Abstract
Probiotics may benefit in cystic fibrosis (CF) as gut dysbiosis is associated with gastrointestinal symptoms and exacerbation of respiratory symptoms in CF. We conducted a systematic review of randomized controlled trials (RCTs) and non-RCTs of probiotic supplementation in children with CF, using the Cochrane methodology, preferred reporting items for systematic reviews (PRISMA) statement, and meta-analysis of observational studies in epidemiology (MOOSE) guidelines. Primary outcomes were pulmonary exacerbations, duration of hospitalization and antibiotics, and all-cause mortality. Secondary outcomes included gastrointestinal symptoms, markers of gut inflammation, and intestinal microbial balance. A total of nine studies (RCTs, 6, non-RCTs, 3; N = 275) with some methodological weaknesses were included in the review. The pooled estimate showed significant reduction in the rate of pulmonary exacerbation (fixed effects model, two parallel group RCTs and one cross-over trial: relative risk (RR) 0.25, (95 % confidence interval (95 % CI) 0.15,0.41); p < 0.00001; level of evidence: low) and decrease in fecal calprotectin (FCLP) levels (fixed effect model, three RCTs: mean difference (MD) −16.71, 95 % CI −27.30,−6.13); p = 0.002; level of evidence: low) after probiotic supplementation. Probiotic supplementation significantly improved gastrointestinal symptoms (one RCT, one non-RCT) and gut microbial balance (decreased Proteobacteria, increased Firmicutes, and Bacteroides in one RCT, one non-RCT).
Conclusion: Limited low-quality evidence exists on the effects of probiotics in children with CF. Well-designed adequately powered RCTs assessing clinically meaningful outcomes are required to study this important issue.
What is Known: • Gut dysbiosis is frequent in children with cystic fibrosis due to frequent exposure to pathogens and antibiotics. • Probiotics decrease gut dysbiosis and improve gut maturity and function. |
What is New: • This comprehensive systematic review shows that current evidence on the safety and efficacy of probiotics in children with cystic fibrosis is limited and of low quality. • Well-designed and adequately powered trials assessing clinically important outcomes are required considering the health burden of cystic fibrosis and the potential benefits of probiotics. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Cystic fibrosis (CF) is a lethal hereditary disorder leading to respiratory infections and gastrointestinal inflammation with a possible association with intestinal dysbiosis [19]. In acute pulmonary exacerbations in CF, there is an increase in pathogens (e.g., Enterobacteriaceae) and decrease in beneficial microbes (e.g., Bifidobacteria) in the gut [19, 20, 36, 56]. The fecal-oral transmission of increased gut pathogens could explain these exacerbations in CF [28, 36]. Because of recurrent bronchopulmonary infections, patients with CF are treated with repeated courses of antibiotics thereby disrupting the gut microbial balance and innate immune mediators [34]. Increased gut colonization by Fusobacterium and Bacteroides also predisposes to gut infection and inflammation in CF [48, 57]. Immune-mediated intestinal inflammation in CF has been reported in several studies [12, 60]. Fecal calprotectin (FCLP) and rectal nitric oxide (RNO) are inflammatory markers that are elevated in CF and other inflammatory bowel diseases [5, 62, 72]. Since endoscopy and biopsy which are the gold standards to diagnose intestinal inflammation are invasive and expensive, non-invasive markers of intestinal inflammation such as FCLP and RNO are increasingly being used to detect intestinal inflammation in CF [5, 8, 9]. Normal concentrations of FCLP range between 0 and 50 μg/g of wet stools; values between 50 and 100 μg/g are intermediate and need to be followed up, whereas values over 100 μg/g indicate intestinal inflammation [22, 51]. Similarly, RNO can be easily measured non-invasively and that nitric oxide (NO) increase is observed in active intestinal inflammation [10].
Probiotics are live microbial agents which when ingested in an adequate amount, provide health benefits to the host [39]. Probiotics decrease gut microbial imbalance [59] and result in more stable and diverse flora by various mechanisms including modulation of gut immunity, competition with gut microorganisms for nutrients, and production of growth substrates/inhibitors [42, 54, 55, 59, 65]. The ability to reduce intestinal dysbiosis is one of the most important mechanisms of benefits of probiotics in inflammatory bowel diseases [1, 37]. Probiotics have direct antimicrobial [50], immune modulatory [2, 50], and anti-inflammatory effects [47, 66] and enhance gut maturity and function [2]. Both FCLP and RNO production decrease after probiotic supplementation [5, 7]. Pulmonary exacerbations (PE) in CF are associated with immune-mediated lung damage [14]. Given the health burden of the disease, and the potential benefits of probiotics [2, 4, 41, 47, 50, 53, 66], we aimed to conduct a systematic review of studies assessing the effects of probiotic supplementation in children with CF.
Methods and participants
We followed the Cochrane methodology [35], the preferred reporting items for systematic reviews (PRISMA) statement [46], and the meta-analysis of observational studies in epidemiology (MOOSE) guidelines [61] for conducting and reporting this systematic review. Ethics approval was not required.
Eligibility criteria
Types of studies
Randomized controlled trial (RCT) as well as non-RCT studies were eligible for inclusion in the review.
Participants
Participants comprised children under 18 years with CF.
Type of interventions
Oral supplementation was given with any probiotic supplementation (with or without prebiotic) in any form and dose and continued for minimum 2 weeks in children with CF. Control intervention was either placebo or standard treatment without probiotic supplementation.
