Abstract
Kawasaki disease (KD) is an acute systemic vasculitis that most commonly causes acquired cardiac disease in children in developed countries. The most highly recommended treatment for KD is 2 g/kg intravenous immunoglobulin (IVIG). There are two types of IVIG, sodium-containing (high-Na) and sodium-trace (low-Na) preparations. However, few studies have compared the effects of these two preparations for superiority. The purpose of this study was to compare outcomes between high and low-Na IVIG preparations in KD children using a national inpatient database in Japan. We used the Diagnostic Procedure Combination database to identify KD patients treated with IVIG between 2010 and 2017. We identified those receiving high and low-Na preparations of IVIG as an initial treatment. Outcomes included proportion of coronary artery abnormalities (CAA), IVIG resistance, adverse effects, length of stay, and medical cost. Propensity score–matched analyses were conducted to compare the outcomes between the two groups. Instrumental variable analyses were performed to confirm the results. We identified 42,345 patients with KD. There were significant differences in proportions of CAA (2.8% vs. 3.2%; p = 0.031) and IVIG resistance (17% vs. 18%, p = 0.001) between the two groups. However, there were no significant differences in length of stay or medical cost. The instrumental variable analysis confirmed the same results as the propensity score analysis.
Conclusion: The present study suggests that high-Na IVIG is potentially effective for reducing the proportion of CAA in KD patients. Prospective studies are warranted to confirm the effectiveness observed in this study.
What is Known: • For treatments of Kawasaki Disease in acute phase, intravenous immunoglobulin have been the most recommended to reduce fever early and prevent complications of coronary artery abnormalities. There are two types of IVIG preparations, sodium-containing IVIG and sodium-trace IVIG. However, few studies have performed comparisons to determine which preparation of IVIG is superior. What is New: • The present findings suggest that high-Na IVIG is associated with reductions in the proportions of CAAs and IVIG resistance in KD patients. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kawasaki disease (KD) is an acute systemic vasculitis that most commonly causes acquired cardiac disease in children in developed countries [1]. The standard treatment for KD is intravenous immunoglobulin (IVIG) and aspirin [2, 3]. There are two types of IVIG preparations, sodium-containing (high-Na) IVIG and sodium-trace (low-Na) IVIG. However, few studies have performed comparisons to determine which preparation of IVIG is superior. The appropriate type of IVIG preparation for treatment of KD patients in the acute phase remains unclear. The purpose of the present study was to compare the outcomes of KD children treated with high-Na and low-Na IVIG preparations using a national inpatient database in Japan.
Methods
Data source
We performed this retrospective cohort study using the Diagnosis Procedure Combination database. The details for this nationwide inpatient database were described elsewhere [4]. The database includes hospitalizations for all factors across all ages and has about 1000 participating hospitals ranging from academic to community hospitals (about 7 million patients every year). All 82 academic hospitals are obliged to participate in the database, while participation by community hospitals is voluntary. Data for about 7 million hospitalized patients of all ages are collected every year, equivalent to about 50% of the total acute-care hospitalizations in Japan. The database includes the following information: unique identifiers of hospitals; patient baseline characteristics; and diagnosis at admission, comorbidities at admission, and complications after admission recorded with text data in Japanese and International Classification of Diseases, Tenth Revision (ICD-10) codes. The database also contains information on medical procedures and treatments, including drug administrations, use of devices, and surgical and nonsurgical procedures, as well as length of stay, discharge status, and medical cost for hospitalization. Diagnoses at and after admission are clearly differentiated in the database. The attending physicians are obliged to record patient data for all diagnoses and comorbidities with reference to the medical records. Furthermore, because accurate reporting is linked with the payment system in Japan, the attending physicians and hospitals are required to accurately report the diagnoses and comorbidities. The present study was approved by the Institutional Review Board of The University of Tokyo (approval number: 3501-(3); 25 December 2017). The requirement for informed consent was waived because of the anonymous nature of the data.
