Abstract
Purpose
Anion exchanger 1 (AE1) is a transmembrane glycoprotein which is abundantly expressed in erythrocyte plasma membrane and mediates the electroneutral exchange of Cl− and HCO3 −. We previously reported that the AE1 protein was unexpectedly expressed in the gastric and colonic cancer and take part in the carcinogenesis of the cancer cells. The aim of the present study is to determine the potential clinical implications of AE1 expression in gastric carcinoma.
Methods
Immunohistochemistry assay was used to determine the expression of AE1 protein. The expression of AE1 in normal and malignant tissues from 286 patients with early and advanced gastric carcinoma was examined. The correlations of AE1 expression with clinicopathological parameters, including age, tumor size, location and subtypes, expression frequency, survival period and lymph metastasis were assessed by Chi-squared test and t test analysis.
Results
AE1 immunoreactivity was negative in normal gastric tissue. Positive immunostaining of AE1 was detected in gastric carcinoma regardless of the location. AE1 was most frequently expressed in the gastric antrum carcinoma compared with gastric body cancer (P = 0.034). Expression of AE1 was significantly associated with bigger tumor size, deeper invasion, shorter survival period, and non-lymph metastasis. In para-cancer tissues of intestinal-type gastric cancer, the expression frequency of AE1 was higher than that in diffuse-type (P = 0.011).
Conclusion
The results showed a strong association of AE1 expression with the onset and progression of the gastric cancer and that may be helpful for improving the tumor classification and the treatment of cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human AE1, also named Band 3, is the most abundant integral membrane protein of the erythrocyte plasma membrane (Tanner 1997). It comprises approximately 25% of the membrane protein of the erythrocyte and mediates the electroneutral exchange of Cl− for HCO3 − across the plasma membrane. AE1 is composed of 911 amino acids and has three functional domains. The N-terminal 403 residues constitute the cytoplasmic domain that interacts with a number of proteins, including ankyrin, protein 4.2, glycolytic enzymes, and deoxyhemoglobin and hereby affecting cell shape and stability, apoptosis, and glycolysis regulation. Residues 404–883 composed of a multispanning membrane domain that are responsible for the anion exchange. The acidic short C-terminal cytoplasmic domain contains the binding sites for carbonic anhydrase II and tumor supressor p16 (Kopito et al. 1989; Salhany et al. 1993; Shen et al. 2007).
AE family consists of multiple members including AE1, AE2, and AE3 (Kopito 1990; Alper et al. 2002, 2006). The three members are expressed in different normal tissues. AE1 is found in the erythrocytes, and an N-terminal truncated form is expressed in the kidney (kAE1) (Pang et al. 2008). Immunohistochemical microscopy demonstrated that the kAE1 is located in the basolateral plasma membranes of all type A intercalated cells in the connecting tubule. In addition, AE1 participates in the adhesion of malaria-infected erythrocytes to endothelial cells. AE1-related diseases has been widely described and they remain a major concern in much of hematic and kidney disease (Jennings and Gosselink 1995; Ito et al. 2006; Akel et al. 2007; Bruce 2008) including the southeast asian ovalocytosis, erythroid spherocytosis, hemolytic anemia, and renal tubular acidosis (RTA); AE2 is found in a variety of tissues and regulates intracellular pH (pHi) by exchanging cytosolic bicarbonate for extracellular chloride, but it is most abundantly expressed in the stomach and regulates the acid secretion. Disorders of AE2 expression and functional activity are involved in carcinogenesis (Yang et al. 2008; Stewart et al. 2007); AE3 is found in brain, retina, and heart (Meier et al. 2007).
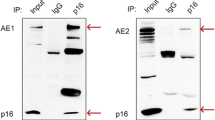
We recently reported that the full-length AE1 was unexpectedly expressed in human gastric and colonic carcinoma and take part in the gastric carcinogenesis in two ways. First, the AE1 interacts with tumor suppressor p16 and sequestrates the protein in cytoplasm that resulted in the disturbance of cell cycle regulation (Fu et al. 2005; Shen et al. 2007). Second, the expression of AE1 in gastric cancer cells with concomitance of alkalization of the cells. In this paper, we focus on the correlation between AE1 expression and clinicopathological parameters of gastric cancer to determine the potential clinical implications of AE1 expressions in gastric carcinoma.