Primary outcomes
Primary outcomes include the following: (1) pulmonary exacerbation as defined by the CF foundation criteria [13, 67], which include at least one major criterion or two minor signs/symptoms with fulfillment of symptom duration. Major criteria include decrease in forced expiratory volume in 1 second (FEV1) of > =10 % from best baseline within past 6 months,unresponsive to albuterol, oxygen saturation < 90 % on room air or > = 5 % decline from previous baseline, new lobar infiltrates or atelectasis on chest radiograph and hemoptysis. Minor signs/symptoms include increased work of breathing or respiratory rate, new or increased adventitial sounds on lung exam, and weight loss CF. Control intervention was either placebo or standard treatment without probiotic supplement in months, increased cough, decreased exercise tolerance or level of activity, and increased chest congestion or change in sputum activity. (2) Duration of hospitalization; (3) duration of antibiotic treatment (oral/intravenous); and (4) all-cause mortality.
Secondary outcomes
Secondary outcomes include the following: (1) GI symptoms/signs including diarrhea, vomiting, constipation, reduced appetite, abdominal distension, flatulence, abdominal pain, jaundice, pale colored stools, steatorrhoea, gastroesophageal reflux (heart burn), and infection [3]; (2) GI inflammation demonstrated by mean FCLP >50 μg/g and RNO >5 μmol/L; (3) plasma/blood cytokines (interleukin (IL)-6, IL-8, tumor necrosis factor (TNF α) as systemic inflammatory markers; (4) intestinal microbial balance assessed by fecal meta-genomic studies; and (5) change in weight/height/body mass index (BMI).
Search strategy
We searched the Cochrane central register of controlled trials (CENTRAL), PubMed, EMBASE, CINAHL databases, proceedings of PAS/SPR and Pediatric Pulmonology conferences, and Psyc INFOS for studies reported from the earliest available online year of indexing until December 2015 using the keywords/MeSH terms (Lactobacillus or Bifidobacterium or Saccharomyces or probiotic agent or probiotics or prebiotics or synbiotics) and cystic fibrosis. No restrictions were applied on study design or language. References of the obtained studies were reviewed to identify additional studies. The international trial registries and Australian Clinical Trials Registry were checked for ongoing/registered trials in this area. Google scholar was searched for articles that might not have been cited in the standard medical databases. Reviewers conducted the literature search independently. Conclusions regarding quality and strength of evidence were based on the grading of recommendations, assessment, development, and evaluation (GRADE) system [33]. Search strategy is summarized in Fig. 1.
Study selection
Reviewers AA and HB independently assessed eligibility for selection of all studies identified using the prespecified search strategy. Any disagreements were resolved by discussion among all reviewers.
Data extraction
Reviewers AA and HB independently completed a prespecified data extraction form for all included studies. For dichotomous outcomes, the number of patients with the event and the number of patients analyzed in each treatment group of each study were entered into the form. For continuous outcomes, we planned to enter the mean and standard deviations (SD). Information about study design and outcomes was verified by all reviewers. Any disagreements were discussed until consensus was achieved. We contacted the investigators for clarification and/or additional data for analysis.
Assessment of risk of bias
We used the Cochrane Neonatal Review Group guidelines to assess the methodological quality of the included RCTs [35]. Additional information was requested from the trial authors to clarify methodology and results if necessary. For each trial, information was sought regarding the method of randomization, allocation concealment and blinding, and reporting of all outcomes of all children enrolled in the trial. Reviewers AA and HB separately assessed each study. Any disagreement was resolved by a group discussion.
The quantitative scoring tool, Newcastle-Ottawa scale (NOS), proposed by Cochrane Collaboration, was adopted for evaluating the methodological quality of the included non-RCTs [71]. The NOS contains three major domains: selection of subjects, comparability between groups, and the outcome measures. The maximum score for each area is 4, 2, and 3 points, respectively. A total score of 3 or lower indicates low methodological quality.
Assessment of publication bias
This was planned to be assessed by a funnel plot [35].
Data synthesis
The assessment of risk of bias and heterogeneity in the included studies, data extraction and synthesis, and pooling of treatment effects was planned according to the standard Cochrane methodology [35]. Meta-analysis was planned if pooling of data was possible and justified. We planned to calculate the I 2 statistic for each analysis to quantify inconsistency across studies and describe the percentage of variability in effect estimates that may be due to heterogeneity than due to sampling error. A narrative synthesis was planned if meta-analysis was not possible due to significant heterogeneity in included studies and/or non-availability of the outcome measures in the desired form.
Results
The literature search retrieved 204 potential relevant citations (Fig. 1). After carefully reviewing the abstracts, 23 duplicate studies were excluded. A total of 168 studies were excluded due to non-fulfillment of the inclusion criteria. We considered 13 studies including 4 RCTs, 4 cross-over trials, and 5 non-RCTs for inclusion in the review. Among them, two RCTs and two non-RCTs were excluded since only abstracts were available and the full texts could not be obtained after a detailed search and trying to contact the authors. Finally, nine studies including four RCTs, two cross-over trials, and three non-RCTs were included in the review. The characteristics of the included studies are described in Tables 1 and 2. These studies enrolled 275 children (RCTs, 235; non-RCTs, 40) and had significant variation in patient age group, probiotic type, dose and duration, and outcome measures (Tables 1 and 2). The characteristics of the four excluded studies available only as abstracts are described in Table 3.
Details of included studies
Bruzzese [7]: This double-blinded RCT recruited 22 study participants (10 in probiotic group and 12 in placebo group) who received either probiotic supplementation or placebo for 1 month. The primary outcome measured was intestinal inflammation defined as increase in FCLP (0–50 μg/g as normal, 50–100 μg/g as intermediate, and >100 μg/g abnormal) and RNO levels (>2.6 μmol/L). Sixty-three percent of children with CF had increased FCLP (>100 μg/g). Mean FCLP in probiotic group after treatment with probiotics decreased significantly (p < 0.05), while mean FCLP in the placebo group after treatment with probiotics did not show significant change (p = 0.3). The correlation between intestinal inflammatory markers and the richness of intestinal microbiota in children with CF was evaluated. Mean difference in the Bacteroides count before and after intervention in the probiotic group was 4.84 × 1010 and in the placebo group was 1.37 × 1010. Mean difference in the Faecalibacterium prausnitzii count before and after intervention in the probiotic group was 2.46 × 1010 and in the placebo group was 0.48 × 1010. Mean difference in the Eubacterium rectale count before and after intervention in the probiotic group was 0.59 × 1010 and in the placebo group was 5.62 × 1010. Probiotic supplementation reduced FCLP and RNO levels significantly and showed a trend towards restoring intestinal microbiota composition though it was not statistically significant.
