Abstract
This study aims to provide an up-to-date meta-analysis of data from studies investigating the risk of bearing a child with autism spectrum disorder (ASD) after being conceived by assisted reproductive technology (ART). The study was conducted according to the PRISMA Statement. PubMed and Scopus databases were searched up to August 2, 2020. Observational studies using a type of conception of assisted reproductive technology and examined as outcome offspring with ASD were included. A random effect model was applied due to the heterogeneity of the studies. Statistical analysis was performed with Stata 13 software. The Newcastle–Ottawa scale was used to assess the methodological quality of the included studies. The search strategy identified 587 potentially relevant studies. A total of 15 studies provided adequate data for statistical comparisons and, therefore, were included in the meta-analysis. Analysis of the subset of studies that examined all offspring and controlled for confounder factors revealed that the use of ART is associated with a higher risk of ASD (RR = 1.11, 95% CI = 1.03–1.19, p < 0.009), while in the case of studies that focused on singletons, a statistically significant association between ART and ASD was not observed (RR = 0.96, 95% CI = 0.82–1.13, p = 0.654).
Conclusion: The present meta-analysis confirmed the existing positive correlation between ART and ASD in offspring, suggesting that ART is correlated with a higher risk for bearing a child with ASD. In contrast, this relationship is not confirmed in singletons. High quality prospective studies with a larger number of participants are still required.
What is Known: • Studies that investigated the association between ART and ASD in offspring have shown conflicting results. • A previous meta-analysis showed that offspring conceived by ART are 1.35 times more likely to develop ASD than offspring spontaneously conceived. | |
What is New: • This investigation separately considered studies with and without adjustment for confounders. • The findings from the two analyses were similar. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autism spectrum disorder is a lifelong complex neurodevelopmental disorder characterized by persistent deficits in three vital domains, social interaction, communication, and repetitive stereotypical behaviors, activities, and interests. The first symptoms are present in early childhood, usually after 36 months and before 3 years old, with different severity levels among individuals with ASD. The impact on the quality of their social life and autonomy and their families’ lives is life-changing [1,2,3]. According to the last estimates from CDC’s Autism and Developmental Disabilities Monitoring Network, 1 in 54 offspring is now born with ASD [4]. Despite many studies and extensive research, the exact etiology and the pathophysiologic mechanisms of ASD remain poorly understood.
Combinations of various heterogeneous causes that contribute to the pathogenesis of ASD have been described. These include prenatal, perinatal, postnatal and environmental factors [5,6,7,8]. Advanced parental age, preeclampsia, multiple pregnancies, pre-term delivery, and low birth weight (LBW) are some risk factors for ASD [9,10,11,12]. In addition, some studies have reported that parents of children with ASD are more likely to have infertility problems [13,14,15]. According to the World Health Organization, infertility affects 8–12% of couples of reproductive age worldwide.
Consequently, the percentage of couples who have offspring by ART has risen sharply over the last decade [16, 17]. ART includes all therapeutic procedures that intervene simultaneously in the gametes of both sexes. In contrast, it does not include therapies that intervene only in the sperm such as intrauterine or artificial insemination or drug treatments of ovarian stimulation which are not followed by ovulation, while medically assisted reproduction (MAR) includes ART procedures and/or the use of infertility medication [18].
Associations between ART and ASD might be anticipated because ART shares common risk factors with ASD such as multiple pregnancies, pre-term delivery, and LBW [19, 20]. Consequently, this field has attracted scientific interest and a large number of studies have attempted to investigate and determine the potential correlation between ART and ASD.
Sandin et al. [21] claimed that ART treatment using ICSI procedures has an increased risk of having offspring with intellectual disability and autistic spectrum disorder. To date, two systematic reviews and a meta-analysis have been published that attempted to combine the literature regarding the correlation between ART and the risk of ASD in offspring. Hvidtjørn et al. [22] conducted a systematic review of 41 studies, of which 31 studies examined all neurodevelopmental disorders and only eight focused on the autism spectrum. Their findings show that only the study of Klemetti et al. [23] resulted in a statistically significant correlation (OR 1.68, 95% CI 1.11–2.58) between ART and the risk of having offspring with a wide range of psychiatric disorders including ASD. In contrast, Maimburg et al. [24] detected a protective effect (OR 0.37, 95% CI 0.14–0.98) of ART concerning the risk of conceived offspring with ASD. Conti et al. [25] performed a systematic review of seven observational studies (five case–control and two cohorts), concluding that there is no significant association between ART and ASD in offspring. The meta-analysis of Liu et al. [26] found a statistically significant correlation (RR 1.35, 95% CI 1.09–1.68), showing that offspring conceived by ART are 1.35 times more likely to develop ASD than offspring spontaneously conceived.
