Abstract
In generalized arterial calcification of infancy (OMIM no. 208000), calcification of the media and proliferation of the intima lead to arterial stenoses. Most affected patients present with untreatable arterial hypertension and die within the first months of life. The disease has recently been linked to mutations in ENPP1. We report two siblings with prolonged survival, both of whom carry the compound heterozygous ENPP1 mutations c.913C>A and c.1164+2T>A. In both siblings, spontaneous regression of arterial calcifications occurred, and antihypertensive treatment could be tapered off gradually. In some patients, the natural course of GACI may be more favourable than previously assumed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Generalized arterial calcification of infancy (GACI) (OMIM no. 208000) is characterized by subendothelial fibroproliferative changes in large and medium-sized arteries and calcification of the media, resulting in stenosis and reduced vascular elasticity [1, 7]. Clinical presentation is variable, and the diagnosis is usually made postnatally. The patients present with heart failure, hypertension or a failure to thrive. Frequently, GACI is diagnosed only at autopsy [1]. Coronary artery involvement can lead to death within the first 6 months of life [6, 15] and, in general, the prognosis is considered to be poor, although occasional long-term survival has been reported [4, 14]. The disease has recently been found to be caused by mutations in the ENPP1 gene [13] which encodes for ecto-nucleotide pyrophosphatase/phosphodiesterase 1 (E-NPP1), an enzyme that generates inorganic pyrophosphate, a potent inhibitor of hydroxyapatite crystal formation [11,12].
We describe two sisters with GACI and prolonged survival. They presented with variable phenotypes, but were proven to carry the same compound heterozygous mutations in ENPP1. Their ENPP1 genotype has already been characterized as part of a previous study (family 3, Table 1 in reference [10]).
Methods
Mutation detection
DNA from the patients was extracted from EDTA blood samples after informed consent was obtained from the parents. We used a set of 26 primer pairs to amplify all ENPP1 exons together with their flanking sequences and about 700 bp of the promoter region. The PCR products were directly sequenced using an ABI PRISM 3,730 DNA Analyzer and a BigDye Terminator v. 1.1 Cycle Sequencing kit according to the manufacturer’s protocol (Applied Biosystems, Foster City, Calif.). Primer sequences are available on request [13].
Case reports
Case 1
The female infant was delivered at 31 weeks of gestation by caesarean section because of acute fetal distress and fetal hydrops. She developed severe systemic hypertension (systolic blood pressure >100–110 mmHg, with peaks of >140 mmHg) refractory to conventional medical treatment, responding only to continuous prostaglandin (PGE1) infusions. The detailed neonatal history is described elsewhere [2]. In addition to arterial calcification, an X-ray of the limbs performed in the first week of life showed peri-articular calcification (Fig. 1). Plasma renin levels were elevated, suggesting renal arterial stenosis [2]. Repeated ultrasound studies did not reveal cerebral, renal or hepatic calcifications. During the second year of life, she developed spastic quadriplegia, presumably as a long-term result of a cardiac arrest that required resuscitation and mechanical ventilation in the neonatal period. Surprisingly, calcifications were no longer visible during the following months on repeated X-rays of the pelvis and lower extremities.
At the age of 2 years, she developed hypophosphatemia (P: 0.93 mmol/l; normal values: 1.25–2.10 mmol/l), with a decreased renal phosphate reabsorption (TmP/GFR) of 0.85 mmol/l (calculated using the formula TmP/GFR=SP–(UP×SCr)/UCr; a normal value for this age is 1.15–2.44 mmol/l [9]). Despite severe hypophosphatemia, no radiological signs of rickets developed. While hypophosphatemia persisted with values as low as 0.80 mmol/l, alkaline phosphatase, parathyroid hormone (PTH) levels and Vitamin D metabolites were always within the normal range (Table 1). Hyperaminoaciduria, metabolic acidosis or significant glucosuria were not noted; urinary β2-microglobulin and urinary calcium/creatinine ratios were within the normal range, thereby ruling out a more complex renal tubular dysfunction. The glomerular filtration rate was also normal on several occasions (Table 1).
Sequence analysis of the ENPP1 gene revealed the missense mutation c.913C>A in exon 8 on one allele leading to the amino acid change Pro305Thre and the mutation c.1164+2T>A in intron 11 on the other allele, which leads to a frame shift [10].
