Abstract
Purpose
Lymphorrhea is an uncommon complication of abdominal surgery. Here, we retrospectively investigate the treatment and prevention of lymphorrhea after radical gastrectomy.
Methods
From January 1995 to January 2007, a total of 1,596 patients who underwent surgery for gastric cancer were investigated. According to the AJCC cancer stage manual, tumor stages of 693 (43.4%) cases were T1 or T2 and 903 (56.6%) cases were T3 or T4. A total of 1,104 (69.2%) patients received grade D1 lymphadenectomy or grade D2, and 492 (30.8%) patients received grade D3 or D4. Ligation was used during the lymphadenectomy in 829 (51.9%) patients, and the electrotome cautery was used in 767 (48.1%) patients. Patients diagnosed of lymphorrhea were treated with total parenteral nutrition (TPN) alone before 2001, and with TPN plus octreotide after 2001.
Results
The incidence of lymphorrhea of patients with D1–2 lymphadenectomy was much lower than those with D3–4 lymphadenectomy (P < 0.01). For patients whose lymphatic vessels were ligated during the operation, the incidence of lymphorrhea was much lower than those lymphatic vessels were electrically cauterized (P < 0.01). No significant difference of incidence of lymphorrhea could be found between patients with T1–2 and T3–4 tumor stages (P > 0.05). Octreotide or TPN administration can reduce the quantity and duration of lymphorrhea,and the combination of Octreotide and TPN has a more significant effect on lymphorrhea than TPN alone (P < 0.01).
Conclusion
The major cause of lymphorrhea following radical gastrectomy was the inappropriate management of lymphadenectomy. Avoiding an extensive lymphadenectomy at surgery and ligating the disrupted lymph vessels would reduce the incidence of lymphorrhea. The combination of Octreotide and TPN is an effective therapeutic modality for lymphorrhea.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Postoperative lymphorrhea has been observed after retroperitoneal lymphadenectomy for urinary oncological surgery (Ferrigini and Novicki 1985), portal system decompression (Maywood et al. 1978), abdominal aortic surgery (Williamson and Provan 1987), renal transplantation (Liu et al. 2006), and after arthrodesis using an anterior approach to the spine (Shen et al. 1989). The incidence of lymphorrhea was approximately 7.4% after oncological abdominal surgery (Kaas et al. 2001), however, only a few cases of lymphorrhea after radical gastrectomy for gastric cancer have been reported. (Iankin. 2006; Tanaka et al. 2004) Lymph node metastases occur during the early stages of gastric cancer, and regional lymphadenectomy is recommended as a part of radical gastrectomy.
Recently, beneficial effects have been reported by the use of octreotide in the treatment of lymphorrhea after axillary node dissection (Carcoforo et al. 2003). Here, we try to investigate the treatment and prevention of lymphorrhea after radical gastrectomy retrospectively.
Materials and methods
We retrospectively reviewed 1,596 patients who underwent surgery for gastric cancer from January 1995 to January 2007. All the patients underwent routine biochemical and physical examinations before operation. Those who died within 2 weeks of the operation and those with anastomotic leakage, abdominal infection, and ileus were excluded from this study. According to the AJCC cancer stage manual (6th edn, 2003), tumor stages of 693 (43.4%) cases were T1 or T2 and 903 (56.6%) cases were T3 or T4. A total of 1,104 (69.2%) patients received grade D1 lymphadenectomy (perigastric lymph node dissection) or grade D2 (standard lymph node dissection), and 492 (30.8%) patients received grade D3 (extended lymph node dissection) or D4 (far-extended lymph node dissection including the paraaortic lymph nodes). Ligation was used during the lymphadenectomy in 829 (51.9%) patients, and the electrotome cautery was used in 767 (48.1%) patients.
Diagnostic criteria of lymphorrhea included: (1) abdominal drainage was more than 200 ml per day and over 3 days after radical gastrectomy; (2) drainage was non-blood and amylase in normal level; (3) the drainage was confirmed as lymph fluid by chylous qualitative and triglyceride tests.