Campo [16]: This was a double-blinded cross-over trial which randomized 30 children with CF to receive probiotic supplementation in period 1 followed by placebo in period 2 or vice versa for 6 months in each period. Primary outcomes measured were FCLP, GI discomfort, inflammatory parameters such as IL-8, IL-6, and TNF-α, and gut microbial balance. The mean FCLP after probiotic consumption decreased significantly. The study found statistically significant improvement in the G1QL1 scoring for GI comfort (p = 0.003) and improved gut microbial balance. The inflammatory parameters such as IL-8, TNF-α, and IL-6 did not show any significant variations (4 vs. 3.6 pg/mL for IL-8, 3.3 vs. 2.7 pg/mL for TNF-α, and 2.3 vs. 2.4 pg/mL for IL-6).
Di Nardo [18]: This double-blinded RCT randomized 61 study participants to receive either probiotic supplementation or placebo for 6 months. The primary outcomes measured were pulmonary exacerbations, hospital admissions, GI, and upper respiratory tract infections (URTI). The study found significant reduction in the rate of pulmonary exacerbation in the probiotic group (number needed to treat, 3 (95 % confidence interval (95 % CI), 2–7)). The number of URTI significantly reduced in the probiotic group (number needed to treat, 6 (95 % CI, 3–102)). There was no significant difference in the number and mean duration of hospitalization between the two groups, GI infection and inflammatory markers. There was no difference detected in the qualitative and quantitative analyses of bacteria in the sputum.
Fallahi [23]: This double-blinded RCT enrolled 47 study participants (24 in probiotic group, 23 in placebo) to receive probiotic supplementation or placebo for 4 weeks. The primary outcome measured was intestinal inflammation defined as FCLP >50 μg/g. The study found 65.9 % of the CF patients with intestinal inflammation at baseline. Mean FCLP showed a significant decrease after treatment with probiotic supplementation (p = 0.031) compared with the placebo. The study reported decrease in intestinal inflammation after probiotic supplementation (58.1 to 27.6 %) by using FCLP as the sole predictor of intestinal inflammation.
Jafari [40]: This RCT enrolled 37 study participants (17 in probiotic group, 20 in placebo group) who received either probiotic supplementation or placebo for 1 month. The primary outcomes measured were quality of life and rate of pulmonary exacerbation. At the start point, quality of life was assessed in all study subjects using the Pediatric Quality of Life Inventory 4.0 short-form questionnaire [68]. The mean total score of parent-reported quality of life was significantly higher in the probiotic group (86.8 vs. 80.2; p = 0.01) at 3 months with no significant difference at 6 months. The mean number of pulmonary exacerbations significantly decreased in the probiotic group after intervention (p = 0.01). The significant improvement in the quality of life at 3 months is questionable since the significance did not persist after 6 months.
Bruzzese [6]: In this cross-over trial, 38 children with CF who were chronically infected with Pseudomonas were randomized to receive probiotic supplementation in period 1 followed by ORS in period 2 or vice versa for 6 months in each period. The primary outcomes measured were incidence of pulmonary exacerbation, hospital admission, FEV1, and modification of body weight. The study found decreased rate of pulmonary exacerbations in both periods, decreased rate of hospital admissions in period 1, increased in mean FEV1, and increased in body weight with probiotic supplementation compared with placebo.
Weiss [70]: This prospective open pilot study included 10 study participants who received probiotic supplementation for 6 months. The primary outcomes included number and severity of PEs, FEV1, body weight by body mass index, bacterial strain in sputum, cell counts in sputum, and sputum IL-8 levels. The study reported decrease in the rate of pulmonary exacerbation. There were no significant changes in FEV1 and BMI, no difference in sputum bacterial strain, no change in sputum cell count and sputum IL-8.
De Infante Pina [38]: This matched pair analysis study recruited 20 children who received probiotic supplementation for 4 weeks. The primary outcomes included water, fat, nitrogen, and sugar content of feces as a measure of stool appearance, stool frequency, and bacterial overgrowth. The study reported improved stool appearance, intestinal comfort, and decrease in the number of daily stools with probiotic treatment. Significant improvement in the intestinal function was noted with probiotic supplementation which was shown by reduction in stool fat, sugar, and nitrogen.
Bruzzese [5]: This prospective matched pair analysis enrolled 60 children (30 with CF and 30 healthy controls, subset of 10 CF children compared before and after probiotic supplementation) who received probiotic supplementation for 4 weeks. The primary outcomes measured were intestinal inflammation defined by FCLP levels (0–50 μg/g normal; 50–100 μg/g as intermediate, and >100 μg/g abnormal) and RNO levels. The study showed a significant decrease in FCLP and RNO levels with probiotic supplementation. The mean FCLP in children with CF (n = 60) was four times higher, almost reaching the levels of children with inflammatory bowel disease compared with controls. Probiotic supplementation reduced FCLP and RNO in vast majority of CF children although in none did the FCLP return to normal.
Ongoing studies
A RCT of Lactobacillus GG (LGG) supplementation (6 × 109 CFU/day × 12 months) in children with CF has recently been completed and is titled as “Probiotics in Cystic Fibrosis.” The study has recruited 110 participants aged 2 to 18 years [31]. The trial evaluates the rate of PE, incidence of hospital admissions, pulmonary function, and markers of intestinal inflammation in participants.