Materials and methods
Subjects
Paraffin-embedded early (n = 104) and advanced (n = 182) gastric cancer samples were collected from surgical resection and endoscopic biopsy in Shanghai Jiao Tong University, Renji hospital from 2001 to 2007. Among the early gastric carcinoma patients, 14 cases have tumors accompanied with lymph node metastasis. The patients with advanced gastric carcinoma were ranged from 32 to 87 years (mean = 74 years). Among advanced gastric carcinoma, 118 cases have tumors accompanied with lymph node metastasis. None of the patients underwent chemotherapy or radiotherapy before surgery. They all agreed to the use of the tumor tissues for clinical research, and the Ethical Committee of Shanghai Jiao Tong University approved the research protocol. We followed up 94 patients in 1–80 months by consulting their case documents through telephone.
Pathology
All tissues were fixed in 4% neutralized formaldehyde, embedded in paraffin, and incised into 4-μm sections. These sections were stained by hematoxylin and eosin (HE) to confirm their histologic diagnosis and other microscopic characteristics. The staging for each gastric carcinoma was evaluated according to TNM staging guidelines. Histologic architecture of gastric carcinoma was expressed in terms of the Lauren’s classification. Furthermore, tumor size and depth of invasion were determined.
Immunohistochemistry
Consecutive slices were dewaxed and rehydrated with xylene or ethanol, and subjected to antigen retrieval by citric acid (pH 6.0). To block endogenous peroxidase activity, the slices were incubated in 0.3% H2O2 for 10 min. Then the samples were incubated overnight at 4°C with anti-AE1 monoclonal antibodies diluted in 10 mM PBS (pH 7.4). After that, the horseradish peroxidase-labeled polymer conjugated with secondary antibody was applied for 15 min and incubated with diaminobenzidine solution (MaxVision™ Kits), followed by light counterstaining with Mayer’s hematoxylin.
Antibody
Antibody employed was monoclonal anti-AE1 (BIII-136, 1:300, Sigma).
Controls
Sections of gastric carcinoma with known positivity for AE1 were used as external positive controls. For negative controls, the primary antibodies were omitted.
Assessment of staining
All sections were evaluated by two independent observers (Wei-Qing Xu and Ling-Jun Song) unaware of the disease outcome. Distinct cytoplasm staining was considered to be positive, regardless of the staining intensity. For AE1 expression, <10% expression was considered as negative.
Statistical analysis
Statistical evaluation was performed using the Chi-squared test and t test to analyze the data. P value less than 0.05 was considered as statistically significant. The SPSS 11.0 software (Chicago, IL) was used to analyze all data.
Results
Different expression patterns of AE1 in gastric carcinoma
The human AE1 protein was not expressed in normal stomach that has been extensively identified (Kudrycki et al. 1990). In this study, we detected the expression of AE1 protein by immunohistochemistry in gastric non-cancer tissues (seven cases) obtained from patients postsurgical operation. Our results supported the previous report and confirmed that the AE1 protein expression was absent in normal human gastric tissues (data not shown).
To determine the potential clinical implications of AE1 expressions in gastric carcinomas or para-cancer tissues (non-cancer cells close to cancer cells), total 286 cases of early and advanced primary carcinomas were analyzed. The different patterns of AE1 expression were detected. In para-cancer tissues of advanced carcinoma, or cancer tissues in the early stage, the large granule profile of AE1 was observed which located in the basolateral side of the glandular epithelium cell. However, the protein was diffusely expressed in cytoplasm of the cancer cells in advanced gastric cancer (Fig. 1). We observed that 123 (67.6%) of 182 cases of advanced gastric carcinoma including cancer and para-cancer tissue had detectable expression of AE1 and that was decreased to 54.4%, while the para-cancer tissue samples were excluded. The AE1 expression in 40 (38.5%) of 104 cases is lower in early stage than that in advanced stage, which means that the latter stage of gastric cancer the higher expression frequency of AE1.
Anatomically, the stomach can be divided into fundus, body and antrum. We have previously reported that gastric cancer located in fundus, body or antrum shown different molecular characters, suggested that the differences in epidemiology, histopathology, and molecular pathology of gastric carcinomas of the three parts (Yang et al. 2008). In this paper, we contrastively analyzed the AE1 expression in gastric carcinoma located in fundus, body, or antrum (Figs. 2, 3a). The results showed that the AE1 was more frequently expressed in antrum carcinoma compared with that in body carcinoma in advanced stage (P = 0.034) (Fig. 3b). In addition, intensity of AE1 expression in antrum carcinoma is stronger than that in fundus or body carcinoma. No difference could be seen in their para-cancer tissues of the gastric cancer in the three parts.
