, reconstruction and reimplantation) was performed for 1 patient with local recurrence in the left ventricle. Tumor resection was performed to obtain grossly negative margins. Table 3 summarizes types of resections for primary malignant cardiac tumors and complications after primary malignant cardiac tumors resection. The three most common complications among patients who underwent primary malignant cardiac tumors resection at our institution were pneumonia (5 patients, 17.2 %), heart failure (2 patients, 6.9 %) and bleeding (2 patients, 6.9 %). There was no death in hospital in the cohort group. Eleven patients received a post-operative neoadjuvant chemotherapy with doxorubicin hydrochloride and ifosfamide.
Overall survival was defined as the time elapsed from the date of diagnostic until death (all causes). Surviving patients were censored at last follow-up date. Survival was estimated using the Kaplan–Meier product limit method, and curves were compared using a log-rank test. The data were managed and analyzed by SPSS software version 16 (SPSS In., Chicago, IL).
Results
Eight primary malignant cardiac tumors considered not operable were all dead at the last follow-up day, and 2 of them received neoadjuvant chemotherapy with doxorubicin hydrochloride and ifosfamide. In the 19 patients who had negative surgical margins, tumor recurrence occurred in 13 patients (8 with local recurrence and 5 with remote metastases) during the follow-up. Only 4 patients were free of disease (no local recurrence or remote metastases) at the time of analysis with a follow-up of 6, 18, 22 and 45 months. Another patient with local recurrence in the left ventricle was still alive 36 months after repeat operation of cardiac autotransplantation. Only 1 patients who had positive surgical margins were alive at the time of analysis with a follow-up of 6 months. Patients rejected to surgery had a lower survival compared with operation receivers (15 vs 23 months, P = 0.011) (Fig. 3). However, there were no significant differences in survival in patients rejected to surgery than in the patients who had positive surgical margins (15 vs 16 months, P = 0.874) (Fig. 4). Patients who had positive surgical margins had a median overall survival duration of only 16 months, whereas patients with negative surgical margins had a median overall survival duration of 27 months (P = 0.002) (Fig. 5). There were no significant differences in survival in patients with receiving a post-operative adjuvant chemotherapy than in the rest of the population (20 vs 25 months, P = 0.150) (Fig. 6).
Discussion
Approximately a quarter of all cardiac tumors exhibit some features of malignancy or behave in a malignant way. 95 % of those are sarcomas, and the other 5 % are lymphomas (Shapiro 2001). To our knowledge, in 1934, the first antemortem diagnosis of a malignant cardiac tumor based on an electrocardiogram and biopsy of a peripheral embolic lesion was reported by Barnes (Barnes et al. 1934). Primary cardiac tumors remained rare and mostly reported in autopsy series and clinical curiosities when diagnosed antemortem until the routine use of echocardiography in 1960s (Schattenberg 1968).
Primary malignant cardiac tumors are thus exceedingly rare diagnoses. Most of the reported series in the literature have not found any sex predilection for these tumors (Perchinsky et al. 1997; Simpson et al. 2008) and specific symptom (Cheitlin et al. 1975). The majority of the patients in our group were symptomatic at the time of diagnosis, such as dyspnea, palpitation, chest pain, syncope or congestive heart failure; however there is no specific one. Physical examination was more commonly nonspecific and reflected only the degree of compromise in the cardiac function. Late systolic murmurs and early diastolic tumor plop might be discovered, which is similar with myxoma. Because of nonspecific symptoms and physical signs, the correct diagnosis is comparatively difficult.
Echocardiography is well known as a fundamental diagnostic tool in intracardiac pathology (Meng et al. 2002; McAllister and Fenoglio 1975). MRI allows better soft-tissue characterization than CT, is the modality of choice for evaluating myocardial and pericardial involvement (Lund et al. 1989), and provides functional information such as flow direction and velocity in large vessels (Araoz et al. 1999). In the diagnosis of cardiac tumors, the use of MDCT and MRI can help differentiate benign from malignant masses. Especially, the use of MDCT is advantageous in providing anatomical information. Recently, the diagnostic yield of MDCT was obviously higher than that of other diagnostic tool. Not only does MDCT allow a correct diagnosis, but it also allows surgical planning and evaluation of possible types of resections. The differences in signal intensity between the heart and the adjacent structures permit MRI to define mediastinal invasion. Central necrosis in sarcomas is demonstrated on contrast MRI’s with gadopentetate dimeglumine. Tissue perfusion imaging with MRI or MDCT can distinguish neoplasm from thrombus and evaluate tumor extent. In our patients, 11 patients were diagnosed as malignant cardiac tumors using MDCT; moreover, the surgical planning was decided from the imaging (Yu et al. 2010, 2011a, b).