Currently, at the age of 11 years, hypertension is well controlled by 2.5 mg Enalapril in the morning and 1.25 mg at night. Echocardiography demonstrates diffuse left ventricular hypertrophy and mild right ventricular hypertrophy, without any signs of valvular calcification.
Case 2
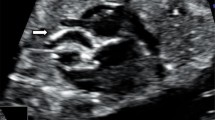
The second child of the family was delivered by cesarean section 8 years later at 37 weeks of gestation. During pregnancy, the fetus had been followed up closely by frequent ultrasound examinations. At 14 weeks, mitralic echogenic foci (Fig. 2) were visualized in the left ventricle. No other signs of calcifications were identified in the heart or great vessels. The findings were confirmed at 2 weeks of gestation. The parents were counselled about the risk of recurrence of the disease, and at 26 weeks, sonography revealed increased echogenicity and thickening of the wall of the abdominal aorta proximal to the iliac bifurcation (Fig. 3). Color and pulsed Doppler studies showed mild stenosis of the aorta without a significant alteration of flow velocities. Follow-up studies at 36 weeks showed progressive disease of the abdominal aorta and hyperechogenicity of the aortic and pulmonary valve annulus with normal intracardiac flows. The infant was born 1 week later with a birth weight of 2,200 g (<10th percentile), a head circumference of 31 cm (<10th percentile) and a length of 47.5 cm (<10th percentile). Apgar scores were 8 and 9 at 1 and 5 min, respectively. She was admitted to the Neonatal Intensive Care Unit for intensive monitoring but had no circulatory problems. Echocardiography confirmed the prenatal findings. A chest/abdomen X-ray was performed on day 7 and showed string-like calcifications of the abdominal aorta extending from T12 to L2 (Fig. 4). During the first months of life she developed arterial hypertension that was well controlled by Captopril 2 mg three times a day. At 3 months of age, echocardiography showed hyperthrophy of the interventricular septum and the posterior wall of the left ventricle. Calcifications were noted in the aortic annulus (Fig. 5). At 8 months of age, the left ventricular wall thickness had increased slightly, while the aortic calcifications had disappeared. An X-ray of the pelvis and the lower extremities did not show peri-articular calcifications or calcifications of the iliac or femoral arteries.
At the age of 3 years, the patient was in good general health and growing along the 10th percentile for weight and height. Psychomotor development was normal. Echocardiography revealed that the vascular calcifications were no longer visible, and the thickness of the left ventricular wall was normal. Arterial hypertension persisted, but was well controlled by 6.25 mg Captopril three times a day. No alterations in calcium/phosphorus metabolism were detected. Mutational analysis of ENPP1 revealed she had the same genotype as her older sister.
Discussion
Generalized arterial calcification of infancy (GACI) is associated with the deposition of hydroxyapatite crystals in the media of the large- and medium-sized muscular arteries and in peri-articular soft tissue [11, 12]. The disease is inherited in an autosomal recessive manner so that it is not uncommon for siblings to be affected [3, 13, 14]. In the past, GACI was also referred to as “Idiopathic Infantile Arterial Calcification (IIAC)” [11, 16]. Recently, mutations in ENPP1 encoding ecto-nucleotide pyrophosphatase/ phosphodiesterase 1 (E-NPP1) have been identified as the underlying genetic defect in most of the cases studied [10]. We therefore suggest re-naming the disease “Generalized Arterial Calcification of Infancy (GACI)”.
In previously reported cases, affected siblings had the same disease phenotype, and most died in early infancy. The clinical expression of the disease in our two sisters is different from previous cases in two respects: (1) both sisters survived the critical period of infancy; (2) the sisters differed from each other in the severity of the phenotype even though they carried the same ENPP1 genotype.
Patient 1 was delivered prematurely and presented with severe circulatory problems in the neonatal period. In the first days of life, she had arterial hypertension, which only responded to PGE1 infusions. She had extensive arterial and peri-articular calcifications and multiple vascular stenoses in infancy. Interestingly, the calcifications regressed spontaneously during childhood and were no longer visible at the age of 8 years. The child, now 11 years old, is one of the longest known survivors in GACI [4].
Spontaneous regression of calcifications in GACI has been reported previously [4, 14]. Apparently, GACI patients who survive the first few months of life have a better prognosis. Our patient has developed hypophosphatemia due to renal phosphate wasting during childhood. In a previous study, severe hypophosphatemic rickets were observed in the father of a GACI patient [13], and hypophosphatemia has been discussed with respect to a compensation of the phenotype in GACI patients [13].