Expression of AE1 in early gastric cancer cells in parts of gastric fundus, body, or antrum and corresponding para-cancer tissues. Immunohistochemical staining with anti-AE1 monoclonal antibody of human early gastric carcinoma and corresponding para-cancer tissue. a, b Fundus carcinoma and para-cancer tissue. c, d Body carcinoma and para-cancer tissue. e, f Antrum carcinoma and para-cancer tissue (×40)
Expression of AE1 in advanced gastric cancer cells in parts of gastric fundus, body, or antrum and corresponding para-cancer tissues. a Immunohistochemical staining of human advanced gastric carcinoma and corresponding para-cancer tissue. a, b Fundus carcinoma and para-cancer tissue. c, d Body carcinoma and para-cancer tissue. e, f Antrum carcinoma and para-cancer tissue (×40). b Expression frequency of AE1 in fundus, body, and antrum carcinoma (left) and para-cancer tissue (right)
Expression of AE1 was correlated with clinicopathological features of gastric carcinoma
Analysis of the associations between AE1 expression and clinicopathological variables was performed by Chi-squared test or t test. The results did not demonstrate any significant association between AE1 expression and age, depth of invasion and TNM staging in advanced gastric cancer. However, there was a strong association between AE1 expression and tumor size, lymph metastasis, and Lauren classification. In the advanced stage, the tumor shown bigger size with average 4.35 ± 0.95 cm3 for AE1 positive carcinoma compared with that (1.67 ± 0.35 cm3) for AE1 negative carcinoma (P = 0.021). As summarized in Table 1, the expression of AE1 was detected in cancer tissues in 59 (50%) of 118 cases that with lymph metastasis. But it was detected in 20 (69%) of 29 cases that without lymph metastasis, suggesting that there was a negative association between AE1 expression and lymph metastasis (P = 0.066). This was emphasized by comparing the expression of AE1 in para-cancer tissue of the patients with or without lymph metastasis (P = 0.023). There was no significant difference in subtypes of cancer tissues between AE1-positive and -negative cases; however, there was a relationship between AE1 expression and intestinal subtypes in para-cancer tissue. The AE1 was detected in 16 (61.5%) of 26 cases of intestinal types and 24 (32.9%) of 73 cases of diffuse types (P = 0.011), suggesting that there was more association between AE1 expression and intestinal types than diffuse types of gastric cancer. In addition, the association of AE1 expression in early cancer tissues and tumor invasion and lymph metastasis were analyzed. As shown in Table 2, 26 (49.1%) of 53 cases of early gastric carcinoma displayed positive expression of AE1 in deeper invasion of the cancer cells which were observed in submucosum layer. However, only 12 (26.7%) of 45 cases showed positive expression of AE1 in cancer tissues which infiltrate till mucous layer (P = 0.023). This indicates that the AE1 expression is associated with the invasion depth in the early stage of gastric cancer.
Follow-up information was available on 94 advanced gastric carcinoma patients after surgical operation for periods ranging from 1 month to 6.7 years (median = 22.5 months) after surgical operation. As shown in Table 3, the patients who expressed AE1 in gastric carcinoma had lower life time when compared with the patients which have no AE1 expression (<5 years) (P = 0.022).
Discussion
Gastric adenocarcinoma (95–98% of gastric cancer) is the fourth most common cancer and a leading cause of cancer-related death worldwide. Despite some advances were achieved in the treatment of advanced gastric cancer (Sipponen 2002; Fukase et al. 2008; Lee et al. 2008; Sugano 2008), these patients still do poorly. Radical surgical resection is still the best treatment option in clinical practice (Lynch et al. 2008). Overall survival of patients with gastric cancer remains disappointing. Our present work showed that the overall 5-year survival rate of people with stomach cancer is 22% which is consistent with other reports (Table 3). This survival rate for gastric cancer is lower than other solid cancers which are considered due to the lack of early diagnostic strategies, the poor understanding of gastric cancer biology and the inadequate medical treatments. Successful approaches to the treatment of gastric cancer are in large part depends on the increasing understanding of their molecular pathway (Vauhkonen et al. 2006).
Gastric cancer is a biologically heterogeneous disease with multigene alteration. Biological prognostic factors are thought to represent a crucial cause for gastric cancer. Several gastric cancer-related factors have been identified (Ernst et al. 2008; Merchant 2008; Poplawski et al. 2008). Stomach is a unique organ and in charge of most of the digestive process; however, no stomach-specific carcinogenic gene was reported. Gastric acid and enzymes plays a key role in the digestive procedure. Increasing evidence indicating that factors inhibiting the acid secretion are involved in the carcinogenesis of gastric cancer (Louise et al. 2005; Argani et al. 2007; Vilkin et al. 2008).