This study aimed to present the results of a new systematic review and meta-analysis of the results of the studies that examined the association of the risk of conceiving offspring with ASD from ART and investigate the possible change of the above correlation after controlling for confounding factors. Also, an attempt was made to investigate separately the above correlation in the offspring of pre-term/full-term neonates, single/multiple births and according to sex.
Methods
The methods and the results of this study were carried out according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (PRISMA) [27]. The review protocol was registered on PROSPERO (International Prospective Register of Systematic Reviews) with CRD (Centre of Reviews and Dissemination) report number CRD42020210749.
Search strategy
A systematic search was conducted to PubMed and Scopus until August 2, 2020, using the following specific keywords along with their combinations: “in vitro fertilization,” “fertilization,” “infertility,” “assisted reproduction technologies,” “intracytoplasmic sperm injection,” “autism,” “autistic,” “Asperger syndrome,” “Rett syndrome,” and “developmental disorder.” The keywords above were used to perform a thorough evaluation of the Medical Subject Headings (MeSH) with language and study population restrictions (only English and human studies). The complementary search was performed by scanning the references of the previous systematic reviews on the topic. The search strategy is shown in the Appendix.
Eligibility criteria
We considered all case–control and cohort studies that used as a type of conception the assisted reproductive technology and examined as outcome offsprings with autism according to the International Classification of Diseases (ICD) or Diagnostic and Statistical Manual of Mental Disorders (DSM). Studies without a control group, with a different outcome, and published in languages other than English were not considered inclusion criteria.
Study selection and data extraction
Two reviewers (M.T.A. and G.N.K.) independently screened the literature, reviewed the full text of all studies considered eligible according to the inclusion criteria and extracted the studies’ data individually. Reviews, editorials, abstracts, letters to the editor, and studies with no adequate data were excluded. For all studies, the following data were recorded into Microsoft Excel spreadsheets: the name of the first author, year of publication, country of origin, the type of ART, the diagnostic criteria of ASD, the number of cases and controls and effect estimates and their corresponding 95% CI, and their adjusted factors in data analysis. In case that the effect estimates were not adjusted, we extracted a crude effect estimate. A third reviewer (P.T.) participated in resolving any queries derived from the process above.
Assessment of methodologic quality
To assess the methodological quality of each study included in the review, we used the quality assessment Newcastle–Ottawa 9-point-scale tool for case–control and cohort studies [28]. Two independent reviewers subsequently evaluated the included articles and scored them according to the criteria that existed in each domain. We assessed three main domains: selection, comparability, and outcome or exposure for cohort and case–control studies, respectively. Allocation of a study as a high, moderate or low quality is done using a star grading system. A study with a NOS score of more than seven stars was regarded as high methodological quality since a standard cut-off score for what is classified as a high-quality study has not been established.
Data analysis
A systematic review was performed for the studies that were regarded as eligible for the inclusion criteria. We also performed a meta-analysis using the studies that provided adequate data for statistical comparison. The relative ratios (ORs) calculated in the prospective and retrospective studies, and the relative risks (RRs), calculated in all studies above except the retrospective studies, show small numerical differences, unless a large extrapolation was observed. As the risk of autism was low, the relative risks and the corresponding 95% confidence interval (95% CI) were used as summary statistics to assess the association between assisted fertilization and the risk of autism in our systematic review and meta-analysis [29]. To assess the statistical significance of pooled RRs we performed a Z-test. The meta-analysis was performed using Stata 13 software [30]. The main analysis, and the subgroup analysis were performed using the random effect model due to the heterogeneity of the studies [31]. The I2 test was used to assess statistical heterogeneity between the analyzed studies (significance level: P ≤ 0.1) and the I2 statistic, applying the following interpretation for I2: < 50%, low heterogeneity, 50–75%, moderate heterogeneity, and > 75%, high heterogeneity [32]. Heterogeneity was investigated using subgroup analysis according to the geographical origin of the study and study type. We also performed sensitivity analysis by excluding each of the analyzed studies at a time in sequence to assess the stability of our results. Additionally, to further explore the source of study heterogeneity, we used the Galbraith plot [33, 34]. The publication bias was assessed using the Egger test and a p-value of < 0.05 stated statistically significant publication bias [35, 36].
Results
Search results
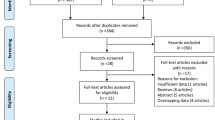
The initial search procedure yielded 587 studies. After removing duplicates, we screened 570 studies by title and abstract, and from them, 496 were excluded subsequently, and 74 studies were screened by full text for eligibility (Fig. 1). Finally, 16 studies [13, 15, 21,22,23,24, 37,38,39,40,41,42,43,44,45,46] were included in our systematic review, and 15 studies [15, 21,22,23,24, 37,38,39,40,41,42,43,44,45,46] provided adequate data that enabled for statistical comparisons and, therefore, were included in our meta-analysis. The detailed flow diagram of the study selection process and the various reasons for exclusion studies are shown in Fig. 1.
