Abstract
The human pathogen Helicobacter pylori expresses two dominant adhesins; the Lewis b blood group antigen binding adhesin, BabA, and the sialic acid-binding adhesin, SabA. These adhesins recognize specific carbohydrate moieties of the gastric epithelium, i.e. the Lewis b antigen, Leb, and the sialyl-Lewis x antigen, sLex, respectively, which promote infection and inflammatory processes in the gastroduodenal tract. To assess the contribution of each of BabA, SabA and the neutrophil activating protein (HP-NAP) in a local inflammation, we investigated the traits of H. pylori mutants in their capacity to interact with and stimulate human neutrophils. We thence found that the SabA adhesin was not only the key inducer of oxidative metabolism (Unemo et al. J Biol Chem 280:15390–15397, 2005), but also essential in phagocytosis induction, as evaluated by flow cytometry, fluorescence microscopy and luminol-enhanced chemiluminescence. The napA deletion resulted in enhanced generation of reactive oxygen species and impaired adherence to the host cells. In conclusion, the SabA adhesin stimulates human neutrophils through selectin-mimicry. Interestingly, HP-NAP modulates the oxidative burst, which could tune the impact of the H. pylori infection for establishment of balanced and chronic inflammation of the gastric mucosa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infections by Helicobacter pylori are well established as the major cause of chronic gastritis, peptic ulcer disease, gastric lymphoma, and gastric carcinoma [21, 23, 36, 54]. The rate of infection in different populations ranges from 25 to 90%. The highest infection rates, i.e. 50% and above have predominantly been shown in studies carried out in countries belonging to the developing world [41, 44, 69]. In industrialized countries the infections often result in a symptom-free form of gastritis (type B gastritis). Among these carriers 10–20% develop more severe diseases, such as gastric MALT lymphoma and gastric adenocarcinoma [10, 21, 31, 64]. Although, H. pylori is one of the most common infectious agents in humans seen worldwide, the pathogenic mechanisms that promote gastric disease to the host are still not fully understood.
Helicobacter pylori is commonly considered to be a non-invasive enteropathogenic bacterium. Nonetheless, intracellular H. pylori have on several occasions been spotted in cultured epithelial cells, animal models and human gastric biopsy specimens [2, 5, 17, 35, 37, 49, 52, 57, 74].
Helicobacter pylori infections are characteristically associated with a dense infiltration of mainly neutrophils into the epithelial surface layer [15, 18, 60], which correlates with the severity of mucosal damage, and subsequent development of gastroduodenal diseases [72, 73]. The NADPH oxidase in neutrophils is the major generator of oxidative metabolites, which promotes local tissue damage. The NADPH-derived oxidants could, however, also play a protective role. Keenan et al. [32] recently found that there was a dramatic increase in tissue damage and neutrophil infiltration in chronic granulomatous disease mice with a targeted disruption of the gp91phox subunit of the NADPH oxidase.
A direct physical interaction in vivo in between H. pylori and leukocytes has been disputed [e.g., 17, 49, 61, 76]. The exact mechanisms for recruitment of neutrophils to the H. pylori-infected gastric mucosa and the interplay between bacteria, neutrophils and mucosal chemokine responses remain to be delineated [4, 11]. However, the mucosal mediated response has been linked to the cytokine C-X-C subfamily, and especially to interleukin 8, IL-8 [11, 58]. Tight binding of H. pylori to the epithelium triggers the secretion of IL-8, which in its extension elicits chemotaxis and activation of neutrophils [12, 28, 50, 59].
There are furthermore strong implications that bacteria-host cell surface carbohydrate molecule interactions play a crucial role in activation of neutrophils [3, 31, 66, 7]. Interestingly, bacterial adhesion mediated by BabA adhesin to Leb in the human gastric epithelium has been shown to give strong IL-8 release, as evaluated by the in vitro explant culture technique [53].
Recently, we also reported that sialylated carbohydrates are up-regulated in inflamed gastric epithelium, and that sialyl-Lewis x (sLex)-gangliosides are utilized by H. pylori for adhesion and tight membrane apposition [43]. Thus, BabA and SabA adhesins of H. pylori likely act synergistically with chemical gradients of bicarbonate/CO2 or urea/ammonium [56] to furnish binding and bacterial orientation in the gastric mucosa.
The present study was undertaken to assess the role of the SabA adhesin binding to sialyl-Lewisx glycoconjugates on human neutrophils [62, 63], in bacterial adhesion and initiation of an inflammatory response. Previous reports suggest that sLex on leukocytes facilitates binding of H. pylori [46] and possibly also a modulation of a prolonged and sustained inflammation [31]. HP-NAP is a dodecameric protein formed from 17 kDa monomers [70], which affects neutrophil recruitment and is considered to be highly involved in H. pylori-associated disease processes [16, 75]. Purified HP-NAP protein has been shown to bind to sialylated and sulphated glycolipids and glycans [68].
By the use of babA, sabA and napA deletion mutants of H. pylori we have been able to address the role of these traits in the interaction with human neutrophils. Our results unequivocally strengthen, that the SabA adhesin is the key molecule in the activation of human neutrophils. The triggering effect is tuned by HP-NAP, which might balance the inflammatory response. Giving rise to a persistent bacteria infection, ensuing a smouldering chronic inflammation of the gastric mucosa.
Materials and methods
Reagents
Polymorphprep™ and Lymphoprep™ were obtained from Axis-Shield PoC AS (Oslo, Norway). Horseradish peroxidase (HRP) was purchased from Boeringer-Mannheim (Mannheim, Germany). Superoxide dismutase (SOD) and catalase were obtained from Roche Diagnostics (Mannheim, Germany). Unless otherwise noted, other reagents and chemicals were purchased from Sigma-Aldrich (St Louis, MO, USA).
Bacterial strains and growth conditions
The bacterial mutant strains of H. pylori used in this study were based on the reference strain J99 [1], belonging to the special group of disease-promoting strains, the so-called “type I strains”. They harbour the virulence-associated genes: vacuolating cytotoxin activity (vacA), and the cytotoxin-associated gene A (cagA). Four mutant strains were constructed from this strain, previously described in detail [43, 71]. Briefly, these four J99 derivatives have the following characteristics: the SabA adhesin knockout mutant, denoted J99sabA; the BabA adhesin mutant, denoted J99babA; the double-mutant in SabA and BabA adhesins, here denoted J99sabAbabA; and a mutant in the neutrophil-activating protein of H. pylori (HP-NAP), here abbreviated J99napA. In the construction of the J99napA::kan mutant, the napA gene was amplified by PCR, using the napA1F (forward) and napA1R (reverse) primers. The PCR fragment was cloned into the pBluescriptSK+/− EcoRV site (Stratagene, La Jolla, CA, USA).