Patient 2 was proven to carry the same mutations in ENPP1 as her affected sister. She presented with a less severe GACI phenotype and did not develop hypophosphatemia. This variant may be related to compensatory mechanisms which start earlier, presumably even in utero, to prevent vascular calcifications. These mechanisms, which certainly account for the difficulty of genotype-phenotype correlations in GACI patients, will have to be defined in future studies.
Bisphosphonate treatment has been proposed by several authors as a means to reduce arterial calcifications in GACI patients [6, 15–18]. The use of bisphosphonates makes sense theoretically, since E-NPP1, the enzyme generating extracellular pyrophosphate (PPi), is deficient in most cases [10], and low extracellular levels of PPi are a diagnostic hallmark of the disease [11]. PPi is known to inhibit hydroxapatite formation in vitro. It is thus tempting to supplement the GACI patient with bisphophonates, which are synthetic PPi analogues. First-generation bisphophonates, such as disodium etidronate, inhibit osteoclast activity and, in high concentrations, also inhibit mineralization. In theory, etidronate may therefore promote the dissolution of vascular calcification, decrease vascular rigidity and support vasodilation.
Nevertheless, the exact dose of bisphosphonates for GACI treatment is not known, and data reported in the literature vary from 5 mg/kg per day of disodium etidronate over a period of a few weeks to 15–35 mg/kg per day over 18 months [11]. In the two siblings reported by Stuart et al. 1990 [15], extensive arterial calcifications developed and both infants died within the first 3 months of life, despite treatment with 20 mg of disodium etidronate three times a day. In other cases, spontaneous resolution of the calcifications has been described [4, 14], and this may in fact reflect the natural course of the disease if the patient survives a critical period. Moreover, in two studies, bisphosphonates seemed to have no effect on the degree of vascular stenoses in GACI [11, 16]. At this point, we feel that bisphosphonate treatment could be considered as ultima ratio in infants with GACI who do not respond to conventional antihypertensive therapy. Since arterial hypertension in our first patient responded well to PGE1 infusions in the neonatal period [2], we did not use bisphosphonates.
Interestingly, as in our cases, studies on two other infants with GACI also found that hypertension persisted even after the resolution of arterial calcifications [11, 16], most likely reflecting persistent renal arterial stenoses and generalized arterial stiffness. In the light of these findings, it seems likely that deficient ENPP-1-mediated pyrophosphate generation is not the only pathogenic principle causing arterial stenoses in GACI.
Prenatal sonography established the diagnosis in the second patient at a very early stage. To date, in GACI, the exact time of onset of vascular calcifications in the fetus is not known. The occurrence of mitralic echogenic foci in the 14th week of gestation visualized in our patient by prenatal ultrasound suggests that the calcification process starts as early as during the first trimester of pregnancy. In the past, families with previously affected siblings underwent prenatal ultrasound as the method of choice to determine the disease state of the next infant [3, 8]. Currently, however, families with known ENPP1 mutations of previously affected children are able to undergo prenatal diagnostic testing in the subsequent pregnancy. This will enable the physician to establish a definite diagnosis in a genetic counseling setting even before signs of vascular calcification become visible on a fetal ultrasound scan. In that respect, since termination of pregnancy might be considered in cases with fetal GACI, we hope that our case reports add to the clinical spectrum of the disease by demonstrating that the phenotype can vary to a great extent even within one family.
In summary, we have presented two sisters with generalized arterial calcification of infancy with prolonged survival. Both carry the same mutations in ENPP1 but differ in the severity of the disease. During follow-up, hypophosphatemia, which developed in one of the sisters affected, was the most striking phenotypic difference between the two. In both of the affected siblings, spontaneous regression of arterial calcifications occurred, which should encourage active treatment in any infant with GACI, with the aim of overcoming the immediate postnatal period.
We conclude that GACI is a phenotypically variable disease that is influenced by compensatory mechanisms yet to be identified. Once the severe circulatory problems of infancy are overcome, disease prognosis may be better than previously thought.
