Abstract
Successful therapy of vasodilatory shock in adults and children with arginine-vasopressin (AVP) has been reported previously. Data on the use of vasopressin in neonates is limited. This retrospective study reports the effects of AVP-treatment in neonates with catecholamine-resistant systemic vasodilatation after cardiopulmonary bypass. From March 2003 through December 2005, 172 neonates underwent open-heart surgery, 17 developed vasopressor-resistant hypotension and were treated with AVP. Thirteen patients had a stage I palliation of single ventricle, two had a Ross-operation and two had an arterial switch operation. All patients received multiple traditional inotropes and vasopressors prior to administration of AVP. AVP was started at median 0.0001 U·kg−1·min−1 (range 0.00005−0.0002) and titrated up to a maximum of median 0.0003 U·kg−1·min−1 (range 0.0001–0.001). AVP led to a significant increase in blood pressure (from 49+/−8 mmHg to 69+/− 7 mmHg) and the requirement of traditional vasopressors decreased significantly. Noperipheral vasoconstriction or ischemia was observed. Four of 13 patients, all with single ventricle palliation, died. In two patients death occurred due to additional complications 6 days after AVP was discontinued. One patient, who was still on AVP, died 42 hours postoperatively after prolonged hypoxemia not responding to inhaled nitric oxide. One patient arrested on the third postoperative day when AVP was almost weaned. Conclusion: In neonates with vasodilatory shock after cardiopulmonary bypass AVP is a potent agent to increase blood pressure when traditional vasopressors are failing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic vasodilatation and severe hypotension can occur due to septic shock or from systemic inflammatory response after cardiopulmonary bypass. Especially neonates who undergo cardiac surgery with long bypass duration are at high risk to develop post bypass systemic inflammatory response syndrome [3, 20, 21]. The postoperative management of neonates with vasodilatory shock is challenging. Established therapies include administration of volume and traditional vasopressors like dopamine, epinephrine and norepinephrine. Septic and post cardiopulmonary bypass-induced severe vasodilatation is associated with inappropriately low vasopressin plasma levels, so this vasopressin deficiency may contribute to the hypotension of vasodilatory shock [3, 9–11, 20, 21]. Decreased vasopressin responsiveness in septic shock contributes to a state of relative vasopressin insufficiency [11, 13]. Low-dose continuous infusions of vasopressin have been used in several trials in adults with vasodilatory shock secondary to sepsis or open-heart surgery [1, 4, 7, 15, 17]. Arginine-vasopressin (AVP) led to resolution of hypotension and reduction or withdrawal of catecholamine and vasopressor support. It improved urine output without causing hyponatremia in adults and children [4, 7, 14–18, 23]. Data on the use of vasopressin in neonates with vasodilatory shock is limited [14, 18] and indications and dosing have not been established. The purpose of this study was to review our experience with the use of AVP in the treatment of vasodilatory shock after cardiac surgery in 17 neonates with catecholamine-resistant hypotension.
Materials and methods
From March 2003 through December 2005, 172 consecutive term neonates (age range 1–28 days) underwent open-heart surgery for correction or palliation of congenital heart disease. During the study period extracorporeal membrane oxygenation was not available at our institution. Seventeen of 172 neonates developed systemic inflammatory response syndrome with hypotension, which was resistant to traditional vasopressors, and therefore were treated with AVP (Pitressin, Parke Davis). The charts of the 17 neonates were reviewed and data retrospectively analyzed. The study group included 11 males and 6 females with a median age of 6 days (range 3 to 12 days) and a median weight of 3.4 kg (range 2.0–3.9 kg) who received AVP for a median duration of 47 hours (range 9–85 hours). Four patients had correction of biventricular cardiac defects (two arterial switch operations and two Ross operations), 13 patients with single ventricle defects underwent stage one palliation (Table 1). Sixteen patients had ductal dependent lesions and received prostaglandins preoperatively. The median bypass duration was 208 minutes (range 172–380 minutes). Modified ultra filtration was performed in all patients. No long acting vasodilator such as phenoxybenzamine was used. In all patients milrinone was routinely initiated when coming off bypass as a continuous infusion at a dose of 0.5 μg·kg−1·min−1. No milrinone-loading dose was given. All patients received multiple inotropes and vasopressors in high doses, including dopamine (n = 16), dobutamine (n = 1), epinephrine (n = 16) and norepinephrine (n = 17) prior to AVP infusion. The milrinone infusion was stopped in all patients prior to AVP infusion. Two patients received amiodarone and were on temporary pacemaker therapy to control tachycardia (one junctional ectopic tachycardia and one atrial flutter, which reoccurred despite cardioversion). To keep the ionized calcium level above 1.2 μmol/l all patients received a continuous infusion of calcium. Delayed sternal closure was performed in 8/17 patients. None of the patients showed signs of systemic infection, when vasodilatory shock was diagnosed. All patients were mechanically ventilated and an indwelling catheter monitored systemic arterial blood pressure. To exclude hypotension due to myocardial failure echocardiography was performed in all patients prior to initiation of AVP. None of the patients showed myocardial dysfunction.