The resulting plasmid was linearized with primers napA2F (forward) and napA2R (reverse), ligated with the kanamycin resistance (KanR) cassette from pILL600 [65], and then used to transform the J99 strain. For transformation, strains were grown for 24 h on agar plates before addition of 2 μg of plasmid DNA. After transformation they were cultivated on non-selective plates for 48 h to allow unrestrictive growth, and then transferred to kanamycin-containing plates.
The transformants were analyzed by PCR using primers napA3F and napA4R, which verified that the KanR cassette was inserted into the napA gene. Western blot analysis of napA mutants using anti-NapA antibodies show that the mutant strains were devoid of HP-NAP expression [71].
The H. pylori strains were maintained as frozen glycerol stocks and were cultured on GC II agar base plates (Becton-Dickinson; Mountain View, CA, USA), supplemented with: 2.5% (w/v) Lab M agar no. 2 (Topley House; Bury, UK), 7% (v/v) horse blood citrate, 8% (v/v) heat-inactivated horse serum, 0.03% (v/v) Iso-Vitalex, 12 μg/ml Vancomycin, 2 μg/ml Amphotericin B, 20 μg/ml nalidixic acid, and a final concentration of HCl to 2.5 mM.
For the growth of the J99sabA and J99babA strains, the plates were also supplemented with 20 μg/ml chloramphenicol. For the J99napA-mutant strain the plates were supplemented with 25 μg/ml kanamycin, and for the J99sabAbabA-mutant the plates were supplemented with both 20 μg/ml chloramphenicol and 25 μg/ml kanamycin, to secure the selective growth of the specific mutants. The bacteria were incubated at 37°C, 5% O2, 85% N2 and 10% CO2 for 2 days prior to use.
Preparation of human neutrophils
Peripheral venous blood was drawn from non-medicated healthy blood donors at Linköping University Hospital, and anti-coagulated with 5 U/ml heparin. Neutrophils were separated and isolated as previously described elsewhere [7, 22]. In brief, the blood was layered over a density gradient consisting of Lymphoprep™ layered over Polymorphprep™, and centrifuged at 480g for 40 min at room temperature. The band rich in polymorphonuclear cells was collected, and contaminating erythrocytes were removed by hypotonic lysis. Neutrophils of about 95% purity were resuspended in Krebs-Ringer phosphate buffer containing 120 mM NaCl, 1.2 mM MgSO4 · 7H2O, 1.7 mM KH2PO4, 8.3 mM Na2HPO4 · 2 H2O, 1 mM CaCl2, 4.9 mM KCl and 10 mM glucose (KRG, pH 7.3). Total cell count was calculated in a Channelyzer 256 (Beckman Coulter Inc., Fullerton, CA, USA). Viability of the cells was >98% as assessed by Trypan blue exclusion. The cells were kept on ice until use.
FITC-labeling of H. pylori
The different mutants and the parent strain of H. pylori J99 were grown as described above, and harvested from the agar plate and suspended in PBS pH 7.3 supplemented with 0.05% Tween 20. After centrifugation (3,500g, 4 min) and resuspension three times in PBS pH 7.3 with 0.05% Tween 20, the optical density (OD) was calibrated to 2.0 at a wavelength of 650 nm using PBS, pH 7.3. This OD corresponded to a concentration of bacteria to 5 × 108/ml, verified by optical quantification in a counting chamber.
The suspension was centrifuged (3,500g, 4 min), and after discarding the supernatant the pellet was resuspended in 2 ml bicarbonate buffer (0.1 M NaHCO3, pH 9.6) containing 0.1 mg/ml FITC. The solution was incubated for 1 h at RT under constant rotation in dark. After three resuspension and washing steps in PBS, pH 7.3, bacteria were resuspended in KRG, pH 7.3, and divided into aliquots. Before freezing, the tubes were sonicated at 80 W for 3 × 30 s to disperse aggregates of H. pylori. Aliquoted bacteria were stored frozen at −80°C until used.
Phagocytosis measurement with fluorescence microscopy
A fluorescence-quenching method [25] that distinguishes between extracellularly and intracellularly located bacteria was used to determine phagocytosis. Neutrophils and bacteria were prepared as previously described. Prior to use the thawed vials of H. pylori strains were sonicated to disrupt clusters of bacteria.
FITC-labeled H. pylori were incubated with neutrophils at various bacteria-to-PMN ratios; 10:1, 25:1, 50:1 and 100:1 for 30 min at 37°C. One drop of the neutrophil-bacteria mixture and one drop of Trypan blue (2 mg/ml in 25 mM citrate–phosphate buffer and 25 mM NaCl, pH 7.4) were placed on a microscopic slide. The procedure was also supplementary performed with ethidium bromide (10 μg/ml) as the quenching agent [26].
The fraction of cells containing fluorescent bacteria, i.e. ingested bacteria, was determined and used as a measure of phagocytosis (phagocytic index). Usually 80–100 cells in each sample were counted, this out of a randomized field of vision in the fluorescence microscope. Fluorescence microscopy was performed using a Zeiss Axioskop microscope equipped with a 50 W mercury vapour lamp fitted with standard filter sets for viewing FITC-fluorescence, i.e. with excitation and emission maxima at 488 and 523 nm, correspondingly (Carl Zeiss, Jena, Germany).
Phagocytosis measured with flow cytometry
To further quantify the phagocytosis of bacteria, we used FACS technology. The method applied is a modification of a previously described protocol [24, 26, 39]. FITC-labeled strains of H. pylori were thawed at room temperature, declumped by sonication and added to freshly prepared neutrophils. The assay was carried out in 2 ml Eppendorf tubes with a final volume of 500 μl.
The samples were incubated in KRG supplemented with 0.1% human serum albumin (HSA). Phagocytosis was allowed to proceed in dark for 30 min at 37°C with periodic agitation and placing the tubes on ice terminated phagocytosis. Different multiplicities of infection (MOI) were used; 25:1, 50:1 and 100:1 (bacteria:neutrophils).
