Abstract
Purpose
We investigated fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT)-assessed metabolic tumor volume (MTV) and total lesion glycolysis (TLG) as prognostic factors in lung adenocarcinoma patients.
Methods
This retrospective study included 106 patients (19 stage I/II and 87 stage III/IV lung adenocarcinoma) who underwent FDG PET/CT before treatment. Standardized uptake value (SUV), MTV, and TLG (MTV × mean SUV) of each malignant lesion were measured. Whole MTV and whole TLG were the summation of all the MTV and TLG values in each patient. Survival analysis and FDG PET/CT parameters regarding epidermal growth factor receptor (EGFR) gene mutation status were evaluated.
Results
Univariate survival analysis of stage III/IV patients identified high whole MTV (≥90), high whole TLG (≥600), and stage IV as significant predictors of poor progression-free survival. For overall survival, high whole MTV (≥90), high whole TLG (≥600), EGFR mutation-negative, and stage IV were significant poor prognostic predictors. After multivariate survival analysis, high whole MTV (P = 0.001), high whole TLG (P = 0.027), and stage IV (P = 0.006) were independent predictors of poor progression-free survival. High whole MTV (P < 0.001), high whole TLG (P = 0.001), and EGFR mutation-negative (P = 0.001) were independent prognostic predictors for poor overall survival. In a survival analysis of stage I/II patients, none was an independent prognostic predictor. No significant differences were found in FDG PET/CT parameters for EGFR mutation-negative and EGFR mutation-positive patients.
Conclusions
Assessment of MTV and TLG by FDG PET/CT in advanced lung adenocarcinoma patients provides useful information regarding prognosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is one of the most common cancers and a main contributor to cancer-related deaths worldwide. Despite cancer prevention, early detection, and treatment, the majority of these patients are diagnosed in an advanced stage at initial presentation, resulting in poor long-term survival (Pirozynski 2006; Siegel and Naishadham 2012; Soerjomataram et al. 2012).
The utility of fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT) for evaluating lung cancer has been validated (Lv et al. 2011; Chao and Zhang 2012; Paul et al. 2012; Ravenel 2012). A number of studies have also used FDG PET/CT to investigate the prognostic value of metabolic activity in lung cancer (Downey et al. 2004; Vesselle et al. 2007; Hoang et al. 2008; Agarwal et al. 2010). Most of these studies, however, used only the maximum standardized uptake value (SUVmax) to measure tumor metabolic activity, and their reported values were somewhat discordant. Nowadays, the metabolic tumor volume (MTV) and total lesion glycolysis (TLG) are measurable by FDG PET/CT using an automated contouring program. These values allow assessment of the entire tumor burden and overall metabolic activity. Tumor burden has been known to predict the prognosis of several malignancies. For example, the predictive role of tumor burden in patients with lymphoma regarding survival and response to chemotherapy has been reported (Jagannath et al. 1986; Gobbi et al. 2011). Additionally, high tumor volume prior to neoadjuvant chemotherapy for breast cancer has negative predictive value in regard to response to treatment (Caudle et al. 2010). Thus, MTV and TLG may provide better prognostic information than SUVmax alone (Chen et al. 2012; Lim et al. 2012; Yoo et al. 2012).
With a clearer understanding of molecular biology, we now know that patients with epidermal growth factor receptor (EGFR) gene mutations in non-small cell lung cancer, particularly adenocarcinoma, have a high response rate to tyrosine kinase inhibitors (TKIs) such as gefitinib and erlotinib (Kosaka et al. 2004; Lynch et al. 2004; Wang et al. 2012; Cufer et al. 2013). Because the EGFR mutation is known to be associated with outcomes in patients with lung cancer, FDG PET/CT might be expected to predict the presence of this mutation (Huang et al. 2010; Na et al. 2010; Mak et al. 2011).
We therefore investigated whether FDG PET/CT-determined parameters (SUVmax, MTV, TLG) can provide prognostic information for patients with lung adenocarcinoma. We also evaluated these FDG PET/CT parameters for their ability to predict EGFR mutation.