A double-blinded placebo-controlled randomized cross-over trial of Bio-25 probiotic supplementation titled “The effects of probiotic on sputum bacteria, sputum inflammation and pulmonary infections in patients with cystic fibrosis” was terminated due to severe allergic reaction in one patient [69]. The study targeted to recruit 12 participants aged 5–40 years with CF. The aim of the trial was to evaluate the effect of probiotic supplementation on the rate of pulmonary infections and the rate of pulmonary exacerbations requiring antibiotic therapy. The effects of probiotic supplementation on sputum bacteria, sputum inflammatory markers, and GI inflammation were the secondary outcomes. It was not clear from the published information on the clinicaltrials.gov website if the allergic reaction occurred in the probiotic arm or the placebo.
Recently, a randomized controlled trial of supplementing 1 g of oral probiotic powder daily for 12 months in children with CF has been registered and is titled “Probiotics and the Early Life effects on intestinal bacteria and inflammation in Children with Cystic Fibrosis (PEARL-CF)” [52]. The study aims to recruit 66 children of age ≤6 years. The trial evaluates the effect of probiotic supplementation on the intestinal microbial profile and intestinal inflammation.
Details of excluded studies (available as abstracts only)
Garriga [26]: This randomized cross-over trial enrolled 33 children with CF to receive probiotic supplementation or placebo (16 received 6 months of probiotics in period 1 followed by placebo in period 2, 17 received 6 months of placebo in period 1 followed by probiotics in period 2). The primary outcome measured were FCLP, IL-1b levels, GI health, and gut microbial balance. The study found improvement in GI health, decrease in FCLP and IL-1B levels, and significant changes in the gut flora (Actinobacteria, Firmicutes, and Bacteroidetes). Further analysis of the trial was limited by non-availability of detailed results since only the abstract was available.
Di Benedetto [17]: This cross-over trial randomized 24 children with CF to receive probiotics in period 1 followed by ORS in period 2 or vice versa for 6 months in each period. The primary outcomes included modification of body weight, incidence of abdominal pain, number of episodes of infection needing antibiotics, steatorrhoea, and serum iron levels. The study showed increase in body weight, decreased incidence of abdominal pain, and decreased number of infection requiring antibiotics with probiotic supplementation. The serum iron levels and the incidence of steatorrhoea did not show significant difference with probiotic supplementation. Further details of the study could not be obtained since only the abstract was available.
Scotto [58]: This prospective cohort study enrolled 20 children (ten CF children and ten healthy control) to receive probiotic supplementation for 1 month. The primary outcomes included intestinal inflammation using FCLP and RNO, respiratory inflammation using NO and IL8 in nasal brushings, and systemic inflammation by serum TNF-α and IL-8. Both FCLP and RNO decreased after probiotic supplementation. Nasal NO and serum TNF-α decreased in 100 % patients after probiotic administration. Further details of the study could not be obtained since only abstract was available. The analysis of respiratory inflammation using nasal NO is questionable.
Navas-Lopez [49]: This prospective cohort study enrolled 42 children (17 in probiotic group, 25 in mesalamine group) to receive probiotics for 1 month. The primary outcomes included intestinal inflammation by FCLP and intestinal permeability by dual test with lactulose and mannitol. No statistically significant difference was found after treatment in both groups.
Primary outcomes
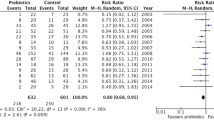
The beneficial effects of probiotic supplementation on the primary outcomes are shown in Tables 1 and 2. The overall pooled estimate suggested that probiotic supplementation decreased the rate of PE in children with CF (MD, 0.25 (95 % CI, 0.15, 0.41); p < 0.00001; heterogeneity: χ 2 = 1.46, I 2 = 32 %; Fig. 2). The remaining data were not pooled due to the significant heterogeneity in outcome measures.
Secondary outcomes
The effect of probiotic supplementation on GI symptoms was reported in one non-RCT. One RCT and one non-RCT reported improvement in gut microbial imbalance, four RCTs and one non-RCT reported on laboratory markers of intestinal inflammation. One RCT reported on plasma/blood cytokine levels and one cross-over trial and one non-RCT reported on the change in body weight after probiotic supplementation (Tables 1 and 2). The overall pooled estimate showed a decrease in the level of FCLP after probiotic supplementation (MD, −16.71 (95 % CI, −27.30, −6.13); p = 0.002; heterogeneity: χ 2 = 6.92, I 2 = 71 %; Fig. 3).
Risk of bias
Four out of six RCTs had some methodological weaknesses. Allocation concealment was unclear in four [6, 7, 23, 40], information about random sequence generation was unclear in two [23, 40], and the risk of attrition bias was high in two trials [6, 16]. The risk of bias summary of the included RCTs is shown in Fig. 4. The mean NOS score of the included non-RCTs was 7 out of 9, indicating that they were of sufficient quality for meta-analysis (Table 4).
Publication bias
Creating a funnel plot was not possible given the inability to pool the data due to the heterogeneity of outcomes reported in the included studies. Therefore, the risk of publication bias could not be ruled out.
Discussion
Our systematic review showed six RCTs and three non-RCTs (N = 275), reporting on the effects of probiotic supplementation in children with CF. Significant improvement in the respiratory parameters (pulmonary exacerbations, infection) and non-respiratory parameters (body weight, abdominal pain, and GI health) was observed. The overall pooled estimate showed a decrease in the rate of pulmonary exacerbations and reduction in the FCLP levels after probiotic supplementation in children with CF.
Of the three studies which analyzed the effect of probiotic supplementation on the rate of pulmonary exacerbation, the pooled estimate was based on two studies. The third study was a cross-over trial and was not pooled to avoid the influence of carryover of the probiotic effect. The pooled estimate was based on the number of exacerbations rather than the number of participants in the study. This was due to small sample size in the included studies and the rarity of the outcome. Hence, the results should be interpreted with caution. A single study showed the outcome of decreased incidence of respiratory tract infection [18] and the rate of hospitalization [6] significantly after probiotic supplementation and hence the data could not be pooled.