The suspension was centrifuged (400g, 4 min), and after discarding the supernatant, the pellet was resuspended in ice-cold KRG, pH 7.3. Samples were then in addition resuspended, centrifuged and washed three times with ice-cold KRG to remove excess of FITC-labelled bacteria. Finally, the pellet of washed cells was resuspended in 500 μl of ice-cold KRG and split into two samples. One half of the suspension was used for measuring phagocytosis, the other half for quantifying the quenching capacity of sample. The mean fluorescent value (MFV; FL1) was determined in each case. Between the sequential steps, the test tubes were maintained on ice, and kept in dark. To make a distinction between extracellularly adhering bacterial cells and those that had been phagocytosed, we used ethidium bromide, at a final concentration of 50 μg/ml, as quenching agent for the FITC. To verify efficient quenching of adherent bacteria, both 10 μM cytochalasin D and 10 μM cytochalasin B were separately used to inhibit phagocytosis.
All measurements were performed using a FACSCalibur (Becton Dickinson, San Jose, CA, USA) equipped with an argon laser operating at an excitation wavelength of 488 nm. Collected data was evaluated using the CellQuest version 3.1f software (Becton Dickinson). For each sample 10,000 events were collected. Events were gated to discard signals that did not qualify as mammalian cells, representing dead cells (high red fluorescence), or big aggregates of bacteria and cells. The average number of cells analyzed was 4,000 in each sample.
The percentage of MFV for FITC (green) out of four independent experiments was used as measure for the activity. Percent ingestion was determined as 100 × [(FL1 after quenching of mutant strain)/(FL1 after quenching of wild type strain)]. The percent adhesion was determined as 100 × [(FL1-before quenching of mutant strain)-(FL1 after quenching of mutant strain)/(FL1-before quenching of wild type strain)-(FL1 after quenching of wild type strain)].
The neutrophil respiratory burst activity
The respiratory burst in neutrophils was determined by luminol-enhanced chemiluminescence (CL) that allows measurement of released reactive oxygen species (ROS). Both extra- and intracellular CL were analyzed in a six-channel Bioluminat LB 9505 instrument (Berthold Co., Wildbad, Germany), using disposable 4 ml polypropene tubes. Neutrophils (1 × 106) were allowed to equilibrate for 5 min at 37°C in KRG, luminol (20 μM), HRP (4 U/ml), thereafter the light emission was recorded continuously for 60 min. After achieving a baseline, the various strains of H. pylori was added in different ratios (25:1, 50:1, 100:1) to the neutrophils. To measure intracellularly produced ROS only, tubes containing SOD (200 U/ml) and catalase (2,000 U/ml) were used instead of HRP to scavenge the extracellularly released superoxide anion and hydrogen peroxide [13, 30].
To rule out the possibility that a soluble component in the supernatant gave the observed results, neutrophils were also incubated with 200 μl of the supernatant from each mutant remaining after the first washing step in the FITC-labelling procedure of the bacterium.
Agglutination of RBCs by SabA and HP-NAP mutants
To test for sialic acid-specific binding characteristics of the bacteria, human red blood cells (RBC) were prepared in the following way. They were treated with bovine pancreas trypsin at a concentration of 0.05 mg/ml [6], or with PBS buffer only, pH 7.4. After this treatment the cells were incubated with 1 mM phenylmetylsulfonylfluoride (PMSF), followed by a three-fold wash in PBS, pH 7.4, and then an incubation with different concentrations (0.02, 0.1, or 0.2 U/ml) of Clostridium perfringens neuraminidase type VI. In parallel, agglutination without neuraminidase treatment was also tested.
The basal density of bacteria used in the assay was 1 × 108 bacteria/ml. A 0.75% RBC suspension was used for the hemagglutination assay and for assessment of the protease/neuraminidase effect. Twenty-five microlitre of bacterial suspension was mixed with the same volume of 0.75% RBCs in round-bottom ELISA plates, and the state of aggregation was visually read after 1 h incubation in RT.
Measurement of binding activity of the strains of H. pylori
The binding assay was performed as previously described [20, 29] with minor modifications. The Lewis b and sialyl-dimeric-Lewis x glycoconjugates (IsoSep AB, Tullinge, Sweden) were labeled with 125I by the chloramines-T method. Bacteria were harvested from agar plate in PBS, pH 7.3. One ml of bacterial suspension (optical density at 600 nm = 0.1) was incubated with 300 ng of 125I-labeled conjugate for 2 h in PBS containing 1% albumin and 0.05% Tween 20. The bacteria were pelleted by centrifugation and the amount of bound radio-labeled conjugates bound to the bacterial pellet was measured by gamma scintillation (1282 Compugamma CS; LKB Wallac, Oy, Finland). Binding experiments were performed in duplicates.
Results
Analysis of expression of the different adhesins by H. pylori wild type and mutants strains
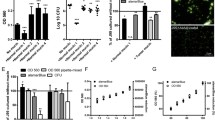
The strain J99 and series of mutants were analyzed for their glycoconjugate binding properties by using soluble neoglycoconjugates, i.e. 125I-labeled semi-synthetic glycoproteins based on albumin were used in RIA-assays [29, 43]. Figure 1a illustrates that the J99babA-mutant and sabAbabA-mutant did not recognize the Leb antigen, since they are devoid of the BabA adhesin. Similarly, in Fig. 1b, the J99sabA-mutant and J99sabAbabA-mutant did not bind their cognate SabA receptor, the sLex antigen. The parent strain J99 binds both antigens, although it binds Leb the best due to the high binding affinity of BabA [43]. Interestingly, the J99 napA-mutant is associated with both Leb and sLex similar to the J99 parent strain, which suggests that the HP-NAP protein does not constitute a part of or else have an exert influence on the BabA or SabA adhesin.
a, b Bacterial binding to soluble neoglycoconjugates. The H. pylori strains were incubated with 125I-labeled Lewis b (a) and sialyl-dimeric-Lewis x glycoconjugates (b), reflecting relative adhesion by wild-type and mutant H. pylori bacteria. Data are presented as mean percent of bound conjugate added
Effect of MOI on the kinetics of generation of ROS
By analyzing the pattern of luminol-enhanced chemiluminescence (CL) in the absence and presence of extracellular SOD and catalase, we can distinguish between total and intracellular production of ROS in the neutrophils. Figure 2 illustrates the rapid kinetics of total CL generation for wild-type H. pylori J99. The kinetics was less sustained when the MOI was increased, which indicates expedient degranulation and activation of the NADPH-oxidase. In contrast, negligible effects were obtained when supernatants of the bacterial cultures were used for priming of hyper-responsiveness. This suggests a low impact of released chemoattractants, such as N-formylated bacterial peptides. Only, some small initial peaks were seen in all instances within the first few minutes of the interaction, superimposed on the general strong CL-signals (Fig. 2).