All included studies were published between 2006 and 2020. Among them, eight studies were cohort [13, 21,22,23, 37, 38, 43, 44] and eight case–control [15, 24, 39,40,41,42, 45, 46]. Six studies were performed in Europe [21,22,23,24, 40, 44], five in America [15, 37,38,39, 41], and five in Asia [13, 42, 43, 45, 46].
Regarding the type of ART, we found that nine studies examined all types of ART [15, 24, 37,38,39, 41, 42, 44, 46], four studies examined in vitro fertilization [13, 21, 23, 40], and three studies assessed in vitro fertilization in combination with ICSI or ovulation induction (Table 1) [22, 43, 45].
Results of systematic review
Association of ART and ASD in offspring
A total of 15 studies evaluated the potential association between ART and the risk of ASD in offspring (Table 1) [15, 21,22,23,24, 37,38,39,40,41,42,43,44,45,46]. The 14 studies that did not check for confounding factors showed that offspring conceived by ART was 0.41–8.61 times more likely to present ASD compared to offspring spontaneously conceived (Table 1) [15, 21,22,23,24, 37,38,39,40,41,42,43, 45, 46]. In 11 studies the control for common confounders such as the mother's age and race, and gestational age was shown to reduce the risk of developing ASD to 0.37–4.98 (Table 1) [15, 21,22,23,24, 37, 38, 40, 41, 44].
Association of ART and ASD in pre-term offspring
One of 16 studies provided data regarding the potential association between ART and the risk of ASD in pre-term offspring and suggested that pre-term offspring conceived by ART are 1.57 times more likely to develop ASD compared to pre-term conceived spontaneously (Table 1) [37]. In two studies, the adjustment for potential confounding factors did not significantly differentiate the observed association [21, 37].
Association of ART and ASD in term offspring
The possible association noted above was assessed in terms of offspring in two studies that check for confounding factors. The results showed that term offspring conceived by ART have a 1–1.5 times higher risk of developing ASD than term spontaneously conceived offspring (Table 1) [15, 21].
Association of ART and ASD in singletons and multiple births
According to three studies that provided data regarding the assessment of association only in singletons, the results indicated that offspring conceived by ART are 0.96–1.2 more likely to develop ASD than the spontaneously conceived pregnancies (Table 1) [21, 23, 40]. Adjustment for confounding factors revealed a reduction of correlation but not significantly (Table 1) [15, 21, 23, 40]. Regarding the assessment of association in multiple births, one study that focused on this category of pregnancies revealed that offspring by ART have a 0.91 times higher risk to develop ASD compared to offspring conceived spontaneously, without a statistically significant difference when the study checked for confounding factors (Table 1) [23].
Association of ART and ASD in pre-term and term singletons
One study that checked for confounding factors concluded that pre-term singletons conceived by ART have a 0.71 times higher risk to develop ASD than offspring conceived spontaneously. The same study found that the risk for the term singletons conceived by ART is 0.89 times higher compared to offspring conceived spontaneously (Table 1) [21].
Association of ART and ASD in males and females
Regarding sex, two studies with or without checking for confounding factors showed that males [13, 22] derived by ART have a lower risk compared to females [22] conceived by ART. The risk for males was calculated to be 1–1.18 compared to 1.55 for females and the adjustment for potential confounding factors did not significantly differentiate the observed association (Table 1).
Results of meta-analysis
From the above correlations, the two groups of studies that examined the association of ART and ASD in offspring and singletons provided sufficient data and were involved in our meta-analysis. Fourteen studies that did not check for potential confounders provided data regarding the risk of the autism spectrum in offspring conceived by ART. According to the findings of these studies, offspring conceived by ART have a statistically significant increased risk to develop autism spectrum compared to offspring spontaneously conceived (RR = 1.37, 95% CI 1.15–1.64, p = 0.001) with no significant publication bias in our study (Egger test = 0.484) (Table 2). The heterogeneity in this group of studies was also significant (I2 = 80.6, p < 0.001). To explore the heterogeneity between studies, we performed sub-group analyses based on the study type and geographical origin of the study. The results revealed a non-statistically significant association between ART and the risk of ASD in studies designed case–control (RR = 1.44, 95% CI 0.94–2.19, p = 0.090) with no publication bias (Table 2).