GI health, gut microbial dysbiosis, and body weight showed improvement after probiotic supplementation; however, the data could not be pooled due to difference in the study design and outcome measures. Hence, the validity of these primary outcome measures could not be ascertained which demands further studies addressing the issues.
The pooled estimate of the FCLP levels was based on decrement in the FCLP levels before and after probiotic supplementation in the treatment group rather than direct comparison between probiotic and placebo groups. There was marked variability in the baseline values of FCLP among the included studies prior to probiotic supplementation. The only study which compared the reduction in FCLP levels between probiotic and placebo groups by Di Nardo et al. showed no significant reduction in the FCLP levels. FCLP is a sensitive marker for intestinal inflammation, but the lack of specificity and baseline variability in the population limits its predictive value in CF [15, 23, 25, 44, 45]. In the absence of a validated marker for gut inflammation, it is important to study the effect of probiotic supplementation on GI signs and symptoms (e.g., diarrhea, constipation, abdominal pain, steatorrhoea) in CF. To our knowledge, this is the first comprehensive systematic review of probiotic supplementation in children with CF—a disease with significant health burden. The strength of our review relates to its comprehensive nature and robust methodology. Significant benefits were observed irrespective of the variations in patient characteristics, the probiotics, and its supplementation protocol. None of the studies reported adverse effects of probiotics. The most important limitations in our systematic review were the large variability in ages of the patients and that most of the studies were not including only children [16, 18, 70], the lack of information of the pancreatic status and disease severity of the patients, and the rather short study duration in most of the studies (4 weeks) [5, 7, 23, 38, 40].
Considering the potential benefits of probiotics in CF [2–4, 7, 14, 35, 41, 46, 47, 61, 66–68] and the health burden of this disease, further research is important in this area. Few issues need to be discussed with regard to the design and conduct of such studies. The duration of probiotic supplementation is important for optimal benefits. Previous studies have shown increased benefits when probiotics are administered for 6 to 12 months in chronic immune-mediated conditions like eczema and Crohn’s disease [29, 32, 43]. Monitoring for complications during long-term probiotic supplementation (e.g., endocarditis, probiotic sepsis) is necessary [21, 24, 63, 64, 73, 74].
Comparing the benefits of single vs. multi-strain probiotics is important. In a systematic review [11], 12/16 included studies showed better benefits following supplementation with a multi-strain compared with single strain probiotics in conditions such as irritable bowel syndrome, diarrhea, atopic disease, respiratory infections, inflammatory bowel disease, and helicobacter pylori infection. Strains with a documented ability to decrease intestinal inflammation and gut dysbiosis (e.g., LGG and Bifidobacterium breve) or multispecies products such as VSL no. 3 may be suitable [27, 30]. Given the low incidence of CF in children, recruitment for adequately powered RCTs will be difficult. Cross-over trials have the disadvantage that the residual effect of the probiotics administered in the initial phase could be a confounding factor when interpreting data from the second phase. Deciding an adequate washout period for individual probiotic strains will be difficult. Considering that early intervention may optimize the benefits, future studies of probiotics in CF need to include infants under 12 months age. Further research is needed to assess the optimal dose, strain selection/combination, and duration of probiotics in children with CF.
In summary, current evidence on the safety and efficacy of probiotics in children with CF is limited and of low quality. Well-designed and adequately powered RCTs assessing clinically important outcomes are required considering the health burden of CF and the potential benefits of probiotics. A comprehensive assessment of gut flora is important to study the pathways of benefits of probiotics in CF. Patient compliance during the study and long-term follow-up need to be ensured.
Abbreviations
- BMI:
-
Body mass index
- CF:
-
Cystic fibrosis
- FCLP:
-
Fecal calprotectin
- FEV:
-
Forced expiratory volume
- GI:
-
Gastrointestinal
- LGG:
-
Lactobacillus GG
- RCT:
-
Randomized controlled trial
- RNO:
-
Rectal nitric oxide
- ORS:
-
Oral rehydration solution
References
AlFaleh K, Anabrees J (2014) Probiotics for prevention of necrotizing enterocolitis in preterm infants. The Cochrane database. Syst Rev 4:Cd005496. doi:10.1002/14651858.CD005496.pub4
Bermudez-Brito M, Plaza-Diaz J, Munoz-Quezada S, Gomez-Llorente C, Gil A (2012) Probiotic mechanisms of action. Ann Nutr Metab 61(2):160–174
Borowitz D, Gelfond D (2013) Intestinal complications of cystic fibrosis. Curr Opin Pulm Med 19(6):676–680
Boyle RJ, Bath-Hextall FJ, Leonardi-Bee J, Murrell DF, Tang ML (2008) Probiotics for treating eczema. Cochrane Database Syst Rev (4):Cd006135. doi:10.1002/14651858.CD006135.pub2