Materials and methods
Patients
Our institutional review board approved this retrospective study. Patient consent was not required. The records of patients with newly diagnosed lung adenocarcinoma who underwent FDG PET/CT before treatment at our center from August 2005 to May 2011 were reviewed. We enrolled a total of 106 patients (43 women and 63 men; age 64 ± 11 years) with lung adenocarcinoma who had no brain metastasis, no previously known history of cancer, and had undergone subsequent clinical follow-up at our center. Because torso FDG PET/CT images were used for the analysis of volume-based parameters, patients with brain metastasis were excluded from the study.
All of the patients underwent bronchoscopy and contrast-enhanced CT of the chest and abdomen within 2 weeks of FDG PET/CT. All primary lung adenocarcinomas were confirmed by biopsy. Distant metastasis was confirmed by histologic examination or by serial imaging follow-up. Additional bone scintigraphy or magnetic resonance imaging was performed to confirm the presence of bone metastasis. The stage was determined using the surgical specimen, except in those who received preoperative neoadjuvant chemotherapy or did not undergo surgery because of distant metastasis. All patients were staged according to the 2010 American Joint Committee on Cancer staging manual 7th edition. Eastern Cooperative Oncology Group (ECOG) performance status was recorded from the patient’s medical record.
Treatment and clinical follow-up
After the pathologic diagnosis and initial staging evaluation, surgery, chemotherapy, and/or radiotherapy was offered to individual patients according to the type and stage of tumor and their medical condition. Patients with stage I or II lung adenocarcinoma underwent surgery, such as sleeve resection, lobectomy, or pneumonectomy. Adjuvant chemotherapy was performed in patients with stage IB–IIB lung adenocarcinoma and a good performance status. For patients with stage IIIA lung adenocarcinoma that could be surgically excised, treatment consisted of surgery followed by chemotherapy and/or radiation. Patients with stage IIIA lung adenocarcinoma that could not be completely excised and patients with IIIB lung adenocarcinoma underwent concurrent or sequential chemoradiation therapy based on their general medical condition. In patients with stage IV lung adenocarcinoma, combination chemotherapy with platinum doublets was the first-line treatment. The chemotherapeutic response was evaluated by contrast-enhanced CT after every two cycles of chemotherapy according to the Response Evaluation Criteria in Solid Tumors. Patients without tumor progression underwent further therapy with the same regimen. Patients with progressive disease underwent second-line treatment, including the EGFR-TKIs (if EGFR mutation +), docetaxel, or pemetrexed. Some were given only supportive care. Follow-up evaluation included a complete physical examination, biochemical screening, and contrast-enhanced CT of the chest and abdomen every 3–4 months. FDG PET/CT, bronchoscopy, or bone scintigraphy was performed whenever clinically indicated.
FDG PET/CT imaging and analysis
All patients fasted for at least 6 h. Blood glucose concentrations were checked before the PET studies (<120 mg/dL for nondiabetic patients and <200 mg/dL for diabetic patients). Subjects in the resting state were given intravenous injections of FDG (4.8 MBq/kg of body weight). PET/CT images were acquired 60 min later using a GEMINI PET/CT scanner (Philips Medical Systems, Cleveland OH, USA). The axes of both PET and CT systems were mechanically aligned. CT was performed from the skull base to the mid-thigh (without intravenous contrast) for attenuation correction and anatomic localization using a standardized protocol of 120 kV, 50 mA, tube-rotation time 0.75 s per rotation, pitch 1.5, and section thickness 5 mm. Immediately after CT, PET images were acquired for 2.5 min per frame using a conventional three-dimensional protocol.
Two experienced nuclear medicine physicians assessed the FDG PET/CT images. Any discrepancies were resolved by consensus. All images were reviewed on a workstation (Extended Brilliance Workspace, Philips Healthcare). Volumetric regions of interest were placed over areas of the malignant lesion. The SUV was defined as the concentration of FDG divided by the injected dose and normalized to the body weight of the patient. SUVmax of the primary tumor (primary tumor SUVmax) and the highest SUVmax on the torso images (torso SUVmax) were obtained. The MTV (in milliliters) was measured from attenuation-corrected torso FDG PET/CT images by an SUV-based automated contouring program (Fig. 1). Initially, the voxels with a threshold of 40 % of the SUVmax in the volume of interest within the contouring margin were incorporated to define the tumor margin accurately. If the tumor margin was not correctly defined, the SUVmax threshold was adjusted. Whole MTV was calculated by adding up the MTVs of all malignant lesions in each patient. The TLG was calculated by multiplying the MTV of each lesion by the corresponding average SUV determined in a selected contouring volume of interest. Whole TLG was calculated by adding up the TLGs of all malignant lesions in each patient.
