Abstract
Purpose: The aims of this analysis were to investigate the clinical features of extraskeletal osteosarcoma (ESOS) and examine the outcome after multi-modal therapy. Methods: The co-operative osteosarcoma study-group database was searched for patients with extraskeletal osteosarcoma. Eligible patients were included in a retrospective analysis of patient, tumour and treatment related variables and outcome. As for conventional osteosarcoma, scheduled treatment included surgery and multi-agent chemotherapy. Results: Seventeen eligible patients were identified with a median age of 44 years (range, 3–65 years). The thigh was the commonest tumour site. Two patients had a history of previous malignancies and two had primary metastases. Median follow-up was 3.2 years (range: 0.6–7.4 years) and at last follow-up, 11 patients were alive in complete remission, 3 patients were alive with disease and 3 patients had died of their disease. Three-year overall actuarial and event-free survival rates were 77% and 56%, respectively. Patients with macroscopically complete surgical remission had an improved overall survival (P=0.0004). Conclusions: The patients in this retrospective study had a surprisingly good survival rate. This may be due to the combination of multi-agent chemotherapy with surgery, and we recommend this approach in the treatment of ESOS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extraskeletal osteosarcoma (ESOS), also referred to extraosseous osteosarcoma, is a soft tissue neoplasm characterised by osteoid production, and which has no attachment to bone or periosteum. ESOS is rare, comprising less than 5% of all osteosarcomas (Jensen et al. 1998). In contrast to skeletal osteosarcoma, which mainly affects adolescents and young adults, ESOS is uncommon in persons under 30-years-old and has been associated with a poor prognosis (Sordillo et al. 1983; Bane et al. 1990; Lee et al. 1995; Jensen et al. 1998). The exact role of chemotherapy in the treatment of ESOS is still being debated, and there is some uncertainty regarding whether ESOS should be treated using regimens designed for conventional osteosarcoma of bone, or by regimens designed for soft tissue sarcomas (Ahmad et al. 2002; Wodowski et al. 2003). In this study, we evaluated the clinical data of 17 patients with ESOS in the database of the Co-operative German–Austrian–Swiss Osteosarcoma Study Group (COSS), who were treated according to COSS polychemotherapy and surgery protocols.
Patients and methods
Patient eligibility
The co-operative osteosarcoma study group (COSS) is an international, multi-centric group which has performed prospective trials since 1977 (Winkler et al. 1984, 1988; Fuchs et al. 1998; Bielack et al. 1999, 2002). The studies focussed on patients under 40 years of age with primary, localised, high-grade central osteosarcoma of bone, but other patients with osteosarcoma variants or related cancers, such as malignant fibrous histiocytoma of bone, were also registered into the group’s database and followed. All studies were approved by the local ethics committee and/or the protocol review committee of the German Cancer Society or the German Ministry for Science and Technology. Before the initiation of treatment, informed consent was obtained from all patients or from their legal guardians.
For this study, we searched the COSS database for patients with histologically verified and previously untreated ESOS. Four of 21 patients with ESOS identified from the database had to be excluded from this analysis, because they were only registered upon relapse, leaving 17 eligible patients from 17 participating institutions in Germany (13), Austria (2) and Switzerland (2), diagnosed between 1986 and 2002, who form the basis of all further analyses. Radiologic images were not available at the COSS study centre and no centralized radiology review was performed. Rather, the classification as “extraskeletal” tumours was made by the institutions registering the patients. In all patients, the histologic diagnosis was made by the local pathologist from biopsy and/or resection material. Moreover, 12 tumour samples were reviewed by members of the COSS pathology panel and two by other reference pathologists, and the diagnosis of osteosarcoma was confirmed in all cases.
Diagnostic staging
The protocols active in the period during which the 17 patients were registered all called for conventional radiography and either computed tomography (CT) or, preferred, magnetic resonance imaging (MRI) of the primary tumour. Procedures prescribed to search for primary metastases were a chest X-ray, a CT scan of the chest and a 99Tc-methylene diphosphonate bone scan. During the follow-up, imaging of the chest and the primary tumour were to be repeated at regular intervals specified in the respective treatment protocols.