Since no significant reduction of between studies heterogeneity was observed, we performed a Galbraith plot to evaluate the source of heterogeneity graphically. According to the Galbraith plot four studies were outside the bounds and were identified as the primary source of heterogeneity. After removing these studies, the heterogeneity was eliminated (I2 = 0.0%, p = 0.852), even though a slight asymmetry in the funnel plot (Fig. 2) was found, there was no publication bias (Egger test = 0.660) and the association between ART and ASD remained statistically significant (RR = 1.23, 95% CI 1.14–1.33, p < 0.001) (Fig. 3) (Table 2).
The assessment of the impact of the individual study on the effect size of our result, by performing a sensitivity analysis suggested that no obvious changes were found after removing each study at the time (data not shown). The group of eleven studies that examined the association of ART and ASD in offspring and controlled for main common confounding factors indicated that there was no statistically significant association between ART and ASD (RR = 1.14, 95% CI 0.95–1.36, p = 0.158) (Table 3). No statistically significant publication bias was observed (Egger test = 0.138). The heterogeneity in this group of studies was also significant (I2 = 83.3, p < 0.001). We proceeded to investigate heterogeneity by performing subgroup analysis, which did not significantly change the above correlation.
Subsequently, because no significant reduction in heterogeneity was observed, we explored the source of study heterogeneity, using the Galbraith plot. After removing the two studies identified from the Galbraith plot, the heterogeneity was eliminated (I2 = 0.0%, p = 0.603). Even though a slight asymmetry in the funnel plot (Fig. 4) was found, no publication bias was noticed (Egger test = 0.556), and the association between ART and ASD in offspring changed to statistically significant (RR = 1.11, 95% CI 1.03–1.19 p = 0.009) (Fig. 5) (Table 3).
To assess the impact of each study on the effect size of our result, we performed a sensitivity analysis that suggested no obvious changes were found after removing each study at the time (data not shown). The meta-analysis of the studies that focused on singletons demonstrated a no statistically significant association between ART and ASD without (Fig. 6) or with control for confounding factors (Fig. 7) (Table 4).
Discussion
This study aimed to provide an up-to-date meta-analysis of available data from studies that assessed the risk of bearing a child with autism spectrum disorder (ASD) after being conceived by assisted reproductive technology (ART). The results of this meta-analysis indicated that ART is associated with a higher risk of ASD in offspring (RR = 1.11, 95% CI = 1.03–1.19, p < 0.009), except in the cases of singletons (RR = 0.96, 95% CI = 0.82–1.13, p = 0.654).
A previous meta-analysis by Liu et al. [26] which included 11 studies, similarly found that offspring conceived by ART are more likely to develop ASD than offspring spontaneously conceived (RR 1.35, 95% CI 1.09–1.68) [26]. Our results are in line with those of Liu et al. [26]; however, our study has several important strengths.
First, the present meta-analysis included new recently published studies and only studies that distinctly examined the assisted reproductive technology, as a way of treating infertility, while Liu et al. [26] included two studies with an unknown way of infertility treatment and one study that generally used MAR.
Second, to assess the crucial role of confounder factors and the possibility to be a direct risk for ASD, we extracted and collected the crude and adjusted data separately from the studies and performed two independent meta-analyses. In contrast, Liu et al. [26] analyzed crude and adjusted data together, extracting a mixed effect size. On account of numerous and varied combinations of confounding factors controlled in each study we could not conduct a subgroup analysis according to each combination and for each type of ART due to the limitation of data. Most studies controlled for many potential environmental factors associated with ASD, like maternal age, maternal race, gestational age, maternal infertility, and parental infertility. The control for, maternal age and race, and gestational age was common in many studies.
It should be noted that our findings from the two meta-analyses confirmed the positive association between ART and ASD in different degrees, which is a field for further investigation, showing that ART may be an independent risk factor for ASD. Specifically, in the present meta-analysis, we found that ART may be associated with a higher risk of having offspring with ASD (RR = 1.23, 95% CI 1.14–1.33, p < 0.001), while when we proceeded to the meta-analysis only those studies that had controlled for confounding factors, the degree of correlation between the ART and ASD decreased (RR = 1.11, 95% CI 1.03–1.19, p = 0.009). To summarize, the results raise important questions about the impact rate of the involvement of the above factors in the risk of ASD in offspring and whether these factors are primary or play a secondary role in the development of autism.
Regarding singletons, we did not detect a positive association (RR = 0.96, 95% CI = 0.82–1.13, p = 0.654), suggesting that multiple pregnancies may be an independent direct risk for ASD. Consequently, more large studies are needed to better identify factors leading to ASD and whether the increased risk is due to the underlying cause of infertility, advanced parental age, or if it is due entirely to ART interventions [47,48,49].