Genetics
Genetic alterations were tested using tissue specimens obtained by biopsy or surgical excision. In all of the cytologic and histologic samples, DNA was extracted from tumor cells that a pathologist microscopically scraped from tumor-rich areas on archived slides. Both cells and tissues were subjected to microdissection with a 26-gauge needle. EGFR mutations in exons 18, 19, 20, and 21 and K-RAS mutations in codons 12, 13, and 61 were analyzed by the pyrosequencing method.
Statistical analysis
The significance of the differences in FDG PET/CT parameters between the EGFR mutation-positive, K-RAS mutation-positive, and wild-type gene groups was determined using analysis of variance. Correlations between stage and whole MTV or TLG were assessed with Pearson tests. For survival analysis, the cutoff values for the categorization of low and high primary tumor SUVmax, torso SUVmax, whole MTV, and whole TLG values were determined using receiver operation characteristic (ROC) curve analysis. The durations of tumor progression-free and overall survivals were evaluated by survival analysis. Univariate analysis of prognostic factors was performed using the Kaplan–Meier method, and the significance of differences between survival curves was tested with the log-rank test. Multivariate survival analysis for independent prognostic factors was performed using a Cox proportional hazards model that included significant univariate variables. PASW statistics version 17.0 for Windows (SPSS, Chicago IL, USA) was used for the analyses. P < 0.05 was considered to indicate significance.
Results
Patient characteristics and genetic analysis
The clinical characteristics of the patients are summarized in Table 1. There was a predominance of patients with advanced-stage disease. The stage of patients significantly correlated with whole MTV (r = 0.44, P < 0.001) and whole TLG (r = 0.41, P < 0.001). EGFR mutations were found in 39.6 % (42/106) of patients. Among EGFR mutation-positive patients, 1, 28, 1, and 12 patients showed EGFR mutations in exons 18, 19, 20, and 21, respectively. Among EGFR mutation-negative patients, 55 had the wild-type gene and nine had a mutation in the K-RAS codon. None of the FDG PET/CT parameters (SUVmax, MTV, TLG) were useful for identifying the EGFR mutation in lung adenocarcinoma (Table 2).
Survival analysis
The median survival for all patients was 20.2 months (range 1.5–76.6 months). At the time of the last follow-up, 35 of 106 patients had survived a minimum of 14.8 months of clinical follow-up (median 27.6 months), and two patients were lost to follow-up after 6.9 months and 14.5 months, respectively. In 35 stage III/IV patients with EGFR mutation, 34 patients received EGFR-TKIs as the second-line treatment (97.1 %). The one patient who did not receive EGFR-TKIs as the second-line treatment had an EGFR mutation in exon 20. Seven stage I/II patients with EGFR mutation did not receive EGFR-TKIs. Multiple lung masses or nodules were found in 29 of 106 lung adenocarcinoma patients. In those cases, primary lung cancer could not be differentiated from lung-to-lung metastasis. Therefore, the prognostic values of lung MTV/TLG and metastatic MTV/TLG could not be evaluated separately. For survival analysis, the cutoff values for the categorization of low and high primary tumor SUVmax, torso SUVmax, whole MTV, and whole TLG values were set at 12.5, 13.0, 90, and 600, respectively. Each cutoff value was selected based on an ROC curve analysis of our data. Considering the adverse effects of aggressive therapy, we chose a high-specificity cutoff point.
Univariate survival analysis found that high whole MTV (≥90), high whole TLG (≥600), advanced stage (≥III), and poor ECOG performance status (≥2) were significant predictors of poor progression-free survival. Univariate survival analysis also showed that high whole MTV (≥90), high whole TLG (≥600), EGFR mutation-negative, advanced stage (≥III), and poor ECOG performance status (≥2) were significant predictors of poor overall survival (Table 3). Primary tumor SUVmax and torso SUVmax were not significant predictors of either progression-free or overall survival. Between patients with K-RAS mutation and wild type, no significant prognostic difference was found in either progression-free survival or overall survival.