Treatment plan
The objective of surgery was complete resection of the primary tumour and, if present, resection of all metastases. There were no specific guidelines for chemotherapy for ESOSs, rather, these were treated according to the COSS protocols for high-grade central osteosarcoma active at the time of enrolment. Details of COSS protocols including chemotherapy administration and modifications have been reported previously (Winkler et al. 1984, 1988; Fuchs et al. 1998; Bielack et al. 1999, 2002). In brief, all COSS protocols active after 1986, when the first patient with ESOS was enrolled, included doxorubicin, cisplatin, ifosfamide and high-dose methotrexate with leucovorin rescue. It was recommended to restrict the use of methotrexate to patients less than 40 years of age. In COSS 96 with risk-stratified therapy, carboplatin and etoposide were to be added in a defined group of high-risk patients with large tumours and very poor response to pre-operative chemotherapy. The protocols called for pre-operative induction chemotherapy and post-operative chemotherapy.
Evaluation methods
Assessment of patient, tumour and treatment-related variables
The following variables, all collected prospectively, were evaluated for their distribution in the patient cohort and for possible correlations with outcome.
Patient age
An age limit of 40 years was used to divide between older and younger patients.
Patient sex
This variable is self-explanatory.
Tumour site
Extraskeletal osteosarcomas of the limbs were compared with those of the trunk.
Tumour size
This was estimated by the greatest tumour dimension. Tumours <5 cm were compared with tumours ≥5 cm.
Primary metastases
Only patients in whom primary metastases were proven by surgery or progression were counted as having primary metastases.
Symptoms and their duration
Most COSS protocols included an assessment of symptom duration. Both the onset of pain and of tumour-associated swelling were documented, and the time interval between first symptom and diagnostic biopsy was calculated to give the symptom duration.
Best local surgical outcome
This was retrospectively classified according to the R-categories as defined by the UICC, with R0 meaning no macroscopical or microscopical residual tumour post-operatively, R1 meaning microscopic and R2 macroscopic tumour residue.
Type of surgery
This was classified as resection, amputation or no operation.
Number of operations
This was the number of local operations until best local surgical outcome was achieved.
Complete surgical remission (local, metastatic and total)
A complete surgical remission was assumed only when all detectable tumour foci were removed during first-line therapy and if this removal was macroscopically complete. This was assessed for both local and metastatic disease. Total complete remission was assumed if macroscopically complete surgical remission of all tumour sites had been achieved.
Tumour response
Response to pre-operative chemotherapy was assessed histologically according to the six-grade scale of Salzer–Kuntschik et al. (1983). The distinction between good and poor response was set at 10% residual viable tumour.
Chemotherapy
The chemotherapeutic agents and the cumulative dose of each agent were recorded for each patient.
Statistical analyses
All 17 eligible patients were evaluated on an intent-to-treat basis. The cumulative probability of survival was calculated using the Kaplan–Meier method (Kaplan and Meier 1958) and the log-rank test (Goldin et al. 1966) was used to compare the difference between survival curves. Overall survival was calculated from the date of the diagnostic biopsy until death from any cause, event-free survival until relapse or death, whichever occurred first. Patients who never achieved a complete surgical remission were assumed to have suffered an event on the first day after biopsy.
Results
Patient characteristics
All 17 eligible patients had high-grade ESOS. Two ESOSs were diagnosed in the 1980s, nine in the 1990s and six in the 2000s. The age of patients ranged between 3 years and 65 years (median, 44 years). There were six patients under 40-years-old (age: 3, 12, 12, 15, 28 and 32 years). Ten patients were male and seven were female (Fig.1).
Two patients had had a previous malignant disease. One patient (Patient 2) had received chemotherapy and a total dose of 40 Gy 10 years previously for non-Hodgkin’s lymphoma of the neck. One patient (Patient 3) had undergone resection, chemotherapy and radiation therapy with 46 Gy 11 years previously for Ewing sarcoma of the left proximal thigh. Both patients developed ESOS within the field of irradiation. Patient 12 was initially diagnosed with myositis ossificans, and a diagnosis of ESOS was only made 7 months later. Fifteen patients had localised disease at diagnosis and two patients had confirmed primary metastases: one patient had liver metastases, and the other had pulmonary and soft tissue metastases.