Participants
We used the database to identify patients who were diagnosed with KD (ICD-10 code: M303) between July 2010 and March 2017. Patients whose first IVIG treatment was started within 5 days of hospitalization and who received at least 2 g/kg IVIG within 3 days of starting their first IVIG treatment were included in the study. We further checked the Japanese text describing the detailed diagnoses in each case to include atypical KD patients and exclude patients with a “suspected” diagnosis of KD, inadequate treatment (<2 g/kg IVIG), and age >6 years. We excluded patients aged >6 years because the appropriate IVIG dose for KD patients with older age remains controversial. The included children with KD in the acute phase were divided into two groups depending on their initial treatment with high-Na IVIG (154 mEq/L Na) or low-Na IVIG (0.09–2.60 mEq/L Na). Patients who received both high-Na and low-Na IVIG were excluded.
Outcomes
The primary outcome was the occurrence of coronary artery abnormalities (CAAs) at the time of discharge in the two groups. CAAs were defined according to the following criteria: (i) recorded diagnosis of CAAs (ICD-10 code: I254); (ii) use of warfarin or clopidogrel; or (iii) cardiac catheterization. The secondary outcomes were IVIG resistance, length of stay, and medical cost. IVIG resistance was defined as use of IVIG at a total dose of ≥4.0 g/kg or a combination of infliximab, cyclosporine, or plasma exchange.
Covariates
The baseline characteristics were age, sex, weight, height, hospital days of illness at initial IVIG, type of hospital, complex chronic conditions [5], transportation by ambulance, activities of daily living at admission, Japan Coma Scale at admission, transportation from other hospital, fiscal year, additional treatment, and hospital volume. The Japan Coma Scale scores were categorized into two groups: alert and not alert. Japan Coma Scale assessment was previously shown to be well-associated with Glasgow Coma Scale assessment [6]. Hospital volume was defined as the mean annual number of all KD patients at each hospital. We categorized the eligible patients into tertiles of hospital volume so that the numbers of patients in the groups were almost equal. Additional treatment was defined as any steroid, infliximab, or cyclosporine, or plasma exchange beyond day 5 after starting the initial IVIG treatment. Because the initial IVIG treatment usually took 1–2 days and a further 1–2 days were required to assess the patient’s response, we chose 5 days for the definition of additional treatment.
Statistical analysis
Categorical variables are shown as number and percentage and were compared using Fisher’s exact test. Continuous variables are shown as mean and standard deviation (SD) or median and interquartile range (IQR). The Mann–Whitney U test was used to compare non-normally distributed variables between the two groups. To obtain meaningful interpretation of variables with skewed distributions, we calculated the median difference with 95% confidence interval (95% CI) using the Hodges–Lehmann estimator.
Propensity score–matched analysis
We conducted 1:1 propensity score–matched analyses to compare the outcomes between the two groups. For the propensity score matching, nearest-neighbor matching without replacement was performed. The caliper width was set at ≤0.2 of the pooled SD of the estimated propensity scores. We examined the covariate balance between the two groups before and after the propensity score matching using the absolute standardized difference. An absolute standardized difference of >10% was regarded as imbalanced [7].
The chi-square test was used to compare the proportions of CAAs and IVIG resistance between the two groups. We also estimated the risk differences and 95% CIs. The Mann–Whitney U test was used to compare length of stay and medical cost.
Instrumental variable analysis
Propensity score–matched analyses cannot eliminate the effects of unmeasured confounders such as laboratory data. To confirm our propensity score–matched analyses, we performed instrumental variable analyses. The key assumptions for instrumental variable analysis are that the instrumental variable (i) is highly correlated with the treatment assignment, (ii) is not correlated with other confounders, and (iii) does not affect patient outcomes except through the treatment [8, 9].
Instrumental variable analysis methods often utilize “physician prescribing preference” as the instrumental variable. This variable reflects the notion that a physician’s prescribing decision depends on not only the patient characteristics but also the physician’s preference for a specific medicine. This means that the physician’s preference is largely independent of the patient characteristics and outcomes and can therefore serve as an instrumental variable. One commonly used method to determine the physician’s preference is to employ the prescription for the last patient treated by the physician as the preference for the current patient [10].