Patients diagnosed of lymphorrhea were treated with total parenteral nutrition (TPN) alone before 2001, and with TPN plus octreotide (longastatina, ITALFARM, Srl, Italy; 0.1 mg three times a day subcutaneously) after 2001. We reviewed the incidence of lymphorrhea in different surgical procedures, the daily amount of lymphorrhea in the first 7 days and the duration of drainage in the two groups.
All values were expressed as mean ± SD, Chi-squared test and Student’s t tests were used to determine the significance of the differences among the groups. Statistical calculations were performed with SPSS (version 12.0). A P value of less than 0.05 was considered statistically significant.
Results
A total of 53 patients (53/1,596, 3.32%) presented with lymphorrhea after radical gastrectomy in our study. The incidence of lymphorrhea of patients with D1–2 lymphadenectomy (22/1,104, 1.99%) was much lower than those with D3–4 lymphadenectomy (31/492, 6.30%) (P < 0.01). In addition, for patients whose lymphatic vessels were ligated during the operation, the incidence of lymphorrhea was much lower than those lymphatic vessels were electrically cauterized (14/815, 1.72% vs. 39/728, 5.36%, respectively) (P < 0.01). However, no significant difference of incidence of lymphorrhea could be found between patients with T1–2 and T3–4 tumor stages (26/667, 3.90% vs. 27/876, 3.08%, respectively) (P > 0.05) (Table 1). Moreover, we found the incidence of lymphorrhea of patients was also closely associated with grade of lymphadenectomy and did not associated with tumor stages either in ligation group (P < 0.05, P > 0.05) or in electrotome cautery group (P < 0.01, P > 0.05) (Table 2).
Patients in the TPN alone group had an average amount of 455.68 ± 45.94 ml per day in the first 7 days after operation and an average duration of 23.32 ± 9.62 days. In comparison, patients in the TPN plus octreotide group had an average amount of 274.84 ± 36.23 ml per day and an average duration of 7.32 ± 2.87 days (P < 0.01, P < 0.01). By day 7, the drains were removed from 66.67% of patients in the TPN plus octreotide group which showed significant difference with TPN alone group (3.12%, P < 0.01) (Table 3).
The side effects of octreotide in our study were minimal which included local irritation at the infection site and general nonspecific gastrointestinal discomfort (anorexia, abdominal cramps, steatorrhea, and diarrhea). Hyperglycemia can pose an important concern lonely with more chronic treatment. None of these adverse effects led to discontinuation of the treatment.
Discussion
Causes of lymphorrhea after gastric cancer surgery
There are numerous causes of lymphorrhea, and the main cause is the surgical procedure used but not tumor stages. We ligated only the large visible disrupted lymph vessels during lymphadenectomy. The lymphatic leaks from the stumps of the small and invisible lymph vessels into the free abdominal cavity resulting in lymphorrhea. The more extensive the lymph node dissected, the higher incidence of lymphorrhea will be. It has been reported in literature that the incidence of lymphorrhea is approximately 1.2–3.0% after retroperitoneal mesenteric vessel pedicle lymph node dissection (Halkic et al. 2003). In this study, the incidence was 1.99% in the patients with D1–2 lymphadenectomy, however, the incidence was as high as 6.30% in the patients with D3–4 lymphadenectomy, indicating that extensive lymph node dissection leads to a higher incidence of lymphorrhea. From this data, we considered that the cause of the higher incidence of lymphorrhea in the patients with D3–4 lymphadenectomy as compared to that in patients with D1–2 lymphadenectomy was the more extensive lymph node dissection in the former, leading to a greater number of injured lymph vessels. The study also showed that the incidence of lymphorrhea was significantly higher in the patients in whom the electrotome cautery was used than in those ligation was used. The electrotome cautery cannot completely seal the large disrupted lymph vessels and lymph vessels with large drainage volumes. The scars on the lymph vessel stumps after cauterization can disintegrate due to the increased lymph fluid pressure, this can lead to the leakage of lymph fluid from the stump. We failed to find any relationship between the incidence of lymphorrhea and tumor stages.