Information on symptoms was available in 11 patients. Four patients reported moderate/severe local swelling only, three patients had pain and four patients had both pain and local swelling. The median duration of symptoms before biopsy was 62 days (range, 5–562 days).
Ten tumours were located in an extremity and seven were axial (Table 1). Tumour size could be estimated in 13 patients by the greatest tumour dimension. Median tumour size was 7.5 cm (range, 0.2–40 cm). Eight tumours were ≥5 cm and five were <5 cm.
Treatment
Details on local and systemic treatment are presented in Table 2. Ten patients underwent resection of the primary tumour, six patients had an amputation and the osteosarcoma of the neck was inoperable. All operated patients achieved macroscopically complete local remission, but a number of operations were often necessary until best margins were achieved: nine patients had one operation, six patients had two operations and one patient had three operations. Microscopically clear margins (R0 resection) were reported for 14 patients, while microscopic contamination (R1 resection) was reported for two. One of these received 66 Gy to the tumour region post-operatively. Both patients with primary metastases had R0 resections of their primary tumours, but neither achieved surgical clearance of their metastases. Therefore, a macroscopically complete surgical remission of all sites was achieved in 14 of the 17 patients.
Sixteen of the 17 patients received chemotherapy. Information about the agents used were available for 15 of these 16. All of these 15 received doxorubicin (median cumulative dose: 180 mg/m2, range: 90–360), ifosfamide (median cumulative dose: 18 g/m2, range: 12–24) and cisplatin (median cumulative dose: 300 mg/m2, range: 240–480) and 8 of them were treated with additional high-dose methotrexate. Two patients received additional agents (Table 2). Only four patients received some pre-operative chemotherapy, but information on response to chemotherapy was only available for one patient, who had a good response (Grade 3, <10% viable tumour) according to the criteria published by Salzer–Kuntschik et al. (1983).
Survival analysis
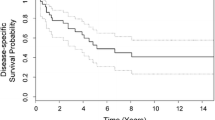
After a median follow-up of 3.2 years (range: 0.6–7.4) for all patients and 3.8 years (same range) for survivors, 14 patients were alive, for a 1 year survival estimate of 94% (standard error SE 6%) and 3- and 5-year survival estimates of 77% (SE 12%) (Fig. 2). Ten of the 14 survivors were alive in first complete remission, 1 was in second complete remission and 3 were alive with disease. Five patients survived in a continuously disease-free condition for more than 5 years.
At the time of analysis (June, 2004), three patients had died, all of them due to progressive disease. These three were the three patients who failed to achieve a macroscopic surgical remission of their primary tumour (one) or primary metastases (two).
Four of 14 patients who achieved a macroscopically complete surgical remission during first-line treatment later developed a recurrence, one of them with additional pulmonary, osseous, skin and soft tissue metastases. One of the local recurrences occurred in a patient with microscopically involved resection margins who had received additional radiotherapy, and one each in patients who had needed one, two or three operations to achieve microscopically free margins.
Event-free survival estimates were 64% at 1 year (SE 12%) and 56% at 3 and 5 years (SE 13%) for all 17 patients (Fig. 2); and 77% at 1 year (SE 12%) and 69% at 3 and 5 years (SE 13%) for the 14 patients who achieved a first complete surgical remission Fig. 3.
Overall survival was not significantly affected (P<0.05) by any evaluated variable except for total macroscopic remission: patients who did not achieve total macroscopic remission were more likely to die because of their diseases (P=0.0004). None of the 5 patients with tumours ≤5 cm had an adverse event.
Discussion
Extraskeletal osteosarcomas is very rare, accounting for approximately only 4% of patients with osteosarcoma (Sordillo et al. 1983; Chung and Enzinger 1987; Bane et al. 1990; Jensen et al. 1998). All of our patients had high-grade osteosarcomas which are more common than the low-grade form (Okada et al. 2003) and as in other series (Sordillo et al. 1983; Chung and Enzinger 1987; Bane et al. 1990; Lee et al. 1995; Jensen et al. 1998) the most common primary tumour site for our patients was the thigh. The other sites for ESOS in our patients, although less common, have also been previously reported, e.g. Cook et al. (1998) report ESOS of the hand, and Silver and Tavassoli (1998) report a series of 50 osteosarcomas of the breast.