Kinetics of the reactive oxygen species (ROS) production in human neutrophils challenged with strain H. pylori J99 at indicated bacteria-to-neutrophil ratios, alternatively to supernatants from both strain J99 and the full series of mutants. The ROS production was measured using a luminol-enhanced chemiluminescence (CL) system. The cells (1 × 106) were incubated for 10 min at 37°C prior to adding supernatants or respective bacteria. The supernatants were obtained as described in experimental procedures. One representative experiment out of at least five independent experiments is displayed
Total ROS production by H. pylori and corresponding series of mutants
Total response in ROS comprises both intracellular release and extracellular discharge of ROS. In general, either the peak or integral value of CL-response can be taken as a quantitative measure of ROS production. They presumably reflect two distinct capacities of the neutrophils, i.e. to mount a rapid response and a sustained reaction, respectively. Either aspect may be important for killing the intruding microorganisms. The total amount of ROS released intra- and extracellularly is reflected by the integral under the curve.
Figure 3a, displaying the integral value, based on at least three separate experiments, reveals several interesting features. First, the J99sabA-mutant and the “double mutant” J99sabAbabA produced the lowest responses. Second, the J99babA-mutant was slightly more effective than the J99 wild-type, which binds both Leb and sLex. It is noteworthy that supportive data for expression of Lewis b on neutrophils is not available. Third, and surprisingly enough, for the J99napA-mutant, i.e. a bacterium devoid of HP-NAP expression, the response was more enhanced than for wild-type bacteria (P<0.01). This might suggest that HP-NAP might exert a “quenching”- rather than a “priming”-type of activity on neutrophil CL-generation.
The production of ROS in human neutrophils in response to activation with strain H. pylori J99 and different mutants. Neutrophils (1 × 106) were preincubated at 37°C prior to stimulation with bacteria, at a bacteria-to-cell ratio of 100:1. The ROS-production was measured by luminol-amplified CL. The bars represent either the integral values (a) or peak values (b) in percent of the wild-type ROS-production. Data are given as mean ± SEM (standard error of the mean) of at least five independent experiments. Statistical significance versus control (i.e. wild-type J99): **P < 0.001; *P < 0.01, by unpaired, two-tailed Student’s t test
The subsequent analysis of peak values for ROS production (Fig. 3b) disguised a similar pattern for the J99sabA-mutant and the J99sabAbabA-mutant. However, here the J99napA-mutant did not differ in CL-reactivity from the J99 wild-type strain by the peak value. The similar response for the parent strain and the J99napA-mutant, indicate that the adhesin-glycan interaction triggers an initial fast response in the neutrophils, followed by a slower, probably intracellular process. This implies that HP-NAP could help balance the ROS production. Other MOIs were also tested in the CL-experiments, but only a representative range is displayed.
By selective scavenging of the extracellularly released ROS with SOD and catalase, intracellular generation of respiratory burst products can be analyzed. Only small differences between the bacterial strains regarding the “integral” and “peak” CL of intracellular generated ROS were detected (data not shown). The signal is dominated by approximately 70% intracellular release. The maximum reduction was seen for the two mutant strains J99sabA and J99napA, i.e. to 63 and 83%, respectively, of the total ROS production elicited by the wild type J99.
Phagocytosis of wild-type and mutant H. pylori by neutrophils analyzed by flow cytometry
The interaction between the bacteria and neutrophils was studied at three relative infection ratios, i.e. at MOIs of 10:1, 25:1 and 100:1. In all three cases the J99sabA-mutant, and the combined J99sabAbabA-mutant showed the lowest relative values (Table 1). This observation underscores that SabA is a critical mediator of H. pylori host cell-contact and subsequent activation of human neutrophils, i.e. is decisive in the generation of ROS (Fig. 4).
Internalization of the wild-type H. pylori J99 strain (thick curve) and the mutant strain J99sabA (thin curve) analyzed by flow cytometry. The phagocytosis curves representing the relative value of neutrophils ingesting FITC-labelled bacteria after quenching with ethidium bromide. A shift to the right indicates a higher total mean fluorescent value (MFV; FL1). The graph shows results of a representative single experiment out of four independent experiments performed (Table 1 )
Pretreatment of the neutrophils with cytochalasin B or D, at final concentrations of 10 μM, substantially lowered the total neutrophil-associated fluorescence at all three investigated ratios (data not shown). These results verify the expected inhibition of phagocytosis, and also serve as a validation of the accuracy of the chosen method.
Worth noting, is that the effects conferred by the mutant phenotypes were most pronounced at the highest MOI, where the J99napA-mutant displayed a distinct behaviour regarding both adherence and ingestion (Table 1). At the lowest MOI (10:1), the J99napA-mutant interestingly had a stimulating effect.
These results suggest that the functional phenotypes of the series of adhesin mutants, including the napA-mutant, are distinct during conditions of bacterial competition for their tropic binding sites, i.e. when approaching MOI ratios of 50:1–100:1. Less discriminating binding modes, especially displayed here at low MOI, tends to level out these typical binding characteristics and related CL-responses (data not shown) for the mutant phenotypes. The relative errors were in a large range, probably due to the individual variation among blood donors, with regard to antigen exposition and excitation states. Moreover, neutrophils having phagocytosed an excessive amount of H. pylori, so called “frustrated phagocytosis”, ensue an overall increased signal in the flow cytometry assay. The degree of frustrated phagocytosing neutrophils can also vary between donors.
Phagocytosis of wild-type and mutant H. pylori by neutrophils assessed with fluorescence microscopy
The difference between the parent H. pylori J99 strain and the J99sabA-mutant in their direct interaction with isolated neutrophils was here visualized by fluorescence microscopy (Fig. 5). It is interesting to notice that in all cases except for the J99sabA-mutant, and the J99sabAbabA-mutant, there was a massive aggregation of bacteria and neutrophils, especially at the highest MOI of 100:1. All the same, the phagocytic index clearly distinguishes the role of the SabA adhesin in the bacteria–cell interaction (Table 2). Very similar results were received from the three strains expressing the SabA adhesin, again stressing how important this lectin is for the physical interaction between H. pylori and human neutrophils.