Multivariate survival analysis indicated that high whole MTV (≥90), high whole TLG (≥600), and advanced stage (≥III) were independent predictors of poor progression-free survival. High whole MTV (≥90), high whole TLG (≥600), EGFR mutation-negative, and advanced stage (≥III) were independent prognostic predictors of poor overall survival (Table 4).
We performed the survival analyses of stage I/II and stage III/IV patients separately. There were 19 stage I/II and 87 stage III/IV patients.
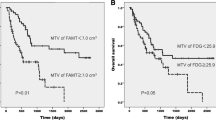
For survival analysis of stage III/IV patients, the cutoff values for the categorization of low and high primary tumor SUVmax, torso SUVmax, whole MTV, and whole TLG values were set at 12.5, 13.0, 90, and 600, respectively. Each cutoff value was selected based on an ROC curve analysis of our data. Univariate survival analysis of stage III/IV patients found that high whole MTV (≥90), high whole TLG (≥600), and stage IV were significant predictors of poor progression-free survival. Univariate survival analysis of stage III/IV patients also showed that high whole MTV (≥90), high whole TLG (≥600), EGFR mutation-negative, and stage IV were significant predictors of poor overall survival (Table 5). The Kaplan–Meier survival curves for progression-free survival of stage III/IV patients according to whole MTV and whole TLG are shown in Fig. 2. The Kaplan–Meier survival curves for overall survival of stage III/IV patients according to EGFR mutation, whole MTV, and whole TLG are shown in Fig. 3.
Multivariate survival analysis of stage III/IV patients indicated that high whole MTV (≥90), high whole TLG (≥600), and stage IV were independent predictors of poor progression-free survival. High whole MTV (≥90), high whole TLG (≥600), and EGFR mutation-negative were independent prognostic predictors of poor overall survival (Table 6). Representative examples from patients with stage IV lung adenocarcinoma with a low and high whole MTV and whole TLG values are shown in Fig. 4.
FDG PET/CT in patients with stage IV lung adenocarcinoma. a A 59-year-old man with low whole MTV (62.6) and whole TLG (452.9). Progression-free survival was 24.3 months, and overall survival was 44.0 months. b A 50-year-old man with high whole MTV (458.5) and whole TLG (1,782.1). Progression-free survival was 4.8 months, and overall survival was 10.3 months
For survival analysis of stage I/II patients, the cutoff values for the categorization of low and high primary tumor SUVmax, torso SUVmax, whole MTV, and whole TLG values were set at 5.5, 5.5, 20, and 70, respectively. Each cutoff value was selected based on an ROC curve analysis of our data. Univariate survival analysis of stage I/II patients found that only a positive smoking history was a significant predictor of poor progression-free survival (P = 0.033). Univariate survival analysis of stage I/II patients also showed that high whole MTV (≥20, P = 0.001), high whole TLG (≥70, P = 0.001), high primary tumor SUVmax (≥5.5, P = 0.005), and high torso SUVmax (≥5.5, P = 0.005) were significant predictors of poor overall survival. However, multivariate survival analysis of stage I/II patients suggested that none of the predictors of overall survival was an independent predictor of a poor prognosis.
Discussion
New diagnostic and treatment strategies are essential to improve survival rates for lung cancer. The variation in lung cancer survival is multifactorial. Stage at presentation, performance status, and genomic patterns seem to have an influence on the outcome for patients with lung cancer. A method for selecting patients who have a better prognosis could influence treatment decisions and potentially reduce therapeutic toxicity. It could ultimately improve survival.
FDG PET/CT is a promising imaging modality that provides complementary prognostic information in contrast to conventional methods. The extent of FDG uptake has been suggested to correlate with tumor aggressiveness in various malignancies (Marom et al. 1999; Patz et al. 2000; Putora et al. 2013; Kitagawa et al. 2003; Higashi et al. 2005; Are et al. 2007; Garin et al. 2009). We found that whole MTV and whole TLG showed a significant correlation with stage and were independent prognostic predictors of both progression-free and overall survival. Therefore, it is possible that whole MTV and whole TLG are related to tumor aggressiveness. However, since pathologic diagnoses were confirmed by needle biopsy only in most of the advanced cases, we could not investigate the association with histological aggressiveness.