Abbreviations
- GACI:
-
Generalized arterial calcification of infancy
- E-NPP1:
-
Ecto-Nucleotide Pyrophosphatase/phosphodiesterase 1
- PPi :
-
Inorganic Pyrophosphate
- TmP/GFR:
-
Maximal tubular phosphate reabsorption per glomerular filtration rate
References
Bird T (1974) Idiopathic arterial calcification in infancy. Arch Dis Child 49:82–89
Ciana G, Colonna F, Forleo V, Brizzi F, Benettoni A, de Vonderweid U (1997) Idiopathic arterial calcification of infancy: effectiveness of prostaglandin infusion for treatment of secondary hypertension refractory to conventional therapy: a case report. Pediatr Cardiol 18:67–71
Eronen M, Pohjavuori M, Heikkila P (2001) Fatal outcome of two siblings with idiopathic arterial calcification diagnosed in utero. Pediatr Cardiol 22:167–169
Marrott PK, Newcombe KD, Becroft DMO, Friedlander DH (1984) Idiopathic infantile arterial calcification with survival to adult life. Pediatr Cardiol 5:119–122
Matos V, van Melle G, Boulat O, Markert M, Bachmann C, Guignard JP (1996) Urinary phosphate/creatinine ratio, calcium/creatinine, and magnesium/creatinine ratios in a healthy pediatric population. J Pediatr 131:252–257
Meradji M, de Villeneuve VH, Huber J, de Bruijn WC, Pearse RG (1978) Idiopathic arterial calcification in siblings: radiologic diagnosis and successful treatment. J Pediatr 92:401–405
Moran JJ (1975) Idiopathic arterial calcification of infancy, a clinicopathological study. Pathol Annu 10: 393–417
Nagar AM, Hanchate V, Tandon A, Thakkar H, Chauybal NG (2003) Antenatal detection of idiopathic arterial calcification with hydrops fetalis. J Ultrasound Med 22:653–659
Payne RB (1998) Renal tubular reabsorption of phosphate (TmP/GFR): indications and interpretations. Ann Clin Biochem 35:201–206
Ruf N, Uhlenberg B, Terkeltaub R, Nürnberg P, Rutsch F (2005) The mutational spectrum of ENPP1 as arising after the analysis of 23 unrelated patients with generalized arterial calcification of infancy. Hum Mutat 25:98
Rutsch F, Schauerte P, Kalhoff H, Petrarulo M, August C, Diekmann L (2000) Low levels of urinary inorganic pyrophosphate indicating systemic pyrophosphate deficiency in a boy with idiopathic infantile arterial calcification. Acta Paediatr 89:1265–1269
Rutsch F, Vaingankar S, Johnson K, Goldfine I, Maddux B, Schauerte P, Kalhoff H, Sano K, Boisvert WA, Superti-Furga A, Terkeltaub R (2001) PC1-nucleoside triphosphate pyrophosphohydrolase deficiency in idiopathic arterial calcification. Am J Pathol 158:543–554
Rutsch F, Ruf N, Vaingankar S, Toliat MR, Suk A, Höhne W, Schauer G, Lehmann M, Roscioli T, Schnabel D, Epplen JT, Knisely A, Superti-Furga A, McGill JH, Filippone M, Sinaiko AR, Vallance H, Hinrichs B, Smith W, Ferre M, Terkeltaub R, Nürnberg P (2003) Mutations in ENPP1 are associated with ‘idiopathic’ infantile arterial calcification. Nat Genet 34:379–381
Sholler GF, Bale PM, Celermajer JM, Kozlowski K (1984) Generalized arterial calcification of infancy: three case reports, including spontaneous regression with long term survival. J Pediatr 105:257–260
Stuart G, Wren C, Bain H (1990) Idiopathic arterial calcification in two siblings: failure of treatment with diphosphonate. Br Heart J 64:156–159
Thiaville A, Smets A, Clercx A, Perlmutter N (1994) Idiopathic infantile arterial calcification: a surviving patient with renal artery stenosis. Pediatr Radiol 24:506–508
Van Dyck M, Proesmans W, Van Hollebeke E, Marchal G, Moerman P (1989) Idiopathic infantile arterial calcification with cardiac, renal and central nervous involvement. Eur J Pediatr 148:374–377
Van Reempts PJ, Boven KJ, Spitaels SE, Roodhooft AM, Vercruyssen EL, Van Acker KJ (1991) Idiopathic arterial calcification of infancy. Calcif Tissue Int 48:1–6
Acknowledgement
F.R. is supported by a grant from the Deutsche Forschungsgemeinschaft.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ciana, G., Trappan, A., Bembi, B. et al. Generalized arterial calcification of infancy: two siblings with prolonged survival. Eur J Pediatr 165, 258–263 (2006). https://doi.org/10.1007/s00431-005-0035-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-005-0035-6