The prevention of lymphorrhea after gastric cancer surgery
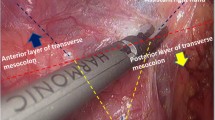
During lymph node dissection for gastric cancer, the cisterna chyli or the surrounding lymph vessel network can be injured. Particularly, in D3–4 dissections, the lymph vessel network around the abdominal aorta, inferior caval vein, and mesenteric vessels can be easily injured. Therefore, during operation, we should carefully dissect and individually ligate the divided tissues. The study indicated that ligation by using a thread could reduce the incidence of lymphorrhea. The electrotome cautery, which only effectively seals the smaller lymph vessels, may lead to lymphorrhea. However, ultrasonically activated shears might reduce the incidence of lymphorrhea (Tsimoyiannis et al. 2002).
It has been reported that as compared to the patients with D1–2 lymphadenectomy, the average daily amount of intra-abdominal fluid collected was higher and the duration of lymphorrhea was longer in patients with D3–4 lymphadenectomy. The postoperative quality of life was significantly decreased with lymphorrhea (Davies et al. 1998). Therefore, selecting a D1–2 lymphadenectomy for early or partially advanced gastric cancer could not only avoid early postoperative recurrence and increase the rate of radical cure but also reduce postoperative lymphorrhea which resulting in a better quality of life.
The treatment of lymphorrhea after gastric cancer surgery
TPN can dramatically decrease the lymph flow in the thoracic duct from 220 ml/(kg h−1) to 1 ml/(kg h−1) (Aalami et al. 2000). Moreover, TPN can restore the nutritional deficits and balance the metabolic impairments imposed by long-standing lymphorrhea. Therefore, TPN are essential in the no operative management of lymphorrhea. The curative effect of an MCT oil low-fat diet was only 36%, and the treatment duration was relatively long, requiring 4–6 months for adequate effect (Bhatia et al. 2001). TPN can reduce the accumulation of intra-abdominal lymph fluid, decreasing the loss of albumin and other nutrients that are induced by abdominal paracentesis, and it can simultaneously supply the necessary nutrition and rectify the fluid and electrolyte balance and hypoproteinemia. A satisfactory effect can be obtained in 3–4 weeks of this treatment.
The mechanism by means of octreotide affects lymphorrhea is unknown. It has also been speculated that somatostatin improves chylous ascites by inhibiting lymph fluid excretion through specific receptors found in the normal lymphatic vessels of the intestinal wall (Shapiro et al. 1996; Reubi et al. 1992). However, somatostatin receptors have been found in lymphatic tissues also outside the gastrointestinal tract (Harris 1990; Ulíbarri et al. 1990; Reubi et al. 1993), so it is possible that there should be a direct action on lymphatic vessels. Somatostatin has previously been shown to decrease the intestinal absorption of fats, lower the triglyceride concentration in the thoracic duct, and attenuate lymph flow in the major lymphatic channels (Collard et al. 2000).
In present study, the mean amount of drainage per day in the first 7 days was significantly lower in the TPN plus octreotide group (P < 0.01) which indicates the therapeutic effect of somatostatin. By day 7, the drains were removed from 66.67% of patients in the TPN plus octreotide group which showed significant difference with TPN alone group (P < 0.01).This result may help in reducing the dose of octreotide and consequently the cost of treatment which might be tailored according to the amount and duration of drainage. The total period of drainage was much lower in the TPN plus octreotide group (P < 0.001). The significant decrease in the amount and duration of drainage association with negligible side effects and less infection rate by use of octreotide may improve patient’s quality of life, shorten hospital stay, and give the chance for early adjuvant treatment to be started.
In conclusion, this study indicates that combination of Octreotide and TPN was an effective therapeutic modality for postoperative lymphorrhea and should be a first-line therapy. This therapy should be initiated as early as possible after the diagnosis.