The criteria for starting AVP was low cardiac output status with hypotension, which was defined as systolic arterial blood pressure below 55 mmHg refractory to fluid replacement and administration of high doses of dopamine, epinephrine and norepinephrine with decreasing urine output or increasing serum lactate levels. Four patients had higher arterial blood pressures (Fig. 1) at initiation of AVP, but were anuric and showed increasing serum lactate levels due to maximum doses of epinephrine and norepinephrine. In these patients AVP was initiated to allow reduction of traditional vasopressors. The average arterial blood pressure over 3 hours prior to initiation of AVP or during time off bypass, if this was less than 3 hours, was calculated and compared to the systolic arterial blood pressure one hour after the effective dose of AVP was reached. Left atrial pressure was continuously monitored via an intracardiac line. The average urine output in millilitres per kilogram per hour was calculated and compared for 6 hours prior to AVP and the first 6 hours after the effective dose was reached. Fluid balance 12 hours prior to and 12 hours after AVP initiation were available in 16/17 patients. Serum sodium concentration, serum lactate levels, creatinine, liver enzymes as well as arterial blood gases were regularly measured. Mixed venous saturations drawn from the pulmonary artery catheter prior to and on AVP were available in 3/4 patients with biventricular circulation. To avoid thrombotic formations in the superior vena cava central venous saturations were not monitored in single ventricle patients. To estimate the level of vasopressor support the inotropic score previously described by Wernovsky et al. [24] was modified. This modified score represents the amount of vasopressors used and was calculated as follows: dopamine+epinephrine×100+norepinephrine×100, all dosages in micrograms per kilogram per minute. The vasopressor score just before initiation of AVP infusion was compared to the vasopressor score 3 and 6 hours after achievement of the effective AVP dose. Data are reported as mean +/− standard deviation (SD). Paired variables were analyzed by the student paired t-test.
Results
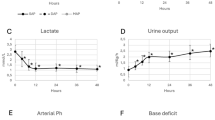
AVP infusion was administered within median 16 hours (range 0–32 hours) in the operating room (n = 1) or in the intensive care unit (n = 16). The AVP infusion rate was titrated up until the systolic arterial pressures were constantly higher than 65 mmHg. This dose was considered to be effective. In 16/17 patients AVP was started at 0.0001 U·kg−1·min−1 (range 0.00005–0.0002) and titrated up to a maximum of 0.0003 U·kg−1·min−1 (range 0.0001–0.001). Due to a calculating mistake one patient received a starting dose of 0.001 U·kg−1·min−1, which subsequently was not titrated up. The effective dose was reached within 1 hour (range 0.5–7 hours). Left atrial pressure before administration of AVP was 11 +/− 2 mmHg and did not change during AVP infusion. Systolic and diastolic arterial blood pressure (SAP) rose significantly in all patients (Fig. 1). In all survivors (n = 13) serum lactate concentration at baseline (3.4 +/− 2.2 mmol/l) was significantly lower than in the four non-survivors (8.3 +/− 1.0 mmol/l) (Fig. 2). Pulmonary arterial pressure, which was directly measured via the pulmonary artery catheter in the four patients with biventricular circulation and indirectly estimated by echocardiography in all single ventricle patients, was not elevated. When the AVP infusion was started, the median arterial oxygen saturation in the single ventricle patients was 67.4%, the median arterial pO2was 34 mmHg, and the median pCO2 was 40 mmHg, which shows that the systemic to pulmonary blood flow was well balanced and systemic arterial hypotension was not caused by unbalanced pulmonary to systemic blood flow.