Our results suggest that whole MTV and whole TLG were more valuable as predictors of prognosis in patients with stage III/IV lung adenocarcinoma than in patients with stage I/II lung adenocarcinoma. In this study, high whole MTV (≥90) and high whole TLG (≥600) were independent negative predictors of both progression-free and overall survival in patients with stage III/IV lung adenocarcinoma. However, in survival analysis of patients with stage I/II lung adenocarcinoma, no independent predictor was identified. This may simply attributable to the small number of stage I/II patients. Some previous studies have reported that MTV or TLG of primary tumor had a significant prognostic value in patients with operable lung cancer (Kim et al. 2012; Lin et al. 2012). Additional studies on patients with earlier-stage disease should clarify the role of these measurements in this population.
For overall survival analysis of stage III/IV patients, high whole MTV (≥90), high whole TLG (≥600), and EGFR mutation-negative were independent prognostic predictors, but stage was not. In this study, all stage III/IV patients with EGFR mutations, except one patient with EGFR mutation in exon 20, received EGFR-TKIs for second-line chemotherapy. The high response rate of lung adenocarcinoma with EGFR mutation to TKIs is well known. Therefore, in stage III/IV lung adenocarcinoma patients, high whole MTV (≥90), high whole TLG (≥600), and the EGFR mutation-negative were better prognostic predictors of poor overall survival than stage.
We also found that SUVmax was not a significant predictor of prognosis. Previous reports suggested that the SUVmax of FDG PET/CT showed a correlation with tumor doubling time and survival in patients with early-stage non-small cell lung cancer (Marom et al. 1999; Patz et al. 2000; Downey et al. 2004). However, in patients with advanced-stage non-small cell lung cancer, the SUVmax of FDG PET/CT did not have a significant relation with survival (Hoang et al. 2008). Our results support those reports as we had a predominance of patients with advanced-stage disease in this study.
Epidermal growth factor receptor mutations have profound value for patients with lung cancer because they can help determine individualized treatment. FDG uptake has been reported to predict the presence of EGFR mutations (Huang et al. 2010; Na et al. 2010; Mak et al. 2011). Early studies, however, reported somewhat discordant values when SUVmax was used for predicting the presence of EGFR mutations. Huang et al. showed that patients with a high SUVmax were likely to have EGFR mutations, whereas other studies have demonstrated that low SUVmax or SUVmax normalized to the SUV of blood in the pulmonary artery correlated with EGFR mutations. A recently published report concluded that FDG uptake was not correlated with EGFR mutations (Putora et al. 2013). We found no significant differences in FDG PET/CT parameters (SUVmax, MTV, TLG) between patients who were EGFR mutation-positive and those who were EGFR mutation-negative. The FDG PET/CT parameters were also not significantly different between the EGFR mutation-positive group, the K-RAS mutation-positive group, and the wild-type gene group. Therefore, we suggest that FDG PET/CT parameters, including SUVmax, MTV, and TLG, may not be useful for predicting the presence of EGFR mutations.
K-RAS mutations are observed at a lower frequency among Asians compared with whites. K-RAS mutations are also found at a lower frequency in never smokers. The presence of K-RAS mutation is considered a marker of poor prognosis and a predictive marker of EGFR-TKIs benefit (Roberts et al. 2010; Roberts and Stinchcombe 2013). Therefore, as with EGFR mutations, we had the clinical question as to whether K-RAS mutations had an association with FDG PET parameters and whether there was a difference in prognosis according to K-RAS mutations. However, the frequency of K-RAS mutation was low in this study (8.5 %, 9/106), and no statistical significance was found.