In the present study, we used “prescription for the last patient” as the instrumental variable. Specifically, when the prescription for the last patient in the same institution was high-Na IVIG, the current patient was assumed to be more likely to receive high-Na IVIG, because only one type of IVIG is typically available within a single facility. Conversely, when the prescription for the last patient in the same institution was low-Na IVIG, the current patient was assumed to be more likely to receive low-Na IVIG. The prescription for the last patient is assumed to be independent of the current patient’s characteristics and not directly related to the outcome. Therefore, this instrumental variable was considered to meet the above-described three key assumptions for an instrumental variable.
We used a two-stage residual inclusion method for both continuous and binary outcome variables [11, 12]. In the first-stage model, we determined the row residual for each patient by calculating the difference between the model-predicted probability of receiving the treatment choice and the actual treatment received. The residuals were included as an additional covariate in the second-stage model. In the second-stage model, the association between treatment choice and outcome was estimated in an unbiased manner, after adjustment for covariates. We used a multivariable linear regression model for continuous outcome variables and a multivariable logistic regression model for binary outcome variables. All instrumental variable analyses were performed using robust standard errors. To assess the validity of the instrumental variable, we tested its association with our main predictor of actual treatment choice using the F-statistic (F-statistic >10 is considered to reflect a valid instrumental variable) [13]. We also determined that the instrumental variable was not associated with the patient background characteristics and outcomes. A two-sided p < 0.05 was considered significant. All statistical analyses were conducted using the Stata software version 16.1 (StataCorp LP, College Station, TX, USA).
Results
Study population
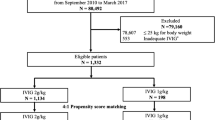
A total of 42,345 patients with KD who met inclusion criteria were identified. Of these, 22,393 patients received high-Na IVIG and 19,952 patients received low-Na IVIG as their initial treatments in the acute phase (Fig. 1). The baseline characteristics before and after the propensity score matching are shown in Table 1.
In the unmatched cohort, patients in the low-Na IVIG group had a lower proportion of academic hospital than those in the high-Na group. The 1:1 propensity score matching between the high-Na IVIG group and low-Na IVIG group created 18,457 matched pairs (Table 1). The characteristics of the patients in the matched cohort were considered well-balanced between the two groups because the absolute standardized differences for all covariates were <10%. The results of comparisons between the high-Na IVIG group and low-Na IVIG group after the propensity score matching are shown in Table 2. In the propensity score–matched cohort, there were significant differences between the high-Na IVIG group and low-NA IVIG group in the proportion of CAAs (2.8% vs. 3.2%; risk difference: 0.39% [95% CI: 0.04–0.74]; (p = 0.029) and proportion of IVIG resistance (17% vs. 18%; risk difference: 1.3% [95% CI: 0.51–2.0]; (p = 0.001). However, there were no significant differences between the two groups in median length of stay (10 days vs. 10 days; median difference: 0 days [95% CI: 0–0]; (p = 0.0723) and median medical cost (6866 US dollars vs. 6848 US dollars); median difference: 3170 US dollars [95% CI: −1550–7880]; (p = 0.1875).
The instrumental variable analyses showed that, compared with the high-Na IVIG group, the low-Na IVIG group was significantly associated with higher proportion of CAAs (odds ratio: 1.35 [95% CI: 1.13–1.61], p < 0.001) and higher proportion of IVIG resistance (odds ratio: 1.18 [95% CI: 1.08–1.28], p < 0.001) (Table 3). The F-statistic was 13,619 and the instrumental variable was considered valid. There were no associations between the instrumental variable and the patient background characteristics (Supplementary Table 1).
Discussion
The present study showed that the high-Na IVIG preparation was associated with reductions in the proportions of CAAs and IVIG resistance for KD patients in the acute phase, using a large nationwide inpatient database in Japan. A previous study on patients with KD in the acute phase showed no significant difference in the proportion of CAAs between the high-Na IVIG group (n = 48) and low-Na IVIG group (n = 30) [14]. The previous study may have failed to show a significant difference between the two groups because of its small sample size.
We confirmed our findings using two analytical methods: propensity score–matched analysis and instrumental variable analysis. Propensity score–matched analysis can reduce potential bias arising from background factors, including disease severity, in the two groups to obtain accurate differences in outcomes caused by the different Na concentrations in the IVIG preparations. However, it is not possible to adjust for unmeasured confounders in a propensity score–matched analysis. Instrumental variable analysis can mimic a random allocation and theoretically balance unmeasured confounders between the two groups.