In this paper, immunohistochemical detection of AE1 showed that the protein was expressed in the cytoplasm of gastric cancer or para-cancer cells. The expression was associated with the tumor size, invasion depth and the survival period indicating that the AE1 is a wicked marker for gastric cancer patients. In regard to the mechanism, our previous work demonstrated that the protein takes part in the carcinogenesis pathway via interacting with tumor suppressor p16INK4A and sequestrate the protein in cytoplasm in gastric cancer cell. It is well defined that AE1 was largely expressed in erythrocyte membrane and involved in RBC volume and pHi regulation. In stomach, the regulation of pHi was completed by several molecules including AE2. Disruption of AE2 resulted in the cellular alkalization and achlorhydria (Gawenis et al. 2004). The alkalization of gastric cancer cell and the achlorhydria are the characters of the gastric cancer. Therefore, it is reasonable to consider that the AE1 might interrupts the exchange of Cl−/HCO3 − across the plasma membrane in gastric cancer cell and therefore influence the acid secretion of the stomach. Achlorhydria is associated with gastric cancer-related bacterial overgrowth and gene expression patterns and hereby associated with the gastric cancer (Friis-Hansen 2006; Argent et al. 2008; Gravalos and Jimeno 2008). Studies indicating that chronic achlorhydria and hypergastrinemia induced the upregulation of growth factors, progressive hyperplasia and incomplete intestinal metaplasia.
It is well known that the invasive growth and metastasis are the hallmarks of malignant cancer. Invasion depth is an important parameter for judging the gastric cancer stage. It is interesting to note that AE1 was inconsistently associated with the invasion and lymph metastasis, suggesting that the AE1 was involved in different mechanisms and molecular pathways in gastric carcinogenesis. This can be supported by some tumor properties in clinical practice. For example, some gastric cancers in early stage show washy invasion and rapid metastasis but others in advanced stage show slower metastasis. It is now considered that the pivotal step in cancer metastasis is active migration of tumor cells. Assemble and movement of cellular skeleton protein is an initiative step for the migration of cancer cell. AE1 interacts with a range of proteins including membrane protein and skeleton protein in erythrocyte and therefore we can consider that these interactions might also occur in gastric cancer cells. Expression of AE1 in the gastric cancer cells could impact the function of the skeleton proteins and therefore affect the movement of the cancer cell.
Laurén method classifies gastric cancer into intestinal and diffuse subtypes that was most successful and widely used. The two types of gastric cancer appear clearly as dissimilar clinical and epidemiological entities. The differentiation of the AE1 expression in para-cancer tissues of the two histopathological subtypes was also found. The AE1 was more frequently expressed in intestinal types of gastric cancer. It is interesting to note that the existence of local precursor lesions such as Helicobacter pylori gastritis, intestinal metaplasia, and achlorhydria are linked with intestinal subtypes, but is poor or do not related to diffuse subtypes of the gastric cancer (Vauhkonen et al. 2005), which implies that achlorhydria is important for intestinal subtypes, but are minor in the development of diffused type. We can speculate that the expression of AE1 is related to the achlorhydria and the latter is a precursor of the intestinal subtypes of gastric cancer. Moreover, this achlorhydria is associated with intestinal metaplasia and bacterial overgrowth, which ultimately leads to the development of gastric cancer.
The normal gastric mucosa in antrum or corpus/fundus is consisted of different epithelial cell types. The distinctness of the cancer located in different parts of stomach is not well investigated. AE2, the AE family member with 70% identity with AE1 was most abundantly expressed in the body part and almost inexistent in antrum of the stomach. The protein regulates the acid secretion and vanished in gastric body cancer tissue, but appeared in diffused pattern in antrum cancer tissue, and suggests that different pathways are involved in the carcinogenesis of gastric cancer. These results may be helpful for improving the tumor classification and treatment of the gastric cancer.