Epigenetic changes might be a conceivable molecular mechanism linking ART with ASD. Many neurodevelopmental and neuropsychiatric disorders, characterized by autistic like features (e.g., ASD, Beckwith–Wiedemann syndrome, and Angelman syndrome) have been observed to be related to epigenetic mechanisms, such as a defect in genetic imprinting [50,51,52]. ART consists of a various in vitro manipulations and interventions at the cellular level, including repeated hormone exposure, retrieval and isolation of gametes, handling and culture of gametes and early embryos, cryopreservation, and embryo transfer procedures that appear to be prone to epigenetic changes [53, 54]. Also several parents with infertility problems were found to carry pre-existing imprinting errors, with the SNRP, UBE3A, H19, and LIT1 genes being some of those involved [55, 56]. Thus, it is crucial to develop a better understanding and determine whether any modified genes responsible for ASD are associated with infertility or the treatments used. As the epigenome is most vulnerable in the early developmental period, the potential imprinting disorders perhaps contribute to these major epigenetic in this early and crucial development time. Therefore, supplementary epigenetic studies will be required to understand the pathogenesis and the association of ART with ASD. Nevertheless, we must consider that the study of epigenetics is complicated because it is not clear at what point these imprinting errors arise and epigenetics of any tissue can occur at any time.
Study limitations
This systematic review and meta-analysis, although performed using strict search strategy and methods, has limitations. First, we did not include data from unpublished studies. However, we assessed the potential presence of publication bias in all respective statistical analyses of the review/meta-analysis. Both prospective and retrospective studies were included. These studies are characterized by different methodological designs with retrospective studies including recall bias. This error was considered, and its consequence on the change of the effect size of assisted reproduction on the risk of having offspring with autism spectrum disorder was tested. We performed subgroup analyses based on the type of studies that participated.
Moreover, as the risk of autism was low, the relative risks and the corresponding 95% confidence interval (95% CI) were used as summary statistics to assess the association between assisted fertilization and the risk of autism in our systematic review and meta-analysis, although the relative ratios (ORs) were calculated in the prospective and retrospective studies, and the relative risks (RRs) were calculated in all studies above, except the retrospective studies. The language restrictions of this review should also be taken into consideration.
Also, we should note that we accepted all the diagnostic criteria for ASD as reported in the original papers. Finally, assisted reproductive technology is quite new in medicine and characterized by numerous and complex therapeutic procedures, making it particularly difficult to identify individual risk factors. Unmeasured and uncontrolled risk factors have the potential to produce biases. In the present study, we could not rule out the effects of infertility causes and their possible effect on ASD outcome. We could not interpret the potential biological mechanism of the association between ART and ASD. Therefore, more studies, mainly cohort, should be included in future reviews to confirm or refute and interpret the correlation of different assisted reproduction techniques with the risk of having offspring with autism spectrum.
Conclusion
This systematic review and meta-analysis evaluated the association between ART and the risk of ASD in offspring. According to our results, ART was associated with a higher risk of ASD except, in the cases of singletons. These results must be interpreted with caution since people requesting ART are usually at an advanced age with different infertility forms, all of which are risk factors for fetal and neonatal abnormalities. Further high-quality prospective studies with a larger number of participants are required to determine the association between ART and ASD.
Abbreviations
- ART:
-
Assisted reproductive technology
- ASD:
-
Autism spectrum disorder
- ICSI:
-
Intracytoplasmic sperm injection
- LBW:
-
Low birth weight
- MAR:
-
Medically assisted reproduction
- NOS:
-
Newcastle-Ottawa Scale
References
Masi A, DeMayo MM, Glozier N, Guastella AJ. An overview of autism spectrum disorder, heterogeneity and treatment options. Neurosci Bull [Internet]. 2017 Apr 17;33(2):183–93. Available from: https://doi.org/10.1007/s12264-017-0100-y
Autism spectrum disorder. In: Diagnostic and Statistical Manual of Mental Disorders DSM-5. Fifth. Washington DC: American Psychiatric Association; 2013. p. 50–9.
Newschaffer CJ, Croen LA, Daniels J, Giarelli E, Grether JK, Levy SE, et al. The epidemiology of autism spectrum.
Data & statistics on autism spectrum disorder | CDC [Internet]. [cited 2020 Aug 23]. Available from: https://www.cdc.gov/ncbddd/autism/data.html
Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, et al. Genetic heritabilty. Arch Gen Psychiatry. 2011;68(11):1095–102.
Landrigan PJ. What causes autism? Exploring the environmental contribution. Curr Opin Pediatr. 2010 Apr;22(2):219–25.
Germain ARD levine susan hanson maureen. 乳鼠心肌提取 HHS Public Access. Physiol Behav. 2017;176(3):139–48.
