Abstract
Music-making is a widespread leisure and professional activity that has garnered interest over the years due to its effect on brain and cognitive development and its potential as a rehabilitative and restorative therapy of brain dysfunctions. We investigated whether music-making has a potential age-protecting effect on the brain. For this, we studied anatomical magnetic resonance images obtained from three matched groups of subjects who differed in their lifetime dose of music-making activities (i.e., professional musicians, amateur musicians, and non-musicians). For each subject, we calculated a so-called BrainAGE score which corresponds to the discrepancy (in years) between chronological age and the “age of the brain”, with negative values reflecting an age-decelerating brain and positive values an age-accelerating brain, respectively. The index of “brain age” was estimated using a machine-learning algorithm that was trained in a large independent sample to identify anatomical correlates of brain-aging. Compared to non-musicians, musicians overall had lower BrainAGE scores, with amateur musicians having the lowest scores suggesting that music-making has an age-decelerating effect on the brain. Unlike the amateur musicians, the professional musicians showed a positive correlation between their BrainAGE scores and years of music-making, possibly indicating that engaging more intensely in just one otherwise enriching activity might not be as beneficial than if the activity is one of several that an amateur musician engages in. Intense music-making activities at a professional level could also lead to stress-related interferences and a less enriched environment than that of amateur musicians, possibly somewhat diminishing the otherwise positive effect of music-making.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cross-sectional and longitudinal studies have shown that learning to play and practice a musical instrument over long periods of time lead to a range of benefits related to intelligence (Schellenberg 2004), verbal skills (Chan et al. 1998; Ho et al. 2003; Seither-Preisler et al. 2014; Moreno et al. 2009), auditory discrimination, and fine motor skills (Forgeard et al. 2008) during childhood but also during adolescence (Tierney et al. 2015) with effects lasting for decades (White-Schwoch et al. 2013). Particularly relevant in terms of transferability into school or career skills and promotable by musical training is self-discipline (Hallam 2010; Jäncke 2013), as enormous investment is required to attain, maintain, refine, and synchronize the underlying actions of music-making such as sight-reading, fine-grained motor control of hands, and sometimes feet, and integrating sensory feedback with feedforward motor actions. Advantageously, these efforts often receive support from positive feedback and reward experiences associated with dopamine releases in the striatal system (Herholz and Zatorre 2012; Altenmüller and Schlaug 2015), knowing to promote motivation and the learning processes involved in music-making (Wise 2004). Furthermore, playing music enriches social interactions, indirectly reinforcing musical behavior but also leading to a health-related impact on the brain (Davidson and McEwen 2012). Together, music-making as a leisure activity is known to be associated with significant health gains, as it provides a valuable enrichment and serves as cognitive stimulator with important consequences of decelerating age-related cognitive decline (Hanna-Pladdy and MacKay 2011; Bugos et al. 2007; Hall et al. 2009) up to reducing the risk and progression of dementia (Verghese et al. 2003), and facilitating plasticity to foster neurorestoration after injury due to its unique abilities to connect auditory inputs with motor cues while tapping into the reward and emotion system (Schlaug 2015; Wan and Schlaug 2010; François et al. 2015).
The brain has been found to be plastic in numerous studies showing that structure and function can be modified depending on experience and behavior, including any kind of sensory and motor activity, environmental exposure, or stimulus deprivation (Jancke 2009b; Markham and Greenough 2004; Schlaug 2001; Wan and Schlaug 2010; Schlaug 2015). These plasticity effects are found in brain networks underlying the specific experience or behavior with effect sizes that are related to the intensity or frequency of the activity engaged in. This strongly argues for the brain’s capacity to optimize required functioning and thus to adapt to specific sensorimotor demands, such as to the ones required for attaining the skills of making music at a high-performance level (Gaser and Schlaug 2003; Wan and Schlaug 2010; Schneider et al. 2002). Regarding brain plasticity, musicians represent an exceptional population given that their brains are engaged in an intense multisensory and motor activity that typically commences early during childhood, a neurodevelopmental stage considered sensitive and highly responsive to internal and external stimulations, and is practiced throughout their life. Therefore, professional musicians are an established in vivo human model for the study of brain plasticity (Jancke 2009a, b; Schlaug 2001; Münte et al. 2002; Wan and Schlaug 2010).
In the present study, we investigated the impact of music-making on the maturing brain. Due to these effects associated with music-making, we expected musicians to show a delay in “brain-aging”. From three samples differing only in musician status (non-musicians, amateur musicians, and professional musicians), we calculated the difference between the subjects’ chronological age and the age of their brains estimated from strongly T1-weighted imaging scans using a kernel method for regression. This method, referred to as BrainAGE framework (Franke et al. 2010), underlies a machine-learning classification algorithm derived from a training sample that determines the multidimensional aging patterns within the whole brain and aggregates them into one single value. Difference scores in which this estimated brain age value falls below the chronological age reflect an age-decelerating effect. Conversely, when the estimated brain age value exceeds the chronological age, the difference score reflects an age-accelerating effect. This gap, referred to as BrainAGE, is a well-validated (Franke and Gaser 2012) and sensitive score, outperforming even biomarkers derived from the cerebrospinal fluid (i.e., Aβ42, total, and phosphorylated tau) in predicting the individual progression of mild cognitive impairment to Alzheimer’s disease (Gaser et al. 2013).