The physicians who made the treatment decisions may have chosen high-Na IVIG for KD patients with low serum Na levels arising from severe inflammation. Although the number of patients for whom high-Na IVIG was selected based on low serum Na levels was likely to be small, serum Na level was one of the unmeasured confounding factors in this study and may have created a bias in the propensity score–matched analyses. However, the same results were obtained in the instrumental variable analyses.
Hyponatremia can be experienced in a variety of inflammatory diseases [15]. KD patients in the acute phase frequently have hyponatremia [16,17,18,19]. The pathogenesis of hyponatremia in acute-phase KD is associated with syndrome of inappropriate antidiuretic hormone secretion mediated by increased cytokine levels in the acute phase [20, 21]. However, the precise mechanism remains unknown. Hyponatremia in patients with acute-phase KD is associated with severe inflammation, which results in Kawasaki shock syndrome, IVIG resistance, and CAA development [22,23,24,25,26].
In a previous study, severe vascular inflammation in acute-phase KD combined with hyponatremia was shown to induce angiotensin II and aldosterone in the renin-angiotensin-aldosterone system, which potentially results in CAAs [27]. Aldosterone might be involved in the vascular injury associated with KD by causing endothelial dysfunction, vasoconstriction, remodeling, oxidative stress, and inflammation [28,29,30,31,32,33].
In summary, hyponatremia may increase the excessive secretion of aldosterone and angiotensin II in the renin-angiotensin-aldosterone system, which results in vascular damage, dysfunction, and remodeling. The use of low-Na IVIG may cause further hyponatremia in patients with hyponatremia-prone KD, and thus, the use of high-Na IVIG is considered safer. This is consistent with case reports on hyponatremia caused by low-Na IVIG [34]. In acute KD patients who are prone to hyponatremia, use of high-Na IVIG may be an important factor to prevent CAA development.
Limitations
There are several limitations to this study. First, because the database comprises administrative claims data, there may be miscoding. To minimize the risk of misclassification regarding the diagnosis of KD, we excluded patients with a “suspected” diagnosis of KD. Second, although a large patient population was included, this was a retrospective observational study that did not include detailed data such as symptoms of KD, laboratory findings, or fever duration for KD patients in the acute phase. In particular, serum Na levels and amounts of Na in the fluid infusion were unmeasured confounders in our propensity score–matched cohort and were not adjusted between the two groups. Therefore, we cannot conclude that the use of high-Na IVIG improved hyponatremia and resulted in the favorable outcomes in the proportions of CAAs and IVIG resistance.
Bias could still exist in the form of unmeasured confounders including laboratory findings, time from KD onset to admission, and day of illness, which were needed to calculate the scoring system to identify IVIG resistance in KD patients. We could not calculate KD scoring systems for IVIG resistance, and the severity of KD was not identified clearly. To adjust for the severity of KD between the two groups, we used baseline characteristics including Japan Coma Scale scores and complex chronic conditions from the database to achieve the propensity score–matched analyses. Furthermore, we carried out instrumental variable analyses and obtained similar results using the two analytical methods. To confirm that the CAAs were not transient and to determine the severity of the CAAs such as diameters and Z-scores, which were not included in the database, we used the history of cardiac catheterization and prescription history of anticoagulants in addition to aspirin. Finally, although this study using a large database found significant differences in the proportions of CAAs and IVIG resistance, the actual effect sizes were small. Further studies are warranted to investigate whether these differences are clinically meaningful.
Conclusions
The present study suggests that high-Na IVIG is potentially associated with reductions in the proportions of CAAs and IVIG resistance in KD patients in the acute phase. Prospective studies are warranted to confirm the effectiveness of high-Na IVIG observed in the present study.