Bruzzese E, Raia V, Gaudiello G, Polito G, Buccigrossi V, Formicola V, Guarino A (2004) Intestinal inflammation is a frequent feature of cystic fibrosis and is reduced by probiotic administration. Aliment Pharmacol Ther 20(7):813–819
Bruzzese E, Raia V, Spagnuolo MI, Volpicelli M, De Marco G, Maiuri L, Guarino A (2007) Effect of lactobacillus GG supplementation on pulmonary exacerbations in patients with cystic fibrosis: a pilot study. Clin Nutr (Edinburgh, Scotland) 26(3):322–328
Bruzzese E, Callegari ML, Raia V, Viscovo S, Scotto R, Ferrari S, Morelli L, Buccigrossi V, Lo Vecchio A, Ruberto E, Guarino A (2014) Disrupted intestinal microbiota and intestinal inflammation in children with cystic fibrosis and its restoration with Lactobacillus GG: a randomised clinical trial. PLoS One 9(2):e87796. doi:10.1371/journal.pone.0087796
Bunn SK, Bisset WM, Main MJ, Gray ES, Olson S, Golden BE (2001) Fecal calprotectin: validation as a noninvasive measure of bowel inflammation in childhood inflammatory bowel disease. J Pediatr Gastroenterol Nutr 33(1):14–22
Canani RB, Cirillo P, Bruzzese E, Graf M, Terrin G, Gaudiello G, De Curtis M, Cucchiara S, Guarino A (2002) Nitric oxide production in rectal dialysate is a marker of disease activity and location in children with inflammatory bowel disease. Am J Gastroenterol 97(6):1574–1576. doi:10.1111/j.1572-0241.2002.05757.x
Canani RB, Terrin G, Rapacciuolo L, Miele E, Siani MC, Puzone C, Cosenza L, Staiano A, Troncone R (2008) Faecal calprotectin as reliable non-invasive marker to assess the severity of mucosal inflammation in children with inflammatory bowel disease. Dig Liver Dis 40(7):547–553. doi:10.1016/j.dld.2008.01.017
Chapman CM, Gibson GR, Rowland I (2011) Health benefits of probiotics: are mixtures more effective than single strains? Eur J Nutr 50(1):1–17
Croft NM, Marshall TG, Ferguson A (1995) Gut inflammation in children with cystic fibrosis on high-dose enzyme supplements. Lancet 346(8985):1265–1267
Cystic Fibrosis Foundation (1994) Mycrobiology and infectious disease in cystic fibrosis. Consensus conferences: concept in care. Bethesda Vol V, sect
Dagli E, Warner JA, Besley CR, Warner JO (1992) Raised serum soluble interleukin-2 receptor concentrations in cystic fibrosis patients with and without evidence of lung disease. Arch Dis Child 67(4):479–481
De Vos M, Louis EJ, Jahnsen J, Vandervoort JG, Noman M, Dewit O, D’Haens GR, Franchimont D, Baert FJ, Torp RA, Henriksen M, Potvin PM, Van Hootegem PP, Hindryckx PM, Moreels TG, Collard A, Karlsen LN, Kittang E, Lambrecht G, Grimstad T, Koch J, Lygren I, Coche JC, Mana F, Van Gossum A, Belaiche J, Cool MR, Fontaine F, Maisin JM, Muls V, Neuville B, Staessen DA, Van Assche GA, de Lange T, Solberg IC, Vander Cruyssen BJ, Vermeire SA (2013) Consecutive fecal calprotectin measurements to predict relapse in patients with ulcerative colitis receiving infliximab maintenance therapy. Inflamm Bowel Dis 19(10):2111–2117
del Campo R, Garriga M, Perez-Aragon A, Guallarte P, Lamas A, Maiz L, Bayon C, Roy G, Canton R, Zamora J, Baquero F, Suarez L (2014) Improvement of digestive health and reduction in proteobacterial populations in the gut microbiota of cystic fibrosis patients using a Lactobacillus reuteri probiotic preparation: a double blind prospective study. J Cyst Fibros 13(6):716–722
Di Benedetto LRV, Pastore A, Albano F, Spagnuolo MI, DeVizia B, Guarino A (1998) Lactobacillus casei strain GG as adjunctive treatment to children with cystic fibrosis. J Pediatr Gastroenterol Nutr 26(5):542
Di Nardo G, Oliva S, Menichella A, Pistelli R, De Biase RV, Patriarchi F, Cucchiara S, Stronati L (2014) Lactobacillus reuteri ATCC55730 in cystic fibrosis. J Pediatr Gastroenterol Nutr 58(1):81–86
Duytschaever G, Huys G, Bekaert M, Boulanger L, De Boeck K, Vandamme P (2011) Cross-sectional and longitudinal comparisons of the predominant fecal microbiota compositions of a group of pediatric patients with cystic fibrosis and their healthy siblings. Appl Environ Microbiol 77(22):8015–8024
Duytschaever G, Huys G, Bekaert M, Boulanger L, De Boeck K, Vandamme P (2013) Dysbiosis of Bifidobacteria and Clostridium cluster XIVa in the cystic fibrosis fecal microbiota. J Cystic Fibros 12(3):206–215
Eren Z, Gurol Y, Sonmezoglu M, Eren HS, Celik G, Kantarci G (2014) Saccharomyces cerevisiae fungemia in an elderly patient following probiotic treatment. Mikrobiyoloji bulteni 48(2):351–355
Fagerberg UL, Loof L, Merzoug RD, Hansson LO, Finkel Y (2003) Fecal calprotectin levels in healthy children studied with an improved assay. J Pediatr Gastroenterol Nutr 37(4):468–472
Fallahi G, Motamed F, Yousefi A, Shafieyoun A, Najafi M, Khodadad A, Farhmand F, Ahmadvand A, Rezaei N (2013) The effect of probiotics on fecal calprotectin in patients with cystic fibrosis. Turk J Pediatr 55(5):475–478
Franko B, Vaillant M, Recule C, Vautrin E, Brion JP, Pavese P (2013) Lactobacillus paracasei endocarditis in a consumer of probiotics. Medecine et maladies infectieuses 43(4):171–173
Garcia-Sanchez V, Iglesias-Flores E, Gonzalez R, Gisbert JP, Gallardo-Valverde JM, Gonzalez-Galilea A, Naranjo-Rodriguez A, de Dios-Vega JF, Muntane J, Gomez-Camacho F (2010) Does fecal calprotectin predict relapse in patients with Crohn’s disease and ulcerative colitis? J Crohn’s Colitis 4(2):144–152