Patients rejected to surgery had a lower survival compared with operation receivers in our study. It seems that the patients can take some advantage from the operation. However, there were no significant differences in survival in patients rejected to surgery than in the patients who had positive surgical margins in our study. It also demonstrated that the poor prognosis of the patients who had positive surgical margins was the same as the patients who did not receive operation. The largest reported series of cardiac malignant tumors consists of 34 patients treated at the Mayo Clinic over a 32-year period (Simpson et al. 2008). The median survival was significantly longer when a complete surgical resection was possible (17 versus 6 months when complete resection was not possible). Similarly, in our study, the median survival was longer in those who had negative surgical margins (27 vs 16 months in those who had positive surgical margins). So, negative surgical margin status was found to be important for survival after malignant cardiac tumors resection. To achieve complete resection, a technique of cardiac autotransplantation, which involves cardiac excision, ex vivo tumor resection with cardiac reconstruction and cardiac reimplantation, has been reported. This approach permits an aggressive and complete resection (Reardon et al. 2006a; Blackmon et al. 2008). Cardiac autotransplantation is a feasible technique for resection of complex left-sided cardiac tumors because ventriculotomy is not appealing due to the need to injure good muscle in entering the ventricle. Recurrent disease after previous resections can be safely treated with this technique. Operative mortality and overall survival seems favorable in this series of patients. Benefits of this technique include improved accessibility and ability to perform a complete tumor resection with reliable cardiac reconstruction (Reardon et al. 2006b). Cardiac autotransplantation was performed for one patient with local recurrence in the left ventricle in our hospital, and the transmitral valve approach has provided excellent exposure for complete intraventricular tumor resection, reconstruction of the interventricular septum and mitral valve replacement. The patient was well with a follow-up of 36 months. The poor results with surgical resection have led to occasional attempts to treat patients with cardiac transplantation, if extracardiac disease is not present. In the largest series, results of cardiac transplantation in patients with malignant tumors (most of which were sarcomas) were evaluated in a review of 21 cases (Gowdamarajan and Michler 2000). Although mean survival was only 12 months, seven patients were free of recurrent malignancy at a mean follow-up of 27 months. In view of the difficulty in determining respectability preoperatively, attempts at resection should be coordinated with donor availability. Should resection prove feasible, a second recipient should be available to avoid wasting a donor heart.
The role of adjuvant chemotherapy after surgery remains to be defined. In some retrospective reports, adjuvant chemotherapy after surgery failed to modify the natural course of the disease and only a surgical complete resection was significantly associated with longer survival (Llombart-Cussac et al. 1998; Donsbeck et al. 1999; Bakaeen et al. 2009). In recent reports, chemotherapy after surgery is considered to obtain satisfactory results (Radulescu et al. 2008; Catton 2008). Bakaeen et al. (2009) concluded multimodal therapy can achieve reasonable survival for patients with resected cardiac sarcomas, and patients with local tumor recurrence or metastatic disease may still benefit from aggressive treatment. However, a retrospective study of the French Sarcoma Group reported series of cardiac malignant tumors consists of 124 patients over a 33-year period (Isambert et al. 2014). They concluded chemotherapy was significantly associated with better overall survival only in nonoperated patients, but not in operated patients. The same as them, in our group, there were no significant differences in survival in patients with receiving a post-operative adjuvant chemotherapy than in the rest of the population. These numbers of our study are too small to derive firm conclusions, but are not encouraging. The role of radiotherapy alone or in the adjuvant setting is also limited. The high doses of radiation used to treat sarcomas in other locations are poorly tolerated by the heart. To our knowledge, there is 1 case report of a patient with an unrespectable high-grade cardiac sarcoma who was cured using hyper fractionated radiotherapy (twice daily) to a total dose of 7,050 centigrays with a radiosensitizer, 5-iododeoxyuridine (Movsas et al. 1998).