Fluorescence microscopy of human neutrophils after phagocytosis of the wild-type H. pylori J99 strain (a) and the J99sabA-mutant (b). FITC-labelled bacteria and non-phagocytosed bacteria were quenched with ethidium bromide or Trypan blue, which may give some red background staining at the cell membranes. Note the large sialic acid-mediated aggregates between the wild-type strain and the neutrophils (a) and in contrast the solitary neutrophils associated with the lectin-deficient J99sabA-mutant (b). White arrowheads indicating ingested H. pylori. Bars 10 μm
Thus, the SabA molecule displays a key role for targeting of bacterial cells to the neutrophils. The massive co-aggregation could also indicate that sialylated receptors are further up-regulated on the neutrophil membrane due to the tight interaction. The signal as such does not tend to require viable bacterial cells, since both frozen-thawed and freshly prepared FITC-labelled bacteria yielded the same effects, i.e. tight neutrophil interaction and aggregation (data not shown).
Analyzes of the influence of HP-NAP on bacterial binding to host cells by use of neuraminidase and protease treated red blood cells
The ROS production proposes a plausible way to assess the contribution of HP-NAP in bacterial interaction with neutrophils. To analyze whether the protein positively or negatively modifies the efficiency of the adhesins to bind and interact with the host cell, a modified hemagglutination method was used. Since the SabA adhesin binds to sialylated antigens, in particular to the sLex glycan, neuraminidase treatment was employed to reduce or remove the surface-presented sialylated residues from the human RBC, thereby abolishing the sialic acid-dependent hemagglutination (sia-HA).
The regular hemagglutination activity was strong for the J99 wild-type strain, and so for both the J99napA- and the J99babA-mutants (Table 3). It was in all instances very sensitive to neuraminidase treatment, where sia-HA was already eliminated at concentrations of 0.1 U/ml, which points to sialic acid-dependent binding mode.
The sia-HA was further analyzed by titration of the neuraminidase at 0.02 U/ml. The sia-HA titres was reduced from 1:8 till 1:4, i.e. conferred a twofold reduction in sia-HA for the J99 wild-type and the babA-mutant. In contrast, a full eightfold reduction in sia-HA was found for the napA-mutant; results proposing that the HP-NAP could facilitate SabA-mediated binding to sialylated antigens on host cells surfaces, especially during conditions of limited availability of sialylated binding sites.
Protease treatment of RBC did not interfere with sialic acid-dependent binding. On the contrary, limited protease treatment fully regained the sia-HA properties for the napA-mutant. In terms of a limited host cell sialylation, as in the case with a low degree of mucosal inflammation, this trait could facilitate SabA-mediated binding of H. pylori. Speculatively, it suggests, that HP-NAP provides a stimulatory modulating effect during less sialylated conditions, whereas HP-NAP instead would confer a quenching mechanism to down-regulate sustained signals when sialylation turns abundant, as suggested by the results on generation of ROS production in Fig. 3.
Discussion
We have recently reported that the SabA adhesin is a key-element in the non-opsonic local immune response in the gastric epithelium [71]. This response is mainly characterized by the recruitment, accumulation and infiltration of activated neutrophils and monocytes in the gastric mucosa. Undoubtedly, such engagement of the leukocytes is an essential step in the initiation and maintenance of the gastric inflammatory process [18].
In the present investigation, we have also further tried to disclose the relative contribution of another virulence trait of the H. pylori, i.e. the neutrophil activating protein (HP-NAP). This molecule is known to be involved in the interaction with and activation of human neutrophils [19, 48, 55], as well as in the scavenging of ROS [9].
How the combined expressions of the virulence proteins help H. pylori to survive in the epithelium lining in the stomach has so far not been resolved. The HP-NAP is considered to be chemotactic, pro-inflammatory and promotes release of nutrients from the mucosa, thus supporting the growth of the bacteria [47, 16].
Recent studies have shown convincingly that H. pylori adhesins BabA and SabA are critical for the establishment of H. pylori locally in normal and inflamed tissue, respectively [29, 43, 71].
We now demonstrate that SabA alone, but not BabA (Fig. 2; Tables 1 and 2) is essential for attachment and activation of human neutrophils by the H. pylori strain J99. The present investigation does not support that the HP-NAP, as a component in a water-soluble extract, promotes ROS generation in neutrophils (Fig. 2). In contrast to our results, Kim et al. [34] have earlier reported activation of the neutrophils by crude water supernatant preparations. On the other hand, this could point towards the highly variable ability of extracts from different H. pylori to stimulate neutrophils [38], or methods chosen for analysis.
Neither did bacteria-associated HP-NAP impel the activation of the oxidative metabolism (Figs. 3a and 3b). Rather, it modified the pattern of generation and total magnitude of respiratory burst products. Further experiments are however, needed for a final assessment of the role of HP-NAP, previously suggested as an important chemoattractant [8, 13, 68]. Could for example HP-NAP through ROS inhibition or modulation (present study) actually promote cell motility?
The ability of the different bacterial strains to elicit an oxidative burst in the neutrophils [32], is clearly reflected in the total chemiluminescence production, comprising both intracellular and extracellular formation of ROS, whether judged by peak or integral values (Figs. 3a and 3b).
To study adherence/phagocytosis of the bacteria, we used both fluorescence microscopy and flow cytometry. By modifying the method described by Heinzelmann et al. [26], flow cytometry was used as an objective measure of phagocytosis [33, 42]. By doing this, we wanted to circumvent the drawbacks with traditional microscopy, which is laborious and sensitive to variations in observer interpretations. Scientifically, results presented in Table 2 fall under a binominal categorisation, in comparison to Table 1 which classifieds as numerical categorisation, based on the 4000 number of cells counted per determination [51].
We were able to consolidate quantitatively the trends in ingestion and adherence between the different bacterial traits (Table 1). At all ratios between bacteria and neutrophils, the SabA adhesin seems to promote the interaction further than any of the other bacterial proteins investigated here. Envisioning that the MOI, i.e. the relative number of intruding H. pylori and host defence cells, reflects conditions that can occur in inflamed tissue, its effect of the magnitude and rate of ROS generation is of specific interest to address (Fig. 2 and Table 1).
The finding of the HP-NAP-mutant phenotype is of principal interest, since both bacterial adherence and ingestion by the leukocytes decreased with higher proportion of bacteria (Table 1). We suggest this is a specific consequence of the loss of the HP-NAP and not due to altered expression of another surface appendage, e.g. SabA; the HP-NAP-mutant and the wild-type strain display similar results in the regular sia-HA analysis, where unlimited neuraminidase was used. The binding results of white blood cells (Table 1) and RBC during limited sialic-acid levels (Table 3) strengthen a concept that HP-NAP participated in adherence to host cells during conditions of limited sialylation. This could be the case in initial and low-grade gastritis, or in situations of competition between bacteria, as modelled in the situation with high MOI series in the flow cytometry analysis (Table 1).