M. Garriga AdB, M. Burreros, P. Guallarte, A. Perez-Aragon, A. Lamas, del Campo R., L. Suarez (2013) Probiotic intake improves the gastrointestinal health of cystic fibrosis patients. Paper presented at the J Cyst Fibros
Ghadimi D, Vrese M, Heller KJ, Schrezenmeir J (2010) Effect of natural commensal-origin DNA on toll-like receptor 9 (TLR9) signaling cascade, chemokine IL-8 expression, and barrier integritiy of polarized intestinal epithelial cells. Inflamm Bowel Dis 16(3):410–427
Gillanders LEJ, Gilpin D, Schneiders T, Tunney MT (2011) The airway microbiome in cystic fibrosis: challenges for therapy. Therapy 8(645e):60
Gionchetti P, Rizzello F, Venturi A, Brigidi P, Matteuzzi D, Bazzocchi G, Poggioli G, Miglioli M, Campieri M (2000) Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology 119(2):305–309
Grimoud J, Durand H, de Souza S, Monsan P, Ouarne F, Theodorou V, Roques C (2010) In vitro screening of probiotics and synbiotics according to anti-inflammatory and anti-proliferative effects. Int J Food Microbiol 144(1):42–50
Guarino A (2015) Effects of LGG administration in children with cystic fibrosis: a randomized controlled trial. Effects of LGG administration in children with cystic fibrosis: a randomized controlled trial: Federico II University
Guslandi M, Mezzi G, Sorghi M, Testoni PA (2000) Saccharomyces boulardii in maintenance treatment of Crohn’s disease. Dig Dis Sci 45(7):1462–1464
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schunemann HJ (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clinical research ed) 336(7650):924–926
Hawrelak JA, Myers SP (2004) The causes of intestinal dysbiosis: a review. Altern med review 9(2):180–197
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clinical researched) 343:d5928. doi:10.1136/bmj.d5928
Hoen AG, Li J, Moulton LA, O’Toole GA, Housman ML, Koestler DC, Guill MF, Moore JH, Hibberd PL, Morrison HG, Sogin ML, Karagas MR, Madan JC (2015) Associations between gut microbial colonization in early life and respiratory outcomes in cystic fibrosis. J Pediatr 167(1):138–147 e131-133
Iannitti T, Palmieri B (2010) Therapeutical use of probiotic formulations in clinical practice. Clin Nutr (Edinburgh, Scotland) 29(6):701–725
Infante Pina D, Redecillas Ferreiro S, Torrent Vernetta A, Segarra Canton O, Maldonado Smith M, Gartner Tizziano L, Hidalgo Albert E (2008) Improvement of intestinal function in cystic fibrosis patients using probiotics. Anales de Pediatria (Barcelona, Spain: 2003) 69(6):501–505
Isolauri E, Salminen S, Ouwehand AC (2004) Microbial-gut interactions in health and disease. Probiotics. Best Pract Res Clin Gastroenterol 18(2):299–313. doi:10.1016/j.bpg.2003.10.006
Jafari SA, Mehdizadeh-Hakkak A, Kianifar HR, Hebrani P, Ahanchian H, Abbasnejad E (2013) Effects of probiotics on quality of life in children with cystic fibrosis; a randomized controlled trial. Iran J Pediatr 23(6):669–674
Jensen H, Dromtorp SM, Axelsson L, Grimmer S (2014) Immunomodulation of monocytes by probiotic and selected lactic acid bacteria. Probiotics Antimicrob Proteins. doi:10.1007/s12602-014-9174-2
Ki Cha B, Mun Jung S, Hwan Choi C, Song ID, Woong Lee H, Joon Kim H, Hyuk J, Kyung Chang S, Kim K, Chung WS, Seo JG (2012) The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Clin Gastroenterol 46(3):220–227
Kim JY, Kwon JH, Ahn SH, Lee SI, Han YS, Choi YO, Lee SY, Ahn KM, Ji GE (2010) Effect of probiotic mix (Bifidobacterium bifidum, Bifidobacterium lactis, Lactobacillus acidophilus) in the primary prevention of eczema: a double-blind, randomized, placebo-controlled trial. Pediatr. Allerg Immunol 21(2 Pt 2):e386–e393. doi:10.1111/j.1399-3038.2009.00958.x
Lasson A, Stotzer PO, Ohman L, Isaksson S, Sapnara M, Strid H (2015) The intra-individual variability of faecal calprotectin: a prospective study in patients with active ulcerative colitis. J Crohn’s Colitis 9(1):26–32
Li F, Sheng XY (2014) Research advances in the role of fecal calprotectin in intestinal development and diseases among children. Zhongguo dang dai er ke za zhi [Chinese Journal of Contemporary Pediatrics] 16(10):1064–1069
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clinical researched) 339:b2700. doi:10.1136/bmj.b2700
Lin YP, Thibodeaux CH, Pena JA, Ferry GD, Versalovic J (2008) Probiotic Lactobacillus reuteri suppress proinflammatory cytokines via c-Jun. Inflamm Bowel Dis 14(8):1068–1083
Macfarlane GT, Blackett KL, Nakayama T, Steed H, Macfarlane S (2009) The gut microbiota in inflammatory bowel disease. Current Pharmaceutical Design BMJ Open 15(13):1528–1536
Navas-Lopez VM, Giron B-AJ, Fernandez-Crehuet F, Serrano Nieto MJ, Vicioso Recio MI, Sierra Salinas C (2013) Intestinal inflammation in cystic fibrosis: lack of effectiveness after treatment with mesalamine and probiotics. In: Ann Nutr Metab. Conference absract: 20th International Congress of Nutrition Granada Spain (63):1694–1695
Ng SC, Hart AL, Kamm MA, Stagg AJ, Knight SC (2009) Mechanisms of action of probiotics: recent advances. Inflamm Bowel Dis 15(2):300–310
Olafsdottir E, Aksnes L, Fluge G, Berstad A (2002) Faecal calprotectin levels in infants with infantile colic, healthy infants, children with inflammatory bowel disease, children with recurrent abdominal pain and healthy children. Acta paediatrica (Oslo, Norway: 1992) 91(1):45–50
Ooi CY (2016) Probioticss and the early life effects on intestinal bacteria and inflammation in children with cystic fibrosis (PEARL-CF): Sydney Children’s Hospitals Network. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=370881. Accessed on 16 Aug 2016