The requirement of vasopressors estimated with the vasopressor score decreased significantly within 3 h continuing through 6 h (Fig. 3) and a significant reduction in volume requirement was noticed (Fig. 4).
The average urine output 6 h prior to AVP (n = 16) increased from 1.33 +/− 0.82 cm3·kg −1·h−1 to an average of 2.17 +/− 0.09 cm3·kg−1·h−1 over the first 6 h after the effective dose was reached (p = 0.02) (Table 1). Serum sodium concentration, serum creatinine levels and liver enzymes remained unchanged during the first 24 h of AVP infusion. Mixed venous saturation, which was available in 3/4 patients with biventricular circulation, increased from 60% +/− 3% to 80% +/− 2%. In the 13 single ventricle patients arterial oxygen saturation raised from baseline 67.4% +/− 9.3 % to 75.8% +/− 7.2 (p = 0.02). In none of the patients were clinical signs of peripheral ischemia or reduced organ perfusion observed throughout continuous infusion of AVP.
Outcome
Thirteen study patients survived to discharge. Four patients died, all after stage one palliation for single ventricle circulation (Table 1). In two patients death was not associated with AVP infusion, as the patients deceased due to additional complications 6 days after AVP was discontinued. In two patients death occurred during AVP infusion: one patient with HLHS died 42 hours postoperatively after prolonged hypoxemia not responding to inhaled nitric oxide. There was no anatomic problem found at autopsy. Another patient with HLHS arrested on postoperative day 4, when AVP was almost weaned. Resuscitation was not successful, the cause of cardiac arrest remained unknown as autopsy did not reveal further information (Table 1).
Discussion
To avoid end-organ failure due to severe hypotension AVP was added to high dose traditional vasopressors in 17 critically ill neonates with vasodilatory shock after cardiopulmonary bypass. In these patients, who were considered to be near death, administration of AVP resulted in an immediate haemodynamic stabilization. In one case with hypoplastic left heart syndrome AVP enabled coming off bypass and leaving the operating room with stable haemodynamics. There is very little experience on the use of vasopressin in neonates with vasodilatory shock [14, 18]. A case series of eleven children (AVP-doses ranged from 0.0003–0.002 U·kg−1·min−1) reported by Rosenzweig et al. [18] included five neonates, three with refractory hypotension after cardiac surgery and two with cardiogenic shock. As the two patients who had poor left ventricular function died despite a transient improvement of blood pressure, vasopressin is probably less appropriate for neonates with ventricular dysfunction. Therefore we took care not to use AVP in patients with cardiac dysfunction. Liedel et al. [14] reported vasopressin therapy for refractory hypotension secondary to sepsis in five children (two neonates) with severe underlying diseases, where AVP doses of 0.001–0.007 U·kg−1·min−1 were used. No studies have been done to determine the appropriate dose of vasopressin in either children or neonates. In adult studies and case reports the doses have ranged from 0.004 to 0.1 U·min−1. Extrapolated from these adult data the dose for children and neonates should range between 0.00006 and 0.001 U·kg−1·min−1. As the appropriate dose of vasopressin in neonates is not known, we started the continuous infusion of vasopressin at the low dose of 0.0001 U·kg−1·min−1 and to avoid side effects we did not exceed a maximum dose of 0.001 U·kg−1·min−1.