The median age of patients in our study was 44 years, which is lower than the median age for patients with ESOS reported in the literature, which ranges from 51–67 years of age (Sordillo et al. 1983; Chung and Enzinger 1987; Bane et al. 1990; Jensen et al. 1998; McCarter et al. 2000; Ahmad et al. 2002). Furthermore, in contrast to the literature, which states that ESOS is rare under the age of 30 years (Sordillo et al. 1983; Bane et al. 1990; Jensen et al. 1998; McCarter et al. 2000), in this series, six patients were under 40 years of age, including four under the age of 20 years. This is likely to be due to the fact that the COSS studies mainly targeted children, adolescents and young adults. We found no significant correlation between age and survival, but the large proportion of younger patients in our study should be remembered when comparing our results with the literature. Most paediatric cases of ESOS are diagnosed during adolescence and in our series, unlike other reports (Wodowski et al. 2003), there is a male preponderance. We are well aware that the lack of a centralized radiology review in our as well as other reports might be a target for criticism, but do not believe that relying on the assessment of treating physician compromizes the overall validity of the data.
Two of our patients had previously received radiation therapy to the area where their ESOS developed. Radiation therapy is a known predisposing factor for the development of bone and soft tissue sarcomas, including ESOS (Chung and Enzinger 1987), and the time interval between radiotherapy and development of sarcomas can be prolonged, with a mean interval of approximately 12 years (Sordillo et al. 1983; Logue and Cairnduff 1991). One patient was diagnosed with myositis ossificans 7 months prior to diagnosis of OS by biopsy. As in this case, ESOS can be mistaken for myositis ossificans (Chung and Enzinger 1987; Lee et al. 1995). However, a number of cases of ESOS developing from myositis ossificans have also been reported (Sordillo et al. 1983; Konishi et al. 2001).
All but one of our patients were treated with multi-agent chemotherapy in combination with surgery, and their treatment was more intensive than in most patients previously reported in the literature. Earlier series, such as Sordillo et al. (1983), mainly studied patients treated by surgery alone, although there was often a subset of patients who received chemotherapy. More recently, Ahmad et al. (2002) from the M.D. Anderson Cancer Center studied 60 patients, of whom 27 with measurable disease were treated with doxorubicin-based chemotherapy (8 patients also received ifosfamide and 13 received cisplatin) and concluded that response rates to doxorubicin-based systemic therapy were low. However, Wodowski et al. (2003) report a case of a 12-year-old patient with pulmonary metastases who responded well to treatment with surgery, radiotherapy and chemotherapy with vincristine, doxorubicin and ifosfamide, and who remains disease-free 2.8 years after diagnosis.
Although the COSS protocols prescribed pre-operative chemotherapy, almost all our patients received only post-operative chemotherapy. One reason for this is that, like in other recent series (Ahmad et al. 2002), many patients had primary surgery without knowledge of the histologic diagnosis, as evidenced by the frequent need for re-operation. Furthermore, there may have been some reluctance to give pre-operative chemotherapy because of the older age of the patients and because the limited information previously available indicated that ESOS has a poor response to chemotherapy (Ahmad et al. 2002).
There was no difference in overall or event-free survival between the patients treated with resection and those treated by amputation. This therefore supports Ahmad et al. (2002), who suggest that limb-sparing surgical techniques can be applied to patients with ESOS. No conclusions can be made regarding radiotherapy, because only one patient received radiotherapy. However, this patient did suffer a local relapse.
The outcome reported here for patients treated with multi-modal therapy compares favourably to previous series of patients treated mainly with surgery alone or with surgery and less intensive chemotherapy. Only three patients died in our series, giving a 77% overall survival estimate at 5 years. In comparison, Lee et al. (1995), Jensen et al. (1998) and McCarter et al. (2000) studied patients treated mainly with surgery, and reported 5-year survival rates ranging from <25% to 50%. Ahmad et al. (2002) reported a 5-year disease-specific survival rate of 46% in a group of 38 patients with localised disease, of whom 24 had some form of chemotherapy (mainly doxorubicin-based).