Froehlich-Santino W, Londono Tobon A, Cleveland S, Torres A, Phillips J, Cohen B, et al. Prenatal and perinatal risk factors in a twin study of autism spectrum disorders. J Psychiatr Res [Internet]. 2014;54(1):100–8. https://doi.org/10.1016/j.jpsychires.2014.03.019
Rao SC. Prevalence of autism spectrum disorder in preterm infants : a meta-analysis. 2017;142(3).
Jenabi E, Karami M, Khazaei S, Bashirian S. The association between preeclampsia and autism spectrum disorders among children : a meta-analysis. 2019;126–30.
Gardener H, Spiegelman D, Buka SL. Perinatal and neonatal risk factors for autism : a comprehensive meta-analysis. 2011;344–55.
Wu S, Wu F, Ding Y, Hou J, Bi J, Zhang Z. Advanced parental age and autism risk in children: a systematic review and meta-analysis. Acta Psychiatr Scand [Internet]. 2017 Jan;135(1):29–41. Available from: https://doi.org/10.1111/acps.12666
Davidovitch M, Chodick G, Shalev V, Eisenberg VH, Dan U, Reichenberg A, et al. Infertility treatments during pregnancy and the risk of autism spectrum disorder in the offspring. Prog Neuro-Psychopharmacology Biol Psychiatry [Internet]. 2018 Aug;86(May):175–9. Available from: https://doi.org/10.1016/j.pnpbp.2018.05.022
Grether JK, Qian Y, Croughan MS, Wu YW, Schembri M, Camarano L, et al. Is infertility associated with childhood autism? J Autism Dev Disord [Internet]. 2013 Mar 10;43(3):663–72. Available from: https://doi.org/10.1007/s10803-012-1598-5
Schieve LA, Drews-Botsch C, Harris S, Newschaffer C, Daniels J, DiGuiseppi C, et al. Maternal and paternal infertility disorders and treatments and autism spectrum disorder: findings from the study to explore early development. J Autism Dev Disord. 2017;47(12):3994–4005.
Vander Borght M, Wyns C. Fertility and infertility: definition and epidemiology. Clin Biochem [Internet]. 2018 Dec;62(March):2–10. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0009912018302200
Care OP. C ommittee opinion. Am Coll Obstet Gynecol WOMEN’S Heal CARE PHYSICIAN. 2016;128(666):6.
Assisted Reproductive Technology (ART) | Reproductive health | CDC [Internet]. [cited 2020 Aug 23]. Available from: https://www.cdc.gov/art/index.html
Adamson D, Baker V. Multiple births from assisted reproductive technologies: a challenge that must be met. Fertil Steril [Internet]. 2004 Mar;81(3):517–22. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0015028203030668
McDonald SD, Han Z, Mulla S, Murphy KE, Beyene J, Ohlsson A. Preterm birth and low birth weight among in vitro fertilization singletons: a systematic review and meta-analyses. Eur J Obstet Gynecol Reprod Biol [Internet]. 2009 Oct;146(2):138–48. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0301211509003753
Sandin S, Hultman C, Reichenberg A. Autism in children born after in vitro fertilization—reply. JAMA [Internet]. 2013 Nov 20;310(19):2101. Available from: https://doi.org/10.1001/jama.2013.278610
Hvidtjørn D, Grove J, Schendel D, Schieve LA, Sværke C, Ernst E, et al. Risk of autism spectrum disorders in children born after assisted conception: a population-based follow-up study. J Epidemiol Community Health. 2011;65(6):497–502.
Klemetti R, Sevóna T, Gissler M, Hemminki E. Health of children born as a result of in vitro fertilization. Pediatrics. 2006;118(5):1819–27.
Maimburg RD, Vaeth M. Do children born after assisted conception have less risk of developing infantile autism? Hum Reprod [Internet]. 2007 Jul 1;22(7):1841–3. Available from: https://doi.org/10.1093/humrep/dem082
Conti E, Mazzotti S, Calderoni S, Saviozzi I, Guzzetta A. Are children born after assisted reproductive technology at increased risk of autism spectrum disorders? A systematic review. Hum Reprod. 2013;28(12):3316–27.
Liu L, Gao J, He X, Cai Y, Wang L, Fan X. Association between assisted reproductive technology and the risk of autism spectrum disorders in the offspring: a meta-analysis. Sci Rep [Internet]. 2017 May 7;7(1):46207. https://doi.org/10.1038/srep46207
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Med [Internet]. 2009 Jul 21;6(7):e1000097. Available from: https://doi.org/10.1371/journal.pmed.1000097
Ottawa Hospital Research Institute [Internet]. [cited 2020 Oct 6]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
Walter S. Choice of effect measure for epidemiological data. J Clin Epidemiol [Internet]. 2000 Sep;53(9):931–9. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0895435600002109
Sterne JAC, Bradburn MJ, Egger M. Meta-analysis in StataTM. Syst Rev Heal Care Meta-Analysis Context Second Ed. 2008;347–69.