References
Akel A, Wagner CA, Kovacikova J, Kasinathan RS, Kiedaisch V, Koka S, Alper SL, Bernhardt I, Wieder T, Huber SM, Lang F (2007) Enhanced suicidal death of erythrocytes from gene-targeted mice lacking the Cl−/HCO3 − exchanger AE1. Am J Physiol Cell Physiol 292(5):C1759–C1767. doi:10.1152/ajpcell.00158.2006
Alper SL (2006) Molecular physiology of SLC4 anion exchangers. Exp Physiol 91(1):153–161. doi:10.1113/expphysiol.2005.031765
Alper SL, Darman RB, Chernova MN, Dahl NK (2002) The AE gene family of Cl−/HCO3 − exchangers. J Nephrol 15:S41–S53
Argani P, Choti MA, Abraham SC (2007) Achlorhydria, parietal cell hyperplasia, and multiple gastric carcinoids: a new disorder. Am J Surg Pathol 31(3):488. doi:10.1097/01.pas.0000213405.95440.a1
Argent RH, Thomas RJ, Aviles-Jimenez F, Letley DP, Limb MC, El-Omar EM, Atherton JC (2008) Toxigenic Helicobacter pylori infection precedes gastric hypochlorhydria in cancer relatives, and H. pylori virulence evolves in these families. Clin Cancer Res 14(7):2227–2235. doi:10.1158/1078-0432.CCR-07-2022
Bruce LJ (2008) Red cell membrane transport abnormalities. Curr Opin Hematol 15(3):184–190. doi:10.1097/MOH.0b013e3282f97b0a
Ernst M, Najdovska M, Grail D, Lundgren-May T, Buchert M, Tye H, Matthews VB, Armes J, Bhathal PS, Hughes NR, Marcusson EG, Karras JG, Na S, Sedgwick JD, Hertzog PJ, Jenkins BJ (2008) STAT3 and STAT1 mediate IL-11-dependent and inflammation-associated gastric tumorigenesis in gp130 receptor mutant mice. J Clin Invest 118(5):1727–1738
Friis-Hansen L (2006) Achlorhydria is associated with gastric microbial overgrowth and development of cancer: lessons learned from the gastrin knockout mouse. Scand J Clin Lab Invest 66(7):607–621. doi:10.1080/00365510600873894
Fu GH, Wang Y, Xi YH, Shen WW, Pan XY, Shen WZ, Jiang XS, Chen GQ (2005) Direct interaction and cooperative role of tumor suppressor p16 with band 3 (AE1). FEBS Lett 579:2105–2110. doi:10.1016/j.febslet.2005.02.063
Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, Terao S, Amagai K, Hayashi S, Asaka M (2008) Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet 372(9636):392–397. doi:10.1016/S0140-6736(08)61159-9
Gawenis LR, Ledoussal C, Judd LM, Prasad V, Alper SL, Stuart-Tilley A, Woo AL, Grisham C, Sanford LP, Doetschman T, Miller ML, Shull GE (2004) Mice with a targeted disruption of the AE2 Cl−/HCO3 − exchanger are achlorhydric. J Biol Chem 279(29):30531–30539. doi:10.1074/jbc.M403779200
Gravalos C, Jimeno A (2008) HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol 19(9):1523–1529. doi:10.1093/annonc/mdn169
Ito D, Koshino I, Arashiki N, Adachi H, Tomihari M, Tamahara S, Kurogi K, Amano T, Ono K, Inaba M (2006) Ubiquitylation-independent ER-associated degradation of an AE1 mutant associated with dominant hereditary spherocytosis in cattle. J Cell Sci 119:3602–3612. doi:10.1242/jcs.03101
Jennings ML, Gosselink PG (1995) Anion exchange protein in Southeast Asian ovalocytes: heterodimer formation between normal and variant subunits. Biochemistry 34(11):3588–3595. doi:10.1021/bi00011a013
Kopito RR (1990) Molecular biology of the anion exchanger gene family. Int Rev Cytol 123:177–199. doi:10.1016/S0074-7696(08)60674-9
Kopito RR, Lee BS, Simmons DM, Lindsey AE, Morgans CW, Schneider K (1989) Regulation of intracellular pH by a neuronal homolog of the erythrocyte anion exchanger. Cell 59(5):927–937
Kudrycki KE, Newman PR, Shull GE (1990) cDNA cloning and tissue distribution of mRNAs for two proteins that are related to the band 3 Cl−/HCO3 − exchanger. J Biol Chem 265(1):462–471
Lee CW, Rickman B, Rogers AB, Ge Z, Wang TC, Fox JG (2008) Helicobacter pylori eradication prevents progression of gastric cancer in hypergastrinemic INS-GAS mice. Cancer Res 68(9):3540–3548. doi:10.1158/0008-5472.CAN-07-6786