Our small series did not show any statistically significant prognostic factors except complete surgical remission. In this series, all three patients who did not achieve complete remission died. The commonest cause of death was distant metastases, which is in agreement with existing studies, with the lung being the most common site (Lee et al. 1995; Jensen et al. 1998). Patient 12 had many metastases (pulmonary, soft tissue, osseous and skin) and this supports the theory that skin metastases may be a sign of widespread disease (Covello et al. 2003). Only one patient (Patient 12) developed distant metastases after diagnosis. In comparison, distant metastases developed in 60% of the patients reported by Jensen et al. (1998).
In the patients from our group who managed to achieve a complete surgical remission, most failures occurred as local recurrences. Sordillo et al. (1983) noted a local recurrence rate of 69% in patients followed for more than 1 year, and more recently Lee et al. (1995) and Jensen et al. (1998) reported local recurrence rates of 36–45%. The local failure rate observed in our series, 4 of 17, was similar to the 20% reported by Ahmad et al. (2002).
Ahmad et al. (2002) suggest that cisplatin-based chemotherapy is not active against ESOS and that the response to doxorubicin is poor. They therefore concluded that ESOS should be viewed as therapeutically distinct from osseous osteosarcoma. However, the more favourable results obtained with more aggressive multi-agent chemotherapy in our series suggest that protocols developed for osseous osteosarcoma may also be effective against ESOS, and we currently recommend that patients with ESOS be treated with polychemotherapy regimens including doxorubicin, ifosfamide, cisplatin and possibly methotrexate and adequate surgery.
References
Ahmad SA, Patel SR, Ballo MT, Baker TP, Yasko AW, Wang X, Feig BW, Hunt KK, Lin PP, Weber KL, Chen LL, Zagars GK, Pollock RE, Benjamin RS, Pisters PWT (2002) Extraosseous osteosarcoma: response to treatment and long-term outcome. J Clin Oncol 20:521–527
Bane BL, Evans HL, Ro JY, Carrasco CH, Grignon DJ, Benjamin RS, Ayala AG (1990) Extraskeletal Osteosarcoma: A clinicopathologic review of 26 cases. Cancer 66:2762–2770
Bielack S, Kempf-Bielack B, Schwenzer D et al (1999) Neoadjuvante Therapie des lokalisierten Osteosarkoms der Extremitäten. Erfahrungen der Cooperativen Osteosarkomstudiengruppe COSS an 925 Patienten. Klin Padiatr 211:260–270
Bielack S, Kempf-Bielack B, Delling G et al (2002) Prognostic factors in high-grade osteosarcoma of the extremities or trunk. An analysis of 1702 patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol 20:776–790
Chung EB, Enzinger FM (1987) Extraskeletal osteosarcoma. Cancer 60:1132–1142
Cook PA, Murphy MS, Innis PC, Yu JS (1998) Extraskeletal osteosarcoma of the hand. J Bone Joint Surg (Am) 80:725–729
Covello SP, Humphreys TR, Lee JB (2003) A case of extraskeletal osteosarcoma with metastasis to the skin. J Am Acad Dermatol 49:124–127
Fuchs N, Bielack S, Epler D et al(1998) Long-term results of the co-operative German-Austrian-Swiss osteosarcoma study group’s protocol COSS-86 of intensive multi-drug chemotherapy and surgery for osteosarcoma of the limbs. Ann Oncol 9:893–899
Goldin A, Serpick AA, Mantel N (1966) Experimental screening procedures and clinical predictability value. Cancer Chemother Rep 50:173–218
Jensen ML, Schumacher B, Jensen OM, Nielsen OS, Keller J (1998) Extraskeletal osteosarcomas: a clinicopathologic study of 25 cases. Am J Surg Pathol 22:588–594
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Konishi E, Kusuzaki K, Murata H, Tsuchihashi Y, Beabout JW, Unni KK (2001) Extraskeletal osteosarcoma arising in myositis ossificans. Skeletal Radiol 30:39–43