Tufanaru C, Munn Z, Stephenson M, Aromataris E. Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. Int J Evid Based Healthc [Internet]. 2015 Sep;13(3):196–207. Available from: http://journals.lww.com/01787381-201509000-00012
Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med [Internet]. 2002 Jun 15;21(11):1539–58. Available from: https://doi.org/10.1002/sim.1186
Anzures-Cabrera J, Higgins JPT. Graphical displays for meta-analysis: an overview with suggestions for practice. Res Synth Methods [Internet]. 2010 Jan;1(1):66–80. Available from: https://doi.org/10.1002/jrsm.6
Series CB. Cochrane handbook for systematic reviews of interventions [Internet]. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al., editors. Cochrane handbook for systematic reviews of interventions. Wiley; 2019. Available from: https://doi.org/10.1002/9781119536604
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ [Internet]. 1997 Sep 13;315(7109):629–34. Available from: https://doi.org/10.1136/bmj.315.7109.629
Publication ASP. Stata Meta-Analysis Reference Manual.
Diop H, Cabral H, Gopal D, Cui X, Stern JE, Kotelchuck M. Early autism spectrum disorders in children born to fertile, subfertile, and ART-treated women. Matern Child Health J [Internet]. 2019;23(11):1489–99. Available from: https://doi.org/10.1007/s10995-019-02770-z
Fountain C, Zhang Y, Kissin DM, Schieve LA, Jamieson DJ, Rice C, et al. Association between assisted reproductive technology conception and autism in California, 1997-2007. Am J Public Health. 2015;105(5):963–71.
Kamowski-Shakibai MT, Magaldi N, Kollia B. Parent-reported use of assisted reproduction technology, infertility, and incidence of autism spectrum disorders. Res Autism Spectr Disord [Internet]. 2015;9:77–95. Available from: https://doi.org/10.1016/j.rasd.2014.10.009
Lehti V, Brown AS, Gissler M, Rihko M, Suominen A, Sourander A. Autism spectrum disorders in IVF children: a national case-control study in Finland. Hum Reprod. 2013;28(3):812–8.
Lyall K, Pauls DL, Spiegelman D, Santangelo SL, Ascherio A. Fertility therapies, infertility and autism spectrum disorders in the Nurses’ Health Study II. Paediatr Perinat Epidemiol. 2012;26(4):361–72.
ÖZBARAN B, KÖSE S, AKYOL ARDIÇ Ü, ERERMİŞ S, KESIKÇI ERGİN H, BİLDİK T, et al. Yardımcı Üreme Teknikleriyle Doğmuş Çocukların ve Annelerinin Psikiyatrik Değerlendirmesi: Klinik Bir Çalışma. Nöro Psikiyatr Arşivi [Internet]. 2013 Mar 5;50(1):59–64. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15891417
Shimada T, Kitamoto A, Todokoro A, Ishii-Takahashi A, Kuwabara H, Kim S-Y, et al. Parental age and assisted reproductive technology in autism spectrum disorders, attention deficit hyperactivity disorder, and Tourette syndrome in a Japanese population. Res Autism Spectr Disord [Internet]. 2012 Jan;6(1):500–7. Available from: https://doi.org/10.1016/j.rasd.2011.07.010
Svahn MF, Hargreave M, Nielsen TSS, Plessen KJ, Jensen SM, Kjaer SK, et al. Mental disorders in childhood and young adulthood among children born to women with fertility problems. Hum Reprod. 2015;30(9):2129–37.
Zachor DA, Ben Itzchak E. Assisted reproductive technology and risk for autism spectrum disorder. Res Dev Disabil [Internet]. 2011;32(6):2950–6. Available from: https://doi.org/10.1016/j.ridd.2011.05.007
Jenabi E, Seyedi M, Hamzehei R, Bashirian S, Rezaei M, Razjouyan K, et al. Association between assisted reproductive technology and autism spectrum disorders in Iran: a case-control study. Clin Exp Pediatr [Internet]. 2020 Sep 15;63(9):368–72. Available from: https://doi.org/10.3345/cep.2020.00073
Pisarska MD, Chan JL, Lawrenson K, Gonzalez TL, Wang ET. Genetics and epigenetics of infertility and treatments on outcomes. J Clin Endocrinol Metab [Internet]. 2019 Jun 1;104(6):1871–86. Available from: https://academic.oup.com/jcem/article/104/6/1871/5245823
Janeczko D, Hołowczuk M, Orzeł A, Klatka B, Semczuk A. Paternal age is affected by genetic abnormalities, perinatal complications and mental health of the offspring (Review). Biomed Reports [Internet]. 2019 Dec 20;12(3):83–8. Available from: https://doi.org/10.3892/br.2019.1266
Das L, Parbin S, Pradhan N, Kausar C, Patra SK. Epigenetics of reproductive infertility. Front Biosci - Sch. 2017;9(4):509–35.