In light of our experience and findings of other groups, we recommend following a treatment strategy for primary malignant cardiac tumors (Fig. 7). When a patient presents with a cardiac tumor, MRI or MDCT of the chest, abdomen and pelvis should be performed to determine the extent of disease. If the patient has localized disease, then the surgeon should determine whether the tumor can be resectable with negative margins, and if so, resect the tumor. However, if the surgeon determines that the tumor cannot be resected with negative margins or if the patient has metastatic disease; a biopsy of the metastatic site or a transvenous biopsy of the cardiac mass should be done to determine the tumor type. If the tumor is a sarcoma, the patient may receive neoadjuvant therapy, which may have an effect against cardiac sarcoma. After that the patient should be reevaluated. If the tumor is likely to be resectable with negative margins, then it should be removed. However, if the tumor is not thought to be resectable with negative margins, surgical resection may be controversial and other techniques, for example, cardiac autotransplantation or cardiac transplantation can be adopted if the patient has no metastatic disease. If the patient has metastatic disease, the second-line neoadjuvant therapy can be adapted.
The current study had several potential limitations, including those inherent to any retrospective study. The single-center nature of the experience also limits the generalizability of surgical outcomes. However, the major strength of the present manuscript is its time spanning and the relatively large size of the cohort in analysis.
In conclusion, primary malignant cardiac tumors remain a rare but lethal disease. To our knowledge, there are insufficient data in the literature to guide treatment decisions for this complex disease. Each patient should be managed on an individual basis, and variety of treatment strategy should be performed. Maximizing the possibility of obtaining negative surgical margins may prolong survival.
References
Araoz PA, Eklund HE, Welch TJ, Breen JF (1999) CT and MR imaging of primary cardiac malignancies. Radiographics 19(6):1421–1434
Bakaeen FG, Jaroszewski DE, Rice DC, Walsh GL, Vaporciyan AA, Swisher SS, Benjamin R, Blackmon S, Reardon MJ (2009) Outcomes after surgical resection of cardiac sarcoma in the multimodality treatment era. J Thorac Cardiovasc Surg 137(6):1454–1460
Barnes AR, Beaver DC, Snell AM (1934) Primary sarcoma of heart: report of a case with electrocardiographic and pathological studies. Am Heart J 9(3):480
Blackmon SH, Patel AR, Bruckner BA, Beyer EA, Rice DC, Vaporciyan AA, Wojciechowski Z, Correa AM, Reardon MJ (2008) Cardiac autotransplantation for malignant or complex primary left-heart tumors. Tex Heart Inst J 35(3):296–300
Burke AP, Cowan D, Virmani R (1992) Primary sarcomas of the heart. Cancer 69(2):387–395
Butany J, Nair V, Naseemuddin A, Nair GM, Catton C, Yau T (2005) Cardiac tumours: diagnosis and management. Lancet Oncol 6(4):219–228
Catton C (2008) The management of malignant cardiac tumors: clinical considerations. Semin Diagn Pathol 25(1):69–75
Cheitlin MD, DeCastro CM, Knowles DM, Fenoglio JJ, McAllister HA (1975) Clinical pathologic conference: heart neoplasm. Am Heart J 90(2):248–254
Donsbeck AV, Ranchere D, Coindre JM, Le Gall F, Cordier JF, Loire R (1999) Primary cardiac sarcomas: an immunohistochemical and grading study with long-term follow-up of 24 cases. Histopathology 34(4):295–304
Fujita Y, Ikebuchi M, Tarui S, Irie H (2009) Successful combined treatment of primary cardiac malignant lymphoma with urgent cardiac operation and chemotherapy. Circ J 73(5):967–969