Our study has several limitations. First and most notably, it was a retrospective review with a limited follow-up period. However, because most of the included patients had a stage III or IV lung adenocarcinoma (87/106, 82.1 %) and a predominance of patients who survived were followed for more than 2 years (24/35, 68.6 %), it is unlikely that the conclusions of the current study would change significantly with longer follow-up. Second, although the histopathology of all of the primary tumors was evaluated, we did not have histological confirmation of all lymph nodes or distant metastases. Such validation by conventional imaging methods and serial follow-up could cause imprecise estimation of the whole MTV and whole TLG. We may have included inflammatory lesions or excluded FDG non-avid malignant lesions. Third, because of insurance coverage in Korea, EGFR-TKIs were not available for first-line treatment, but as second-line treatment for the majority of patients who had EGFR mutation-positive lung adenocarcinoma. Previous studies demonstrated that patients receiving EGFR-TKIs as first-line treatment for EGFR mutation-positive non-small cell lung cancer had a significantly longer progression-free survival than those with standard doublet chemotherapy (Mok et al. 2009; Maemondo et al. 2010; Mitsudomi et al. 2010). This may be the reason why EGFR mutation status was an independent prognostic predictor for overall survival only—not for progression-free survival.
In conclusion, high whole MTV and whole TLG assessed on FDG PET/CT images are independent prognostic factors for poor progression-free and poor overall survival in patients with stage III/IV lung adenocarcinoma. Furthermore, in stage III/IV lung adenocarcinoma patients, whole MTV, whole TLG, and the presence of EGFR mutation are more accurate prognostic predictors of overall survival than stage. However, the role of FDG PET/CT parameters, including MTV and TLG, for predicting EGFR mutation status in lung adenocarcinoma is limited. Therefore, assessment of MTV and TLG by FDG PET/CT provides potentially useful information regarding prognosis in patients with advanced lung adenocarcinoma.
References
Agarwal M, Brahmanday G, Bajaj SK, Ravikrishnan KP, Wong CY (2010) Revisiting the prognostic value of preoperative (18)F-fluoro-2-deoxyglucose ((18)F-FDG) positron emission tomography (PET) in early-stage (I & II) non-small cell lung cancers (NSCLC). Eur J Nucl Med Mol Imaging 37(4):691–698. doi:10.1007/s00259-009-1291-x
Are C, Hsu JF, Ghossein RA, Schoder H, Shah JP, Shaha AR (2007) Histological aggressiveness of fluorodeoxyglucose positron-emission tomogram (FDG-PET)-detected incidental thyroid carcinomas. Ann Surg Oncol 14(11):3210–3215. doi:10.1245/s10434-007-9531-4
Caudle AS, Gonzalez-Angulo AM, Hunt KK, Liu P, Pusztai L, Symmans WF et al (2010) Predictors of tumor progression during neoadjuvant chemotherapy in breast cancer. J Clin Oncol 28(11):1821–1828. doi:10.1200/JCO.2009.25.3286
Chao F, Zhang H (2012) PET/CT in the staging of the non-small-cell lung cancer. J Biomed Biotechnol 2012:783739. doi:10.1155/2012/783739
Chen HH, Chiu NT, Su WC, Guo HR, Lee BF (2012) Prognostic value of whole-body total lesion glycolysis at pretreatment FDG PET/CT in non-small cell lung cancer. Radiology 264(2):559–566. doi:10.1148/radiol.12111148
Cufer T, Ovcaricek T, O’Brien ME (2013) Systemic therapy of advanced non-small cell lung cancer: major-developments of the last 5-years. Eur J Cancer 49(6):1216–1225. doi:10.1016/j.ejca.2012.11.021
Downey RJ, Akhurst T, Gonen M, Vincent A, Bains MS, Larson S et al (2004) Preoperative F-18 fluorodeoxyglucose-positron emission tomography maximal standardized uptake value predicts survival after lung cancer resection. J Clin Oncol 22(16):3255–3260. doi:10.1200/JCO.2004.11.109