Osborn DA, Sinn JK (2007) Probiotics in infants for prevention of allergic disease and food hypersensitivity. Cochrane Database Syst Rev (4):Cd006475. doi:10.1002/14651858.CD006475.pub2
O’Toole PW, Cooney JC (2008) Probiotic bacteria influence the composition and function of the intestinal microbiota. Interdiscip Perspect Infect Dis 2008:175285. doi:10.1155/2008/175285
Preidis GA, Saulnier DM, Blutt SE, Mistretta TA, Riehle KP, Major AM, Venable SF, Finegold MJ, Petrosino JF, Conner ME, Versalovic J (2012) Probiotics stimulate enterocyte migration and microbial diversity in the neonatal mouse intestine. Gastroenterology 26(5):1960–1969
Rogers GB, Carroll MP, Hoffman LR, Walker AW, Fine DA, Bruce KD (2010) Comparing the microbiota of the cystic fibrosis lung and human gut. Gut Microbes 1(2):85–93. doi:10.4161/gmic.1.2.11350
Sánchez-Calvo JM, Garcia-Castillo M, Lamas A, Rodriguez-Baños M, Máiz L, Suárez L, Baquero F, Canton R, del Campo R (2008) Gut microbiota composition in cystic fibrosis patients: molecular approach and classical culture. J Cyst Fibros 7(Suppl. 2):S50
Scotto G (2011) Globalization and infectious diseases: the past and future. Le infezioni in medicina: rivista periodica di eziologia, epidemiologia, diagnostica, clinica e terapia delle patologie infettive 19(1):56–61
Shoaib A, Dachang W, Xin Y (2015) Determining the role of a probiotic in the restoration of intestinal microbial balance by molecular and cultural techniques. Genet Mol Res: GMR 14(1):1526–1537
Smyth RL, Croft NM, O’Hea U, Marshall TG, Ferguson A (2000) Intestinal inflammation in cystic fibrosis. Arch Dis Child 82(5):394–399
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 283(15):2008–2012
Summerton CB, Longlands MG, Wiener K, Shreeve DR (2002) Faecal calprotectin: a marker of inflammation throughout the intestinal tract. Eur J Gastroenterol Hepatol 14(8):841–845
Tena D, Martinez NM, Losa C, Fernandez C, Medina MJ, Saez-Nieto JA (2013) Acute acalculous cholecystitis complicated with peritonitis caused by lactobacillus plantarum. Diagn Microbiol Infect Dis 76(4):510–512
Tena D, Losa C, Medina MJ, Saez-Nieto JA (2014) Peritonitis caused by Bifidobacterium longum: case report and literature review. Anaerobe 27:27–30
Thomas CM, Versalovic J (2010) Probiotics-host communication: modulation of signaling pathways in the intestine. Gut Microbes 1(3):148–163
Thomas CM, Hong T, van Pijkeren JP, Hemarajata P, Trinh DV, Hu W, Britton RA, Kalkum M, Versalovic J (2012) Histamine derived from probiotic Lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS One 7(2):e31951. doi:10.1371/journal.pone.0031951
Treggiari MM, Rosenfeld M, Mayer-Hamblett N, Retsch-Bogart G, Gibson RL, Williams J, Emerson J, Kronmal RA, Ramsey BW (2009) Early anti-pseudomonal acquisition in young patients with cystic fibrosis: rationale and design of the EPIC clinical trial and observational study. Contemp Clin Trials 30(3):256–268
Varni JW, Burwinkle TM, Seid M (2006) The PedsQL 4.0 as a school population health measure: feasibility, reliability, and validity. Qual Life Res: an International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation 15(2):203–215
Weiss B, Efrati O (2014) The effects of probiotics on sputum bacteria, sputum inflammation and pulmonary infections in patients with cystic fibrosis: a double-blind placebo controlled trial: Sheba Medical Center. https://clinicaltrials.gov/ct2/show/NCT01201434. Accessed on 16 Aug 2016
Weiss B, Bujanover Y, Yahav Y, Vilozni D, Fireman E, Efrati O (2010) Probiotic supplementation affects pulmonary exacerbations in patients with cystic fibrosis: a pilot study. Pediatr Pulmonol 45(6):536–540
Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P (2013). The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
Werlin SL, Benuri-Silbiger I, Kerem E, Adler SN, Goldin E, Zimmerman J, Malka N, Cohen L, Armoni S, Yatzkan-Israelit Y, Bergwerk A, Aviram M, Bentur L, Mussaffi H, Bjarnasson I, Wilschanski M (2010) Evidence of intestinal inflammation in patients with cystic fibrosis. J Pediatr Gastroenterol Nutr 51(3):304–308. doi:10.1097/MPG.0b013e3181d1b013
Wong VW, Won GL, Chim AM, Chu WC, Yeung DK, Li KC, Chan HL (2013) Treatment of nonalcoholic steatohepatitis with probiotics. A proof-of-concept study. Ann Hepato 12(2):256–262
Zein EF, Karaa S, Chemaly A, Saidi I, Daou-Chahine W, Rohban R (2008) Lactobacillus rhamnosus septicemia in a diabetic patient associated with probiotic use: a case report. Ann Biol Clin 66(2):195–198
Author’s contribution
AA is responsible for literature review, analysis, and writing the first draft. HB is responsible for literature review and analysis. SR is responsible for synthesis and checking the first and the final versions of the manuscript. SP is responsible for concept, supervision, interpretation of results, and checking the first and the final versions of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Beat Steinmann
Rights and permissions
About this article
Cite this article
Ananthan, A., Balasubramanian, H., Rao, S. et al. Probiotic supplementation in children with cystic fibrosis—a systematic review. Eur J Pediatr 175, 1255–1266 (2016). https://doi.org/10.1007/s00431-016-2769-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-016-2769-8