Louise MJ, Anastasia A, Carlos AR, Zachary S, Gary ES, Marian LM (2005) Gastric achlorhydria in H/K-ATPase-deficient (Atp4a(-/-)) mice causes severe hyperplasia, mucocystic metaplasia and upregulation of growth factors. J Gastroenterol Hepatol 20(8):1266–1278. doi:10.1111/j.1440-1746.2005.03867.x
Lynch HT, Kaurah P, Wirtzfeld D, Rubinstein WS, Weissman S, Lynch JF, Grady W, Wiyrick S, Senz J, Huntsman DG (2008) Hereditary diffuse gastric cancer: diagnosis, genetic counseling, and prophylactic total gastrectomy. Cancer 112(12):2655–2663. doi:10.1002/cncr.23501
Meier S, Hübner CA, Groeben H, Peters J, Bingmann D, Wiemann M (2007) Expression of anion exchanger 3 influences respiratory rate in awake and isoflurane anesthetized mice. J Physiol Pharmacol 58(Suppl 5):371–378
Merchant JL (2008) What lurks beneath: IL-11, via Stat3, promotes inflammation-associated gastric tumorigenesis. J Clin Invest 118(5):1628–1631
Pang AJ, Bustos SP, Reithmeier RA (2008) Structural characterization of the cytosolic domain of kidney chloride/bicarbonate anion exchanger 1 (kAE1). Biochemistry 47(15):4510–4517. doi:10.1021/bi702149b
Poplawski T, Tomaszewska K, Galicki M, Morawiec Z, Blasiak J (2008) Promoter methylation of cancer-related genes in gastric carcinoma. Exp Oncol 30(2):112–116
Salhany JM, Cordes KA, Schopfer LM (1993) Kinetics of conformational changes associated with inhibitor binding to the purified band 3 transporter. Direct observation of allosteric subunit interactions. Biochemistry 32(29):7413–7420. doi:10.1021/bi00080a011
Shen WW, Wu J, Cai L, Liu BY, Gao Y, Chen GQ, Fu GH (2007) Expression of anion exchanger 1 sequestrates p16 in the cytoplasm in gastric and colonic adenocarcinoma. Neoplasia 9(10):812–819. doi:10.1593/neo.07403
Sipponen P (2002) Gastric cancer: pathogenesis, risks, and prevention. J Gastroenterol 37(Suppl 13):39–44. doi:10.1007/BF02990098
Stewart AK, Kurschat CE, Alper SL (2007) Role of nonconserved charged residues of the AE2 transmembrane domain in regulation of anion exchange by pH. Pflugers Arch 454(3):373–384. doi:10.1007/s00424-007-0220-8
Sugano K (2008) Gastric cancer: pathogenesis, screening, and treatment. Gastrointest Endosc Clin N Am 18(3):513–522. doi:10.1016/j.giec.2008.05.003
Tanner MJ (1997) The structure and function of band 3 (AE1): recent developments. Mol Membr Biol 14(4):155–165. doi:10.3109/09687689709048178
Vauhkonen M, Vauhkonen H, Sajantila A, Sipponen P (2005) Differences in genomic instability between intestinal- and diffuse-type gastric cancer. Gastric Cancer 8(4):238–244. doi:10.1007/s10120-005-0346-3
Vauhkonen M, Vauhkonen H, Sipponen P (2006) Pathology and molecular biology of gastric cancer. Best Pract Res Clin Gastroenterol 20(4):651–674. doi:10.1016/j.bpg.2006.03.016
Vilkin A, Levi Z, Morgenstern S, Shmuely H, Gal E, Hadad B, Hardi B, Niv Y (2008) Higher gastric mucin secretion and lower gastric acid output in first-degree relatives of gastric cancer patients. J Clin Gastroenterol 42(1):36–41
Yang Y, Wu PP, Wu J, Shen WW, Wu YL, Fu AF, Zheng L, Jin XL, Fu GH (2008) Expression of anion exchanger 2 in human gastric cancer. Exp Oncol 30(1):81–87
Author information
Authors and Affiliations
Corresponding author
Additional information
W.-Q. Xu and L.-J. Song have been equally contributed to this work.
Rights and permissions
About this article
Cite this article
Xu, WQ., Song, LJ., Liu, Q. et al. Expression of anion exchanger 1 is associated with tumor progress in human gastric cancer. J Cancer Res Clin Oncol 135, 1323–1330 (2009). https://doi.org/10.1007/s00432-009-0573-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-009-0573-9