Gowdamarajan A, Michler RE (2000) Therapy for primary cardiac tumors: is there a role for heart transplantation? Curr Opin Cardiol 15(2):121–125
Isambert N, Ray-Coquard I, Italiano A, Rios M, Kerbrat P, Gauthier M, Blouet A, Chaigneau L, Duffaud F, Piperno-Neumann S, Kurtz JE, Girard N, Collard O, Bompas E, Penel N, Bay JO, Guillemet C, Collin F, Blay JY, Le Cesne A, Thariat J (2014) Primary cardiac sarcomas: a retrospective study of the French Sarcoma Group. Eur J Cancer 50(1):128–136
Iwa N, Masuda K, Yutani C, Kobayashi TK (2009) Imprint cytology of primary cardiac sarcomas: a report of 3 cases. Ann Diagn Pathol 13(4):239–245
Llombart-Cussac A, Pivot X, Contesso G, Rhor-Alvarado A, Delord JP, Spielmann M, Türsz T, Le Cesne A (1998) Adjuvant chemotherapy for primary cardiac sarcomas: the IGR experience. Br J Cancer 78(12):1624–1628
Lund JT, Ehman RL, Julsrud PR, Sinak LJ, Tajik AJ (1989) Cardiac masses: assessment by MR imaging. AJR 152(3):469–473
McAllister HA Jr, Fenoglio JJ Jr (1975) Cardiac involvement in Whipple’s disease. Circulation 52(1):152–156
Meng Q, Lai H, Lima J, Tong W, Qian Y, Lai S (2002) Echocardiographic and pathologic characteristics of primary cardiac tumors: a study of 149 cases. Int J Cardiol 84(1):69–75
Messias P, Bernardo J, Antunes MJ (2008) Primary left atrial haemangioendothelioma. Interact CardioVasc Thorac Surg 7(5):945–946
Miralles A, Bracamonte I, Sconcul H, Diaz del Castillo R, Akhtar R, Bors V, Pavie A, Gandjbackhch I, Cabrol C (1991) Cardiac tumors: clinical experience and surgical results in 74 patients. Ann Thorac Surg 52(4):886–895
Movsas B, Teruya-Feldstein J, Smith J, Glatstein E, Epstein AH (1998) Primary cardiac sarcoma: a novel treatment approach. Chest 114(2):648–652
Perchinsky MJ, Lichtenstein SV, Tyers GF (1997) Primary cardiac tumors: forty years’ experience with 71 patients. Cancer 79(9):1809–1815
Radulescu D, Pripon S, Radulescu LI, Constantea NA, Gulei I (2008) A rare case of primitive right atrium angio-sarcoma with favorable outcome, in a young female. Case report and literature review. Rev Med Chil 136(10):1311–1316
Reardon MJ, Malaisrie SC, Walkes JC, Vaporciyan AA, Rice DC, Smythe WR, DeFelice CA, Wojciechowski ZJ (2006a) Cardiac autotransplantation for primary cardiac tumors. Ann Thorac Surg 82:645–650
Reardon MJ, Walkes JC, Defelice CA, Wojciechowski Z (2006b) Cardiac autotransplantation for surgical resection of a primary malignant left ventricular tumor. Tex Heart Inst J 33(4):495–497
Schattenberg TT (1968) Echocardiographic diagnosis of left atrial myxoma. Mayo Clin Proc 43(9):620–627
Shapiro LM (2001) Cardiac tumours: diagnosis and management. Heart 85(2):218–222
Silverman NA (1980) Primary cardiac tumors. Ann Surg 191(2):127–138
Simpson L, Kumar SK, Okuno SH, Schaff HV, Porrata LF, Buckner JC, Moynihan TJ (2008) Malignant primary cardiac tumors: review of a single institution experience. Cancer 112(11):2440–2446
Yu L, Shi E, Gu T (2010) Fibrosarcoma of the left superior pulmonary vein with extension to the left atrium. J Card Surg 25(6):698
Yu L, Shi E, Gu T (2011a) Primary pulmonary artery endothelial sarcoma. J Card Surg 26(2):217–218
Yu L, Shi E, Gu T, Xiu Z, Fang Q, Wang C (2011b) Intravenous leiomyomatosis with intracardiac extension: a report of two cases. J Card Surg 26(1):56–60
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, L., Gu, T., Shi, E. et al. Primary malignant cardiac tumors. J Cancer Res Clin Oncol 140, 1047–1055 (2014). https://doi.org/10.1007/s00432-014-1651-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-014-1651-1