The splanchnic vascular effect of vasopressin is a matter of debate [2]. Experimental animal studies reported neutral or even beneficial effects when low doses of AVP were used in endotoxemic shock [6, 8]. On the other hand, van Haren et al. demonstrated that vasopressin leads to gastro-intestinal hypoperfusion in norepinephrine-dependent patients with septic shock [22]. In our patients one major concern was not to induce excessive splanchnic vasoconstriction in neonates with a history of preoperatively reduced splanchnic perfusion due to diastolic run-off into the pulmonary artery caused by persistent arterial duct. None of our patients developed signs of necrotizing enterocolitis due to mesenteric ischemia like distension of the intestinal loops in abdominal radiography or air in the portal system on ultrasound examination. Subsequently, enteral nutrition was well tolerated in all survivors. There was no cutaneous ischemia observed in our patients. Liedel et al. [14] observed peripheral vasoconstriction without skin necrosis at a dose exceeding 0.007 U·kg−1 min−1. Besides being a potent vasoconstrictor, AVP has been reported to cause endothelium-dependent pulmonary vasodilation [5, 19]. This effect remains controversial as Leather et al. demonstrated that arginine-vasopressin caused pulmonary vascular constriction and adversely affected right ventricular function in an experimental model [12]. For that reason AVP may not be the drug of choice when the right ventricle is failing and pulmonary hypertension is present.
As a significant difference in baseline lactate levels was found between survivors and non-survivors it could be speculated not to leave patients in hypotension and low cardiac output status too long and to initiate AVP earlier, when the condition of the patient is deteriorating. With increasing experience at our institution the clinicians in charge have started the AVP infusion earlier.
Conclusion
In selected neonates with maintained cardiac function but vasodilatory shock refractory to traditional vasopressors after cardiac surgery vasopressin seems to be a potent agent to increase blood pressure and avoid end-organ failure. As mixed-venous oxygen saturation rose in patients with biventricular circulation an increase in cardiac output was suspected. One of the major findings in this study is that AVP did not cause any ischemic side effects at doses up to 0.001 U·kg−1·min−1. In neonates with vasodilatory shock AVP can be considered as a treatment option before initiating ECMO support. Since indications, dosing and duration of intravenous vasopressin therapy have not been established, its cautious use in neonates is recommended.
Limitations
We are aware of the fact that this study has some limitations. First of all, it was not a prospective study with a strict protocol. The decision to initiate AVP, as well as the rate of increase and the maximum dose was made by the clinician in charge following the above-mentioned criteria. Vasopressin levels prior to treatment and after the establishment of an appropriate dose of AVP were not measured.
Cardiac output and systemic vascular resistance were not measured during AVP infusion, as we do not use Swan-Ganz catheters in neonates. Nonetheless, we are convinced that the observed effects of AVP therapy were striking and did significantly impact outcome in these critically ill patients.
References
Argenziano M, Chen JM, Choudhri AF, Cullinane S, Garfein E, Weinberg AD, Smith CR, Rose EA, Landry DW, Oz MC (1998) Management of vasodilatory shock after cardiac surgery: identification of predisposing factors and use of a novel pressor agent. J Thorac Cardiovasc Surg 116:973–980
Asfar P, Radermacher P, Hauser B (2006) Vasopressin and splanchnic blood flow: vasoconstriction does not equal vasoconstriction in every organ. Intensive Care Med 32:21–23
Ashraf SS, Tian Y, Zacharrias S, Cowan D, Martin P, Watterson K (1997) Effects of cardiopulmonary bypass on neonatal and paediatric inflammatory profiles. Eur J Cardiothorac Surg 12:862–868
Booth JV, Schinderle D, Welsby IJ (2002) Pro: vasopressin is the vasoconstrictor of choice after cardiopulmonary bypass. J Cardiothorac Vasc Anesth 16:773–775