Our results further strengthen the central role of the SabA adhesin in provoking an inflammatory response, which is dependent on the bacterial load. This also supports previous findings of the expression of SabA binding sites and bacterial association with inflamed gastric tissue [43].
In summary, present and previous findings speak for the following model of H. pylori colonization and disease promotion: (1) the BabA adhesion is a prerequisite for the initial colonization [29, 40]; (2) the HP-NAP helps recruit inflammatory cells [16, 47, 55] and protects bacteria from ROS [9] by regulating the amount of ROS generated (this study), and (3) SabA, together with the bacterial load determine the magnitude of the inflammatory response [71, this study], and (4) HP-NAP might facilitate SabA-mediated binding when sialylated antigens are less expressed such as during low-grade inflammation (this study).
The impact of slow and balanced versus rapid and fulminate sLex-mediated binding for development of disease remains to be assessed. So, H. pylori might have dual systems for initiation and termination of leukocyte activity, thereby optimizing its interaction with labile and sensitive immunological cells in the local and inflamed mucosa.
References
Alm RA, Ling LS, Moir DT, King BL, Brown ED, Doig PC, Smith DR, Noonan B, Guild BC, deJonge BL, Carmel G, Tummino PJ, Caruso A, Uria-Nickelsen M, Mills DM, Ives C, Gibson R, Merberg D, Mills SD, Jiang Q, Taylor DE, Vovis GF, Trust TJ (1999) Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397:176–180
Amieva MR, Salama NR, Tompkins LS, Falkow S (2002) Helicobacter pylori enter and survive within multivesicular vacuoles of epithelial cells. Cell Microbiol 4:677–690
Ångstrom J, Teneberg S, Milh MA, Larsson T, Leonardsson I, Olsson BM, Halvarsson MO, Danielsson D, Näslund I, Ljungh A, Wadström T, Karlsson KA (1998) The lactosylceramide binding specificity of Helicobacter pylori. Glycobiology 8:297–309
Baggiolini M, Dewald B, Moser B (1997) Human chemokines: an update. Annu Rev Immunol 15:675–705
Björkholm B, Zhukhovitsky V, Löfman C, Hulten K, Enroth H, Block M, Rigo R, Falk P, Engstrand L (2000) Helicobacter pylori entry into human gastric epithelial cells: a potential determinant of virulence, persistence, and treatment failures. Helicobacter 5:148–154
Borén TWT, Normark S, Gordon JI, Falk PG (1997) Methods for the identification of H. pylori host receptors. In: Clayton CL, Mobley HTL (eds) Helicobacter pylori protocols. Humana Press Inc, New Jersey pp 205–224
Bøyum A (1968) A one-stage procedure for isolation of granulocytes and lymphocytes from human blood. General sedimentation properties of white blood cells in a 1 g gravity field. Scand J Clin Lab Invest Suppl 97:51–76
Bylund J, Dahlgren C (2002) Problems in identifying microbial-derived neutrophil activators, focusing on Helicobacter pylori. Trends Microbiol 10:12–14
Cooksley C, Jenks PJ, Green A, Cockayne A, Logan RP, Hardie KR (2003) NapA protects Helicobacter pylori from oxidative stress damage, and its production is influenced by the ferric uptake regulator. J Med Microbiol 52:461–469
Cotran RS, Kumar V, Collins T (1999) Robbins pathology basis of disease, 6th edn. W.B. Saunders Company, London 315:787–802
Crabtree JE (2001) Cytokine responses in Helicobacter pylori infection. In: Achtman M, Suerbaum S (eds) Helicobacter pylori: molecular and cellular biology. Horizon Scientific Press, Norfolk, pp 63–83
Crabtree JE, Covacci A, Farmery SM, Xiang Z, Tompkins DS, Perry S, Lindley IJ, Rappuoli R (1995) Helicobacter pylori induced interleukin-8 expression in gastric epithelial cells is associated with CagA positive phenotype. J Clin Pathol 48:41–45
Dahlgren C, Karlsson A (1999) Respiratory burst in human neutrophils. J Immunol Methods 232:3–14
Dixon M (1996) Pathological consequences of Helicobacter pylori infection. Scand J Gastroenterol Suppl 215:21
Dixon MF, Genta RM, Yardley JH, Correa P (1996) Classification and grading of gastritis. The updated sydney system. International workshop on the histopathology of gastritis, Houston 1994. Am J Surg Pathol 20:1161–1181
Dundon WG, Nishioka H, Polenghi A, Papinutto E, Zanotti G, Montemurro P, Del GG, Rappuoli R, Montecucco C (2002) The neutrophil-activating protein of Helicobacter pylori. Int J Med Microbiol 291:545–550
el-Shoura SM (1995) Helicobacter pylori: I. Ultrastructural sequences of adherence, attachment, and penetration into the gastric mucosa. Ultrastruct Pathol 19:323–333
Ernst PB, Gold BD (2000) The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu Rev Microbiol 54:615–640
Evans DJ Jr, Evans DG, Takemura T, Nakano H, Lampert HC, Graham DY, Granger DN, Kvietys PR (1995) Characterization of a Helicobacter pylori neutrophil-activating protein. Infect Immun 63:2213–2220
Falk P, Borén T, Haslam D, Caparon M (1994) Bacterial adhesion and colonization assays. Methods Cell Biol 45:165–192
Feldman RA (2001) Epidemiologic observation and open questions about disease and infection caused by Helicobacter pylori. In: Achtman M, Suerebaum S (eds) Helicobacter pylori: molecular and cellular biology. Horizon Scientific Press, Norfolk pp 29–51
Ferrante A, Thong YH (1980) Optimal conditions for simultaneous purification of mononuclear and polymorphonuclear leucocytes from human blood by the Hypaque-Ficoll method. J Immunol Methods 36:109–117
Graham DY (2000) Helicobacter pylori infection is the primary cause of gastric cancer. J Gastroenterol 35(Suppl 12):90–97
Hartmann P, Becker R, Franzen C, Schell-Frederick E, Romer J, Jacobs M, Fatkenheuer G, Plum G (2001) Phagocytosis and killing of Mycobacterium avium complex by human neutrophils. J Leukoc Biol 69:397–404