Lee JSY, Fetsch JF, Wasdhal DA, Lee BP, Pritchard DJ, Nascimento AG (1995) A review of 40 patients with extraskeletal osteosarcoma. Cancer 76:2253–2259
Logue JP, Cairnduff F (1991) Radiation induced extraskeletal osteosarcoma. Br J Radiol 64:171–172
McCarter MD, Lewis JJ, Antonescu CR, Brennan MF (2000) Extraskeletal osteosarcoma: analysis of outcome of a rare neoplasm. Sarcoma 4:119–123
Okada K, Ito H, Miyakoshi N, Sageshima M, Nishida J, Itoi E. (2003) A low-grade extraskeletal osteosarcoma. Skeletal Radiol 32:165–169
Salzer-Kuntschik M, Brand G, Delling G (1983) Determination of the degree of morphological regression following chemotherapy in malignant bone tumors. Pathologe 4:135–141
Silver SA, Tavassoli FA (1998) Primary osteogenic sarcoma of the breast: A clinicopathologic analysis of 50 cases. Am J Surg Pathol 22:925–933
Sordillo PP, Hajdu SI, Magill GB, Golbey RB (1983) Extraosseous osteogenic sarcoma: a review of 48 patients. Cancer 51:727–734
Winkler K, Beron G, Kotz R et al (1984) Neoadjuvant chemotherapy for osteogenic sarcoma: results of a cooperative German/Austrian study. J Clin Oncol 2:617–623
Winkler K, Beron G, Delling G et al (1988) Neoadjuvant chemotherapy of osteosarcoma: results of a randomized cooperative trial (COSS 82) with salvage chemotherapy based on histological tumor response. J Clin Oncol 6:329–337
Wodowski K, Hill DA, Pappo AS, Shochat SJ, Kun LE, Spunt SL (2003) A chemosensitive pediatric extraosseous osteosarcoma: case report and review of the literature. J Pediatr Hematol Oncol 25(1):73–77
Acknowledgements
The studies that form the basis of this work were supported by Deutsche Krebshilfe, Bundesministerium für Forschung und Technologie and Fördergemeinschaft Kinderkrebszentrum Hamburg. S.Y. Goldstein-Jackson received support from the European Community action scheme for the mobility of university students (Erasmus). The authors wish to thank all patients who contributed to the COSS studies and to acknowledge the physicians, nurses, data managers and support staff of the collaborating centres for their active participation. They would like to thank Mathias Kevric for his expert data management. The patients included in this study were from the following hospitals: Universitätsklinikum Münster, Medizinische Klinik und Poliklinik A, Germany; Kinder-Poliklinik, Päd. Onkologie/KMT, Munich; Germany; Medizinische Universitätsklinik, Abteilung Onkologie/Hämatologie, Hamburg, Germany; St. Anna Kinderspital, Vienna, Austria; Medizinische Universitätsklinik I, Cologne, Germany; Medizinische Universitätsklinik, Abteilung Onkologie/Hämatologie, Graz, Austria; Orthopädische Universitätsklinik Balgrist, Zürich, Switzerland; Katharinen – Hospital, Stuttgart; Germany; Medizinische Klinik& Poliklinik V, Heidelberg, Germany; Medizinische Universitätsklinik, Würzburg, Germany; Mutterhaus der Borromäerinnen, Trier, Germany; Medizinische Universitätsklinik I, Abt.: Hämatologie/Internistische Onkologie, Regensburg, Germany; Medizinische Klinik II mit Schwerpunkt Onkologie und Hämatologie, Charité Campus Mitte, Berlin, Germany; Universitätsklinikum Schleswig-Holstein, II. Medizinische Klinik und Poliklinik, Kiel, Germany; Kantonspital Aarau, Abteilung Pädiatrische Onkologie, Aarau, Switzerland; Diakonissenkrankenhaus, Medizinische Klinik II, Stuttgart, Germany; Medizinische Universitätsklinik, Abteilung Hämatologie/Onkologie, Rostock, Germany
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Goldstein-Jackson, S.Y., Gosheger, G., Delling, G. et al. Extraskeletal osteosarcoma has a favourable prognosis when treated like conventional osteosarcoma. J Cancer Res Clin Oncol 131, 520–526 (2005). https://doi.org/10.1007/s00432-005-0687-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-005-0687-7