References
Aalami OO, Allen DB, Organ CH Jr (2000) Chylous ascites: a collective review. Surgery 128:761–778. doi:10.1067/msy.2000.109502
Bhatia C, Pratap U, Slavik Z (2001) Octreotide therapy: a new horizon in treatment of iatrogenic chyloperitoneum. Arch Dis Child 85:234–235. doi:10.1136/adc.85.3.234
Carcoforo P, Soliani G, Maestroni U, Donini A, Inderbitzin D, Hui TT et al (2003) Octreotide in the treatment of lymphorrhea after axillary node dissection: a prospective randomited controlled trial. J Am Coll Surg 196:365–369. doi:10.1016/S1072-7515(02)01757-X
Collard JM, Laterre PF, Boemer F, Reynaert M, Ponlot R (2000) Conservative treatment of postsurgical lymphatic leaks with somatostatin-14. Chest 117:902–905. doi:10.1378/chest.117.3.902
Davies J, Johnston D, Sue-Ling H, Young S, May J, Griffith J et al (1998) Total or subtotal gastrectomy for gastric carcinoma? A study of quality of life. World J Surg 22:1048–1055. doi:10.1007/s002689900515
Ferrigini RG, Novicki D (1985) Chylous ascites complicating genitourinary oncological surgery. J Urol 134:774–776
Halkic N, Abdelmoumene A, Suardet L, Mosimann F (2003) Postoperative chylous ascites after radical gastrectomy: a case report. Minerva Chir 58:389–391
Harris AG (1990) Future medical prospects for sandostatin. Metabolism 39:180–185. doi:10.1016/0026-0495(90)90241-4
Iankin AV (2006) Tachocomb plates efficacy in prevention of lymphorrhea in radical operations for gastric cancer. Khirurgiia (Mosk) 11:44–46
Kaas R, Rustman LD, Zoetmulder FA (2001) Chylous ascites after oncological abdominal surgery. Eur J Surg Oncol 27:187–189. doi:10.1053/ejso.2000.1088
Liu WC, Kuo MC, Wu WJ, Hwang SJ, Chen HC (2006) Chylous ascites after renal transplantation—a case report. Nephrol Dial Transplant 21:3336–3337. doi:10.1093/ndt/gfl354
Maywood BT, Goldstein L, Busuttil RW (1978) Chylous ascites after a Warren shunt. Am J Surg 135:700–702. doi:10.1016/0002-9610(78)90139-3
Reubi JC, Horisberger U, Waser B, Gebbers JO, Laissue J (1992) Preferential location of somatostatin receptors in germinal centers of human gut lymphoid tissue. Gastroenterology 103:1207–1214
Reubi JC, Waser B, Horisberger U, Krenning E, Lamberts SW, Gebbers JO et al (1993) In vitro autoradiographic and in vivo scintigraphic localization of somatostatin recepotors in human lymphatic tissue. Blood 82:2143–2151
Shapiro AM, Bain VG, Sigalet DL, Kneteman NM (1996) Rapid resolution of chylous ascites after liver transplantation using somatostatin analog and total parenteral nutrition. Transplantation 61:1410–1411. doi:10.1097/00007890-199605150-00023
Shen YS, Cheung CY, Nilsen PT (1989) Chylous leakage after arthrodesis using anterior approach to the spine. J Bone Joint Surg Am 71:1250–1251
Tanaka K, Ohmori Y, Mohri Y, Tonouchi H, Suematsu M, Taguchi Y et al (2004) Successful treatment of refractory hepatic lymphorrhea after gastrectomy for early gastric cancer, using surgical ligation and subsequent OK-432 (Picibanil) sclerotherapy. Gastric Cancer 7:117–121. doi:10.1007/s10120-004-0276-5
Tsimoyiannis EC, Jabarin M, Tsimoyiannis JC, Betzios JP, Tsilikatis C, Glantzounis G (2002) Ultrasonically activated shears in extended lymphadenectomy for gastric cancer. World J Surg 26:158–161. doi:10.1007/s00268-001-0199-9
Ulíbarri JI, Sanz Y, Fuentes C, Mancha A, Aramendia M, Sánchez S (1990) Reduction of lymphorrhgaia from ruptured thoracic duct by somatostatin. Lancet 336:258. doi:10.1016/0140-6736(90)91793-A
Williamson C, Provan JL (1987) Chylous ascites following aortic surgery. Br J Surg 74:71–72. doi:10.1002/bjs.1800740126
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sheng-Zhang, L., Hong-Fei, T., Zhong-Lin, N. et al. Treatment and prevention of lymphorrhea after radical gastrectomy of gastric cancer. J Cancer Res Clin Oncol 135, 613–616 (2009). https://doi.org/10.1007/s00432-008-0495-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-008-0495-y