Hed J (1977) The extinction of fluorescence by crystal violet and its use to differentiate between attached and ingested microorganisms in phagocytosis. FEMS Microbiol Lett 1:357–361
Heinzelmann M, Gardner SA, Mercer-Jones M, Roll AJ, Polk HC Jr (1999) Quantification of phagocytosis in human neutrophils by flow cytometry. Microbiol Immunol 43:505–512
Hirmo S, Kelm S, Schauer R, Nilsson B, Wadström T (1996) Adhesion of Helicobacter pylori strains to alpha-2,3-linked sialic acids. Glycoconj J 13:1005–1011
Hofman V, Ricci V, Galmiche A, Brest P, Auberger P, Rossi B, Boquet P, Hofman P (2000) Effect of Helicobacter pylori on polymorphonuclear leukocyte migration across polarized T84 epithelial cell monolayers: role of vacuolating toxin VacA and cag pathogenicity island. Infect Immun 68:5225–5233
Ilver D, Arnqvist A, Ögren J, Frick IM, Kersulyte D, Incecik ET, Berg DE, Covacci A, Engstrand L, Borén T (1998) Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science 279:373–377
Karlsson A, Dahlgren C (2002) Assembly and activation of the neutrophil NADPH oxidase in granule membranes. Antioxid Redox Signal 4:49–60
Karlsson KA (2000) The human gastric colonizer Helicobacter pylori: a challenge for host-parasite glycobiology. Glycobiology 10:761–771
Keenan JI, Peterson RA 2nd, Hampton MB (2005) NADPH oxidase involvement in the pathology of Helicobacter pylori infection. Free Radic Biol Med 38:1188–1196
Kennedy CJ, Rakoczy PE, Constable IJ (1996) A simple flow cytometric technique to quantify rod outer segment phagocytosis in cultured retinal pigment epithelial cells. Curr Eye Res 15:998–1003
Kim JS, Jung HC, Kim JM, Song IS, Kim CY (2000) Helicobacter pylori water-soluble surface proteins activate human neutrophils and up-regulate expression of CXC chemokines. Dig Dis Sci 45:83–92
Ko GH, Kang SM, Kim YK, Lee JH, Park CK, Youn HS, Baik SC, Cho MJ, Lee WK, Rhee KH (1999) Invasiveness of Helicobacter pylori into human gastric mucosa. Helicobacter 4:77–81
Kuipers EJ (1997) Helicobacter pylori and the risk and management of associated diseases: gastritis, ulcer disease, atrophic gastritis and gastric cancer. Aliment Pharmacol Ther 11(Suppl 1):71–88
Kwok T, Backert S, Schwarz H, Berger J, Meyer TF (2002) Specific entry of Helicobacter pylori into cultured gastric epithelial cells via a zipper-like mechanism. Infect Immun 70:2108–2120
Leakey A, La Brooy J, Hirst R (2000) The ability of Helicobacter pylori to activate neutrophils is determined by factors other than H. pylori neutrophil-activating protein. J Infect Dis 182:1749–1755
Lin JC, Chang FY, Fung CP, Xu JZ, Cheng HP, Wang JJ, Huang LY, Siu LK (2004) High prevalence of phagocytic-resistant capsular serotypes of Klebsiella pneumoniae in liver abscess. Microb Infect 6:1191–1198
Lindén S, Nordman H, Hedenbro J, Hurtig M, Borén T, Carlstedt I (2002) Strain- and blood group-dependent binding of Helicobacter pylori to human gastric MUC5AC glycoforms. Gastroenterology 123:1923–1930
Logan RPH, Hirschl AM (1996) Epidemiology of Helicobacter pylori infection. Curr Opin Gastroenterol 12:1–5
Lun S, Willson PJ (2004) Expression of green fluorescent protein and its application in pathogenesis studies of serotype 2 streptococcus suis. J Microbiol Methods 56:401–412
Mahdavi J, Sondén B, Hurtig M, Olfat FO, Forsberg L, Roche N, Ångström J, Larsson T, Teneberg S, Karlsson KA, Altraja S, Wadström T, Kersulyte D, Berg DE, Dubois A, Petersson C, Magnusson KE, Norberg T, Lindh F, Lundskog BB, Arnqvist A, Hammarström L, Boren T (2002) Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science 297:573–578
Marshall BJ (2002) Helicobacter in the year 2000. WWW-document. URL: http://www.barryjmarshall.com/helicobacter/hpy2 k/frMain.htm
Marshall BJ, Warren JR (1984) Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1:1311–1315
Miller-Podraza H, Bergström J, Teneberg S, Milh MA, Longard M, Olsson BM, Uggla L, Karlsson KA (1999) Helicobacter pylori and neutrophils: sialic acid-dependent binding to various isolated glycoconjugates. Infect Immun 67:6309–6313
Namavar F, Sparrius M, Veerman EC, Appelmelk BJ, Vandenbroucke-Grauls CM (1998) Neutrophil-activating protein mediates adhesion of Helicobacter pylori to sulfated carbohydrates on high-molecular-weight salivary mucin. Infect Immun 66:444–447
Nishioka H, Baesso I, Semenzato G, Trentin L, Rappuoli R, Del Giudice G, Montecucco C (2003) The neutrophil-activating protein of Helicobacter pylori (HP-NAP) activates the MAPK pathway in human neutrophils. Eur J Immunol 33:840–849
Noach LA, Bosma NB, Jansen J, Hoek FJ, van Deventer SJ, Tytgat GN (1994a) Mucosal tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8 production in patients with Helicobacter pylori infection. Scand J Gastroenterol 29:425–429
Noach LA, Rolf TM, Tytgat GN (1994b) Electron microscopic study of association between Helicobacter pylori and gastric and duodenal mucosa. J Clin Pathol 47:699–704
Nuutila J, Lilius EM (2005) Flow cytometric quantitative determination of ingestion by phagocytes needs the distinguishing of overlapping populations of binding and ingesting cells. Cytometry Part A 65:93–102
Oh JD, Karam SM, Gordon JI (2005) Intracellular Helicobacter pylori in gastric epithelial progenitors. Proc Natl Acad Sci USA 102:5186–5191
Olfat FO, Naslund E, Freedman J, Boren T, Engstrand L (2002) Cultured human gastric explants: a model for studies of bacteria-host interaction during conditions of experimental Helicobacter pylori infection. J Infect Dis 186:423–427
Ota A, Genta RM (1997) Morphological characterisation of the gastric mucosa during infection with H. pylori. In: Ernst PB, Michetti P, Smith PD (eds) The immunbiology of H. pylori: from pathogenesis to prevention. Lippincott-Raven Publishers, Philadelphia, pp 15–27