Garin E, Le Jeune F, Devillers A, Cuggia M, de Lajarte-Thirouard AS, Bouriel C et al (2009) Predictive value of 18F-FDG PET and somatostatin receptor scintigraphy in patients with metastatic endocrine tumors. J Nucl Med 50(6):858–864. doi:10.2967/jnumed.108.057505
Gobbi PG, Valentino F, Bassi E, Coriani C, Merli F, Bonfante V et al (2011) Chemoresistance as a function of the pretherapy tumor burden and the chemotherapy regimen administered: differences observed with 2 current chemotherapy regimens for advanced Hodgkin lymphoma. Clin Lymphoma Myeloma Leuk 11(5):396–402. doi:10.1016/j.clml.2011.04.008
Higashi K, Ito K, Hiramatsu Y, Ishikawa T, Sakuma T, Matsunari I et al (2005) 18F-FDG uptake by primary tumor as a predictor of intratumoral lymphatic vessel invasion and lymph node involvement in non-small cell lung cancer: analysis of a multicenter study. J Nucl Med 46(2):267–273
Hoang JK, Hoagland LF, Coleman RE, Coan AD, Herndon JE 2nd, Patz EF Jr (2008) Prognostic value of fluorine-18 fluorodeoxyglucose positron emission tomography imaging in patients with advanced-stage non-small-cell lung carcinoma. J Clin Oncol 26(9):1459–1464. doi:10.1200/JCO.2007.14.3628
Huang CT, Yen RF, Cheng MF, Hsu YC, Wei PF, Tsai YJ et al (2010) Correlation of F-18 fluorodeoxyglucose-positron emission tomography maximal standardized uptake value and EGFR mutations in advanced lung adenocarcinoma. Med Oncol 27(1):9–15. doi:10.1007/s12032-008-9160-1
Jagannath S, Velasquez WS, Tucker SL, Fuller LM, McLaughlin PW, Manning JT et al (1986) Tumor burden assessment and its implication for a prognostic model in advanced diffuse large-cell lymphoma. J Clin Oncol 4(6):859–865
Kim K, Kim SJ, Kim IJ, Kim YS, Pak K, Kim H (2012) Prognostic value of volumetric parameters measured by F-18 FDG PET/CT in surgically resected non-small-cell lung cancer. Nucl Med Commun 33(6):613–620. doi:10.1097/MNM.0b013e328351d4f5
Kitagawa Y, Sano K, Nishizawa S, Nakamura M, Ogasawara T, Sadato N et al (2003) FDG-PET for prediction of tumour aggressiveness and response to intra-arterial chemotherapy and radiotherapy in head and neck cancer. Eur J Nucl Med Mol Imaging 30(1):63–71. doi:10.1007/s00259-002-0978-z
Kosaka T, Yatabe Y, Endoh H, Kuwano H, Takahashi T, Mitsudomi T (2004) Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res 64(24):8919–8923. doi:10.1158/0008-5472.CAN-04-2818
Lim R, Eaton A, Lee NY, Setton J, Ohri N, Rao S et al (2012) 18F-FDG PET/CT metabolic tumor volume and total lesion glycolysis predict outcome in oropharyngeal squamous cell carcinoma. J Nucl Med 53(10):1506–1513. doi:10.2967/jnumed.111.101402
Lin Y, Lin WY, Kao CH, Yen KY, Chen SW, Yeh JJ (2012) Prognostic value of preoperative metabolic tumor volumes on PET-CT in predicting disease-free survival of patients with stage I non-small cell lung cancer. Anticancer Res 32(11):5087–5091
Lv YL, Yuan DM, Wang K, Miao XH, Qian Q, Wei SZ et al (2011) Diagnostic performance of integrated positron emission tomography/computed tomography for mediastinal lymph node staging in non-small cell lung cancer: a bivariate systematic review and meta-analysis. J Thorac Oncol 6(8):1350–1358. doi:10.1097/JTO.0b013e31821d4384
Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW et al (2004) Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 350(21):2129–2139. doi:10.1056/NEJMoa040938
Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H et al (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362(25):2380–2388. doi:10.1056/NEJMoa0909530
Mak RH, Digumarthy SR, Muzikansky A, Engelman JA, Shepard JA, Choi NC et al (2011) Role of 18 F-fluorodeoxyglucose positron emission tomography in predicting epidermal growth factor receptor mutations in non-small cell lung cancer. Oncologist 16(3):319–326. doi:10.1634/theoncologist.2010-0300