Abbreviations
- ADL:
-
Activities of daily living
- ASD:
-
Absolute standardized difference
- CAA:
-
Coronary artery abnormalities
- CI:
-
Confidence interval
- IQR:
-
Interquartile range
- IVIG:
-
Intravenous immunoglobulin
- KD:
-
Kawasaki disease
- SD:
-
Standard deviation
- USD:
-
US dollar
References
Burns JC, Glodé MP (2004) Kawasaki syndrome. Lancet. 364:533–544
Saji T, Ayusawa M, Miura M, Kobayashi T (2012) The clinical guideline for medical treatment of acute stage Kawasaki disease. Pediatr Cardiol Cardiac Surg 28:S1–S28
McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, Baker AL, Jackson MA, Takahashi M, Shah PB, Kobayashi T, Wu MH, Saji TT, Pahl E, American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Surgery and Anesthesia; and Council on Epidemiology and Prevention (2017) Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 135:e927–e999
Isogai T, Matsui H, Tanaka H, Fushimi K, Yasunaga H (2016) Early β-blocker use and in-hospital mortality in patients with Takotsubo cardiomyopathy. Heart. 102:1029–1035
Simon TD, Berry J, Feudtner C, Stone BL, Sheng X, Bratton SL, Dean JM, Srivastava R (2010) Children with complex chronic conditions in inpatient hospital settings in the United States. Pediatrics. 126:647–655
Todo T, Usui M, Takakura K (1991) Treatment of severe intraventricular hemorrhage by intraventricular infusion of urokinase. J Neurosurg 74:81–86
Austin PC, Stuart EA (2015) Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 34:3661–3679
Brookhart MA, Rassen JA, Schneeweiss S (2010) Instrumental variable methods in comparative safety and effectiveness research. Pharmacoepidemiol Drug Saf 19:537–554
Aso S, Yasunaga H (2020) Introduction to instrumental variable analysis. Ann Clin Epidemiol 2:69–74
Brookhart MA, Wang PS, Solomon DH, Schneeweiss S (2006) Evaluating short-term drug effects using a physician-specific prescribing preference as an instrumental variable. Epidemiology. 17:268–275
Terza JV, Basu A, Rathouz PJ (2008) Two-stage residual inclusion estimation: addressing endogeneity in health econometric modeling. J Health Econ 27:531–543
Terza JV, Bradford WD, Dismuke CE (2008) The use of linear instrumental variables methods in health services research and health economics: a cautionary note. Health Serv Res 43:1102–1120
Staiger D, Stock JH (1997) Instrumental variables regression with weak instruments. Econometrica. 65:557
Kaneko K, Hirabayashi M, Tateiwa A, Shimo T, Teranishi K, Tanaka S et al (2010) Immunoglobulin preparations affect hyponatremia in Kawasaki disease. Eur J Pediatr 169:957–960
Swart RM, Hoorn EJ, Betjes MG, Zietse R (2011) Hyponatremia and inflammation: the emerging role of interleukin-6 in osmoregulation. Nephron Physiol 118:45–51
Laxer RM, Petty RE (1982) Hyponatremia in Kawasaki disease. Pediatrics. 70:655
Miura K, Harita Y, Takahashi N, Tsurumi H, Yasudo H, Isojima T, Hirata Y, Inuzuka R, Takizawa K, Toyofuku E, Nishimoto H, Takamizawa M, Ando T, Sugawa M, Yanagisawa A, Inatomi J, Nogimori Y, Kinumaki A, Namai Y, Hattori M, Oka A (2020) Nonosmotic secretion of arginine vasopressin and salt loss in hyponatremia in Kawasaki disease. Pediatr Int 62:363–370
Watanabe T, Abe Y, Sato S, Uehara Y, Ikeno K, Abe T (2006) Hyponatremia in Kawasaki disease. Pediatr Nephrol 21:778–781
Shin JI, Kim JH, Lee JS, Kim DS, Choi JY, Sul JH (2006) Kawasaki disease and hyponatremia. Pediatr Nephrol 21:1490–1491 author reply 1492
Lim GW, Lee M, Kim HS, Hong YM, Sohn S (2010) Hyponatremia and syndrome of inappropriate antidiuretic hormone secretion in kawasaki disease. Korean Circ J 40:507–513
Mori J, Miura M, Shiro H, Fujioka K, Kohri T, Hasegawa T (2011) Syndrome of inappropriate anti-diuretic hormone in Kawasaki disease. Pediatr Int 53:354–357
Kobayashi T, Inoue Y, Takeuchi K, Okada Y, Tamura K, Tomomasa T, Kobayashi T, Morikawa A (2006) Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation. 113:2606–2612