Satin B, Del Giudice G, Della Bianca V, Dusi S, Laudanna C, Tonello F, Kelleher D, Rappuoli R, Montecucco C, Rossi F (2000) The neutrophil-activating protein (HP-NAP) of Helicobacter pylori is a protective antigen and a major virulence factor. J Exp Med 191:1467–1476
Schreiber S, Konradt M, Groll C, Scheid P, Hanauer G, Werling HO, Josenhans C, Suerbaum S (2004) The spatial orientation of Helicobacter pylori in the gastric mucus. Proc Natl Acad Sci USA 101:5024–5029
Semino-Mora C, Doi SQ, Marty A, Simko V, Carlstedt I, Dubois A (2003) Intracellular and interstitial expression of Helicobacter pylori virulence genes in gastric precancerous intestinal metaplasia and adenocarcinoma. J Infect Dis 187:1165–1177
Sharma SA, Tummuru MK, Miller GG, Blaser MJ (1995) Interleukin-8 response of gastric epithelial cell lines to Helicobacter pylori stimulation in vitro. Infect Immun 63:1681–1687
Shimoyama T, Everett SM, Dixon MF, Axon AT, Crabtree JE (1998) Chemokine mRNA expression in gastric mucosa is associated with Helicobacter pylori cagA positivity and severity of gastritis. J Clin Pathol 51:765–770
Sobala GM, Crabtree JE, Dixon MF, Schorah CJ, Taylor JD, Rathbone BJ, Heatley RV, Axon AT (1991) Acute infection: clinical features, local and systemic immune response, gastric mucosal histology, and gastric juice ascorbic acid concentrations. Gut 32:1415–1418
Steer HW (1975) Ultrastructure of cell migration throught the gastric epithelium and its relationship to bacteria. J Clin Pathol 28:639–646
Stocks SC, Albrechtsen M, Kerr MA (1990) Expression of the CD15 differentiation antigen (3-fucosyl-N-acetyl-lactosamine, LeX) on putative neutrophil adhesion molecules CR3 and NCA-160. Biochem J 268:275–280
Stocks SC, Kerr MA (1993) Neutrophil NCA-160 (CD66) is the major protein carrier of selectin binding carbohydrate groups LewisX and sialyl LewisX. Biochem Biophys Res Commun 195:478–483
Suerbaum S (2000) Genetic variability within Helicobacter pylori. Int J Med Microbiol 290:175–181
Suerbaum S, Josenhans C, Labigne A (1993) Cloning and genetic characterization of the Helicobacter pylori and Helicobacter mustelae flaB flagellin genes and construction of H. pylori flaA- and flaB-negative mutants by electroporation-mediated allelic exchange. J Bacteriol 175:3278–3288
Teneberg S, Jurstrand M, Karlsson KA, Danielsson D (2000) Inhibition of nonopsonic Helicobacter pylori-induced activation of human neutrophils by sialylated oligosaccharides. Glycobiology 10:1171–1181
Teneberg S, Leonardsson I, Karlsson H, Jovall PA, Ångström J, Danielsson D, Näslund I, Ljungh A, Wadström T, Karlsson KA (2002) Lactotetraosylceramide, a novel glycosphingolipid receptor for Helicobacter pylori, present in human gastric epithelium. J Biol Chem 277:19709–19719
Teneberg S, Miller-Podraza H, Lampert HC, Evans DJ Jr, Evans DG, Danielsson D, Karlsson KA (1997) Carbohydrate binding specificity of the neutrophil-activating protein of Helicobacter pylori. J Biol Chem 272:19067–19071
Thomas JE, Dale A, Harding M, Coward WA, Cole TJ, Weaver LT (1999) Helicobacter pylori colonization in early life. Pediatr Res 45:218–223
Tonello F, Dundon WG, Satin B, Molinari M, Tognon G, Grandi G, Del Giudice G, Rappuoli R, Montecucco C (1999) The Helicobacter pylori neutrophil-activating protein is an iron-binding protein with dodecameric structure. Mol Microbiol 34:238–246
Unemo M, Aspholm-Hurtig M, Ilver D, Bergström J, Boren T, Danielsson D, Teneberg S (2005) The sialic acid binding SabA adhesin of Helicobacter pylori is essential for nonopsonic activation of human neutrophils. J Biol Chem 280:15390–15397
Warren JR, Marshall BJ (1983) Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 1:1273–1275
Westblom T, Czinn SJ, Nedrud JG (1999) Gastroduodenal disease and Helicobacter pylori. Pathophysiology, diagnosis and treatment. Curr Top Microbiol Immunol. Springer, Berlin Heidelberg New York
Wyle FA, Tarnawski A, Schulman D, Dabros W (1990) Evidence for gastric mucosal cell invasion by C. pylori an ultrastructural study. J Clin Gastroenterol 12(Suppl 1):S92–S98
Zanotti G, Papinutto E, Dundon W, Battistutta R, Seveso M, Giudice G, Rappuoli R, Montecucco C (2002) Structure of the neutrophil-activating protein from Helicobacter pylori. J Mol Biol 323:125–130
Zu Y, Cassai ND, Sidhu GS (2000) Light microscopic and ultrastructural evidence of in vivo phagocytosis of Helicobacter pylori by neutrophils. Ultrastruct Pathol 24:319–323
Acknowledgments
This work was supported by grants from the Program of Infection and Vaccinology (CP/KEM and MA/TB) and the Program of Inflammation (MF), Foundation for Strategic Research (SSF); the ELFA Research Foundation (CP); the Swedish Society for Medical Research (CP); the Swedish Research Council, Project Nos. 621-2001-3570, 521-2001-6565, 11218 (KEM/TB), the King Gustav V 80-Year Foundation (KEM); Swedish Cancer Society, Project No 4101-B00-03XAB (TB); Umeå University Biotechnology Fund (TB), County Council of Västerbotten (TB), the JC Kempe and Seth M Kempe Memorial Foundation (TB), the Mizutani Foundation Glycoscience Award 2003 (TB). We are grateful to Dr. Per-Eric Lindgren for constructive criticism of the material.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Petersson, C., Forsberg, M., Aspholm, M. et al. Helicobacter pylori SabA adhesin evokes a strong inflammatory response in human neutrophils which is down-regulated by the neutrophil-activating protein. Med Microbiol Immunol 195, 195–206 (2006). https://doi.org/10.1007/s00430-006-0018-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-006-0018-x