Marom EM, McAdams HP, Erasmus JJ, Goodman PC, Culhane DK, Coleman RE et al (1999) Staging non-small cell lung cancer with whole-body PET. Radiology 212(3):803–809
Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J et al (2010) Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 11(2):121–128. doi:10.1016/S1470-2045(09)70364-X
Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N et al (2009) Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361(10):947–957. doi:10.1056/NEJMoa0810699
Na II, Byun BH, Kim KM, Cheon GJ, du Choe H, Koh JS et al (2010) 18F-FDG uptake and EGFR mutations in patients with non-small cell lung cancer: a single-institution retrospective analysis. Lung Cancer 67(1):76–80. doi:10.1016/j.lungcan.2009.03.010
Patz EF Jr, Connolly J, Herndon J (2000) Prognostic value of thoracic FDG PET imaging after treatment for non-small cell lung cancer. AJR Am J Roentgenol 174(3):769–774. doi:10.2214/ajr.174.3.1740769
Paul NS, Ley S, Metser U (2012) Optimal imaging protocols for lung cancer staging: CT, PET, MR imaging, and the role of imaging. Radiol Clin North Am 50(5):935–949. doi:10.1016/j.rcl.2012.06.007
Pirozynski M (2006) 100 years of lung cancer. Respir Med 100(12):2073–2084. doi:10.1016/j.rmed.2006.09.002
Putora PM, Fruh M, Muller J (2013) FDG-PET SUV-max values do not correlate with epidermal growth factor receptor mutation status in lung adenocarcinoma. Respirology 18(4):734–735. doi:10.1111/resp.12083
Ravenel JG (2012) Evidence-based imaging in lung cancer: a systematic review. J Thorac Imaging 27(5):315–324. doi:10.1097/RTI.0b013e318254a198
Roberts PJ, Stinchcombe TE (2013) KRAS mutation: should we test for it, and does it matter? J Clin Oncol 31(8):1112–1121. doi:10.1200/JCO.2012.43.0454
Roberts PJ, Stinchcombe TE, Der CJ, Socinski MA (2010) Personalized medicine in non-small-cell lung cancer: is KRAS a useful marker in selecting patients for epidermal growth factor receptor-targeted therapy? J Clin Oncol 28(31):4769–4777. doi:10.1200/JCO.2009.27.4365
Siegel R, Naishadham D (2012) Jemal A (2012) Cancer statistics for Hispanics/Latinos. CA Cancer J Clin 62(5):283–298. doi:10.3322/caac.21153
Soerjomataram I, Lortet-Tieulent J, Parkin DM, Ferlay J, Mathers C, Forman D et al (2012) Global burden of cancer in 2008: a systematic analysis of disability-adjusted life-years in 12 world regions. Lancet 380(9856):1840–1850. doi:10.1016/S0140-6736(12)60919-2
Vesselle H, Freeman JD, Wiens L, Stern J, Nguyen HQ, Hawes SE et al (2007) Fluorodeoxyglucose uptake of primary non-small cell lung cancer at positron emission tomography: new contrary data on prognostic role. Clin Cancer Res 13(11):3255–3263. doi:10.1158/1078-0432.CCR-06-1128
Wang M, Zhao J, Zhang LM, Li H, Yu JP, Ren XB et al (2012) Combined erlotinib and cetuximab overcome the acquired resistance to epidermal growth factor receptors tyrosine kinase inhibitor in non-small-cell lung cancer. J Cancer Res Clin Oncol 138(12):2069–2077. doi:10.1007/s00432-012-1291-2
Yoo SW, Kim J, Chong A, Kwon SY, Min JJ, Song HC et al (2012) Metabolic tumor volume measured by F-18 FDG PET/CT can further stratify the prognosis of patients with stage IV non-small cell lung cancer. Nucl Med Mol Imaging 46(4):286–293
Acknowledgments
This work was supported by Konkuk University.
Conflict of interest
We declare that we have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chung, H.W., Lee, K.Y., Kim, H.J. et al. FDG PET/CT metabolic tumor volume and total lesion glycolysis predict prognosis in patients with advanced lung adenocarcinoma. J Cancer Res Clin Oncol 140, 89–98 (2014). https://doi.org/10.1007/s00432-013-1545-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-013-1545-7