Muta H, Ishii M, Egami K, Hayasaka S, Nakamura Y, Yanagawa H, Matsuishi T (2005) Serum sodium levels in patients with Kawasaki disease. Pediatr Cardiol 26:404–407
Hashimoto I (2019) Indicators of unresponsiveness after initial i.v. immunoglobulin treatment in acute Kawasaki disease. Pediatr Int 61:641–646
Nakamura Y, Yashiro M, Uehara R, Watanabe M, Tajimi M, Oki I, Ojima T, Sonobe T, Yanagawa H (2004) Use of laboratory data to identify risk factors of giant coronary aneurysms due to Kawasaki disease. Pediatr Int 46:33–38
Taddio A, Rossi ED, Monasta L, Pastore S, Tommasini A, Lepore L, Bronzetti G, Marrani E, Mottolese BD’A, Simonini G, Cimaz R, Ventura A (2017) Describing Kawasaki shock syndrome: results from a retrospective study and literature review. Clin Rheumatol 36:223–228
Park S, Eun LY, Kim JH (2017) Relationship between serum sodium level and coronary artery abnormality in Kawasaki disease. Korean J Pediatr 60:38–44
Pacurari M, Kafoury R, Tchounwou PB, Ndebele K (2014) The renin-angiotensin-aldosterone system in vascular inflammation and remodeling. Int J Inf Secur 2014:689360
Tian J, An X, Niu L (2017) Correlation between NF-κB signal pathway-mediated caspase-4 activation and Kawasaki disease. Exp Ther Med 13:3333–3336
Maury CP, Salo E, Pelkonen P (1988) Circulating interleukin-1 beta in patients with Kawasaki disease. N Engl J Med 319:1670–1671
Yin W, Wang X, Ding Y, Peng H, Liu YL, Wang RG, Yang YL, Xiong JH, Kang SX (2011) Expression of nuclear factor-κBp65 in mononuclear cells in Kawasaki disease and its relation to coronary artery lesions. Indian J Pediatr 78:1378–1382
Wang Y, Wang W, Gong F, Fu S, Zhang Q, Hu J, Qi Y, Xie C, Zhang Y (2013) Evaluation of intravenous immunoglobulin resistance and coronary artery lesions in relation to Th1/Th2 cytokine profiles in patients with Kawasaki disease. Arthritis Rheum 65:805–814
Briet M, Schiffrin EL (2013) Vascular actions of aldosterone. J Vasc Res 50:89–99
Nguyen MK, Rastogi A, Kurtz I (2006 Jun) True hyponatremia secondary to intravenous immunoglobulin. Clin Exp Nephrol 10:124–126
Availability of data and material
Data cannot be made publicly available for ethical reasons as the data are patient data. The data are available to interested researchers upon request to the corresponding author, pending ethical approval.
Code availability
This study was analyzed using the standard packages of the Stata software version 16.1 (StataCorp LP, College Station, TX, USA).
Funding
This work was supported by grants from the Ministry of Health, Labour and Welfare, Japan (19AA2007 and 20AA2005) and the Ministry of Education, Culture, Sports, Science and Technology, Japan (20H03907).
Author information
Authors and Affiliations
Contributions
TS and NM conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript. SA, TY, and KS conceptualized and designed the study, and coordinated and critically reviewed the manuscript for important intellectual content. HM, KF, and HY designed the data collection instruments, collected data, carried out the initial analyses, and reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval, Consent to participate, Consent for publication
This article does not contain any studies with human participants or animals performed by any of the authors. Informed consent was waived because all data were de-identified. This study was approved by the Institutional Review Board of The University of Tokyo (approval number: 3501-(3) (December 25, 2017)).
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Peter de Winter
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Suzuki, T., Michihata, N., Aso, S. et al. Sodium-containing versus sodium-trace preparations of IVIG for children with Kawasaki disease in the acute phase. Eur J Pediatr 180, 3279–3286 (2021). https://doi.org/10.1007/s00431-021-04096-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-021-04096-x