Evora PR, Pearson PJ, Schaff HV (1993) Arginine vasopressin induces endothelium-dependent vasodilatation of the pulmonary artery. V1- receptor-mediated production of nitric oxide. Chest 103:1241–1245
Guszman JA, Rosado AE, Kruse JA (2003) Vasopressin vs. norepinephrine in endotoxic shock: systemic, renal and splanchnic hemodynamic and oxygen transport effects. J Appl Physiol 95:803–809
Holmes CL, Walley KR, Chittock DR, Lehmann T, Russell JA (2001) The effects of vasopressin on hemodynamics and renal function in severe septic shock: a case series. Intensive Care Med 27:1416–1421
Knotzer H, Maier S, Dunser MW, Hasibeder WR, Hausdorfer H, Brandner J, Torgersen C, Ulmer H, Friesenecker B, Iannetti C, Pajk W (2006) Arginine vasopressin does not alter mucosal tissue oxygen tension and oxygen supply in an acute endotoxemic pig model. Intensive Care Med 32:170–174
Landry DW, Levin HR, Gallant EM, Ashton RC, Seo S, D’Alessandro D, Oz MC, Oliver JA (1997) Vasopressin deficiency contributes to the vasodilatation of septic shock. Circulation 95:1122–1125
Landry DW, Oliver JA (2001) The pathogenesis of vasodilatory shock. N Engl J Med 345:588–595
Landry DW, Oliver JA (2006) Vasopressin and relativity: on the matter of deficiency and sensitivity. Crit Care Med 34:1275–1276
Leather HA, Segers P, Berends N, Vandermeersch E, Wouters PF (2002) Effects of vasopressin on right ventricular function in an experimental model of acute pulmonary hypertension. Crit Care Med 30:2548–2552
Leone M, Boyle WA (2006) Decreased vasopressin responsiveness in vasodilatory septic shock-like conditions. Crit Care Med 34:1126–1130
Liedel JL, Meadow W, Nachman J, Koogler T, Kahana MD (2002) Use of vasopressin in refractory hypotension in children with vasodilatory shock: five cases and a review of the literature. Pediatr Crit Care Med 3:15–18
Luckner G, Dunser MW, Jochberger S, Mayr VD, Wenzel V, Ulmer H, Schmid S, Knotzer H, Pajk W, Hasibeder W, Mayr AJ, Friesenecker B (2005) Arginine vasopressin in 316 patients with advanced vasodilatpry shock. Crit Care Med 33:2713–2714
Masutani S, Senzaki H, Ishido H, Taketazu M, Matsunaga T, Kobayashi T, Sasaki N, Asano H, Kyo S, Yokote Y (2005) Vasopressin in the treatment of vasodilatory shock in children. Pediatr Int 47:132–136
Morales DLS, Gregg D, Helman DN, Williams MR, Naka Y, Landry DW, Oz MC (2000) Arginine vasopressin in the treatment of 50 patients with postcardiotomy vasodilatory shock. Ann Thorac Surgery 69:102–106
Rosenzweig EB, Starc TJ, Chen JM, Cullinane S, Timchak DM, Gersony WM, Landry DW, Galantowicz ME (1999) Intravenous arginine-vasopressin in children with vasodilatory shock after cardiac surgery. Circulation 100:182–186
Sai Y, Okamura T, Amakata Y, Toda N (1995) Comparison of responses of canine pulmonary artery and vein to angiotensin II, bradykinin and vasopressin. Eur J Pharmacol 282:235–241
Seghaye MC, Grabitz RG, Duchateau J, Busse S, Dabritz S, Koch D, Alzen G, Hornchen H, Messmer BJ, Von Bernuth G (1996) Inflammatory reaction and capillary leak syndrome related to cardiopulmonary bypass in neonates undergoing cardiac operations. J Thorac Cardiovasc Surg 112:687–697
Stiller B, Sonntag J, Dahnert I, Alexi- Meskishvilli V, Hetzer R, Fischer T, Lange PE (2001) Capillary leak syndrome in children who undergo cardiopulmonary bypass: clinical outcome in comparison with complement activation and C1 inhibitor. Intensive Care Med 27:193–200
van Haren FMP, Rozendaal FW, van der Hoeven JG (2003) The effect of vasopressin on gastric perfusion in catecholamine-dependent patients in septic shock. Chest 124:2256–2260
Vasudevan A, Lodha R, Kaba SK (2005) Vasopressin infusion in children with catecholamine-resistant septic shock. Acta Paediatr 94:380–383
Wernovsky G, Wypij D, Jonas RA, Mayer JE Jr, Hanley FL, Hickey PR, Walsh AZ, Chang AC, Castaneda AR, Newburger JW, Wessel DL (1995) Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants: a comparison of low-flow cardiopulmonary bypass and circulatory arrest. Circulation 92:2226–2235
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lechner, E., Hofer, A., Mair, R. et al. Arginine-vasopressin in neonates with vasodilatory shock after cardiopulmonary bypass. Eur J Pediatr 166, 1221–1227 (2007). https://doi.org/10.1007/s00431-006-0400-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-006-0400-0