Fett-Conte AC, Bossolani-Martins AL, Rosan DBA. Etiology of autism the complexity of risk factors in autism spectrum disorder. In: Autism spectrum disorder - recent advances [Internet]. InTech; 2015. Available from: http://www.intechopen.com/books/autism-spectrum-disorder-recent-advances/etiology-of-autism-the-complexity-of-risk-factors-in-autism-spectrum-disorder
Loke YJ, Hannan AJ, Craig JM. The role of epigenetic change in autism spectrum disorders. Front Neurol. 2015;6(MAY):1–18.
Deangelis AM, Martini AE, Owen CM. Assisted reproductive technology and epigenetics. Semin Reprod Med. 2018;36(3–4):221–32.
Hiura H, Okae H, Chiba H, Miyauchi N, Sato F, Sato A, et al. Imprinting methylation errors in ART. Reprod Med Biol [Internet]. 2014 Oct;13(4):193–202. Available from: https://doi.org/10.1007/s12522-014-0183-3
Chen W, Peng Y, Ma X, Kong S, Tang S, Zhao Y, et al. Epigenetic effects of assisted reproductive technology in human offspring 1 2. Available from: https://doi.org/10.1101/816157
Horsthemke B, Ludwig M. Assisted reproduction: the epigenetic perspective. Hum Reprod Update [Internet]. 2005 Oct 1;11(5):473–82. Available from: http://academic.oup.com/humupd/article/11/5/473/606511/Assisted-reproduction-the-epigenetic-perspective
Whitelaw N, Bhattacharya S, Hoad G, Horgan GW, Hamilton M, Haggarty P. Epigenetic status in the offspring of spontaneous and assisted conception. Hum Reprod [Internet]. 2014 Jul 1;29(7):1452–8. Available from: https://doi.org/10.1093/humrep/deu094
Author information
Authors and Affiliations
Contributions
MTA: conceived the study, participated in its design, collection, and interpretation of the data; she also performed the data collection and extraction, as well as the statistical analyses, and helped draft the manuscript. GNK: contributed to the data collection and extraction, in the analysis of the results, and in drafting the manuscript. PT: contributed to the data collection and extraction, as well as in the analysis of the results. CD: participated in the manuscript’s design and coordination. EZ: participated in the manuscript’s design and coordination. IS: participated in the manuscript’s design and coordination. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
All analyses were based on previous published studies; thus, no ethical approval was required.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Gregorio Paolo Milani
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
The search string that we used to Pubmed was: (((((in vitro fertilization[MeSH Terms]) OR (fertilization[MeSH Terms])) OR (infertility[MeSH Terms])) OR (assisted reproduction technologies[MeSH Terms])) OR (intracytoplasmic sperm injection[MeSH Terms]) AND ((humans[Filter]) AND (English [Filter]))) AND (((((autism[MeSH Terms]) OR (autistic[MeSH Terms])) OR (asperger syndrome[MeSH Terms])) OR (rett syndrome[MeSH Terms])) OR (developmental disorder[MeSH Terms]) AND ((humans[Filter]) AND (English [Filter]))). The search string that was applied to Scopus database was: ( ( TITLE-ABS-KEY ( in AND vitro AND fertilization) OR TITLE-ABS-KEY ( fertilization) OR TITLE-ABS-KEY ( infertility) OR TITLE-ABS-KEY ( assisted AND reproduction AND technologies) OR TITLE-ABS-KEY ( intracytoplasmic AND sperm AND injection))) AND ( ( TITLE-ABS-KEY ( autism) OR TITLE-ABS-KEY ( autistic) OR TITLE-ABS-KEY ( asperger AND syndrome) OR TITLE-ABS-KEY ( rett AND syndrome) OR TITLE-ABS-KEY ( developmental AND disorder))) AND ( LIMIT-TO ( DOCTYPE, "ar")) AND ( LIMIT-TO ( LANGUAGE, "English")) AND ( EXCLUDE ( EXACTKEYWORD, "Nonhuman")).
Rights and permissions
About this article
Cite this article
Andreadou, M.T., Katsaras, G.N., Talimtzi, P. et al. Association of assisted reproductive technology with autism spectrum disorder in the offspring: an updated systematic review and meta-analysis. Eur J Pediatr 180, 2741–2755 (2021). https://doi.org/10.1007/s00431-021-04187-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-021-04187-9