Materials and methods
Participants
We recruited three samples of healthy subjects without any neurological or psychiatric conditions, differing only in musician status (non-musicians, amateur musicians, and professional musicians). All subjects gave written informed consent to a protocol that was approved by the institutional review board of Beth Israel Deaconess Medical Center. The professional musicians (N = 42) were performing artists, full-time music teachers, or full-time conservatory students. The amateur musicians (N = 45) played a musical instrument regularly, but their main profession or education was outside the field of music. The amateur musicians and non-musicians (N = 38) were students at local colleges/universities or worked at medical institutions in the local area. Compared to amateur musicians, professional musicians started earlier with musical training, performed more years of music playing, and were more engaged in music performance at the moment and during their lifetime so far (Table 1). The three groups were comparable regarding, age (non-musicians: M = 25.16, SD = 4.80, range 17–39; amateur musicians: M = 24.33, SD = 3.89, range 17–34; professional musicians: M = 24.33, SD = 3.89, range 18–39; F 2,112 = 2.42, P = 0.093), and sex distribution (males: 23, 27 and 22, respectively; χ 22 = 0.71, P = 0.72), educational and other leisure engagements, as sports (categorized as: none, one, or two activities) were equally performed across the three groups (χ 24 = 3.49, P = 0.48). Apart from music-making, no one performed any other activity that required skilled sensorimotor functions and was conducted at a professional level.
Data acquisition and preprocessing
High-resolution anatomical (T1-weighted) images (voxel size, 1 mm3) of the whole brain were acquired on a 1.5 T Siemens Vision whole-body scanner (Erlangen, Germany) using a magnetization prepared rapid acquisition gradient echo sequence. Preprocessing of the T1-weighted images was done using the SPM8 package (http://www.fil.ion.ucl.ac.uk/spm) and the VBM8 toolbox (http://dbm.neuro.uni-jena.de), running under MATLAB. All T1-weighted images were corrected for bias-field inhomogeneities, then spatially normalized and segmented into gray matter, white matter, and cerebrospinal fluid within the same generative model (Ashburner and Friston 2005). The images were spatially normalized using an affine registration and smoothed with an 8-mm full-width-at-half-maximum kernel and resampled to a spatial resolution of 4 mm.
BrainAGE framework
On the processed gray matter images, the BrainAGE framework was applied utilizing a machine-learning pattern recognition method, namely, relevance vector regression (RVR) (Tipping 2001). For training the brain age estimation model as well as for predicting individual brain ages, we used “The Spider” (http://www.kyb.mpg.de/bs/people/spider/main.html), a freely available toolbox running under MATLAB. The pipeline is illustrated in Fig. 1. As training sample (N = 631; 270 males and 261 females), we used the public available IXI (http://brain-development.org) and OASIS database (Marcus et al. 2007). We only included subjects with an age between 20 and 70 years and removed data that exceeded one standard deviation based on a check for sample homogeneity using the VBM8 toolbox. This guaranteed that the aging pattern was trained with data that covered a large age range, but were still similar in homogeneity to our samples. The individual BrainAGE scores were adjusted by subtracting the median BrainAGE score (m = 2.44) of the non-musician control.

[Image modified from Franke and Gaser (2012)]
Depiction of the BrainAGE concept. a Model of healthy brain-aging is trained with the chronological age and preprocessed structural MRI data of a training sample (left; with an exemplary illustration of the most important voxel locations that were used by the age regression model). Subsequently, the individual brain ages of previously unseen test subjects are estimated based on their MRI data [blue; picture modified from Schölkopf and Smola (2002)]. b Difference between the estimated and chronological age results in the BrainAGE score. Consequently, positive BrainAGE scores indicate accelerated brain-aging
Statistical analyses
Analysis was done using the SPSS software (SPSS 21 for Windows; http://www.spss.com). The BrainAGE scores were subjected to parametric tests (ANOVA and t tests). Since “years of training” over a lifespan cannot be considered normally distributed (one-sample Kolmogorov–Smirnov test; professional musicians: P = 0.021; amateur musicians: P = 0.001), we used a more conservative, non-parametric test (Spearman’s rank order) to perform correlation analyses.
Results
The impact of musician status on brain-aging
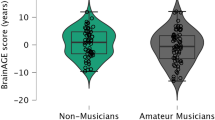
Figure 2 shows the individual BrainAGE scores plotted separately for each group (non-musicians: M = −0.48, SD = 6.85, N = 38; amateur musicians: M = −4.51, SD = 5.60, N = 45; professional musicians: M = −3.70, SD = 6.57, N = 42) which were matched with regard to age, sex, educational, and amount of other leisure activities such as sports. A one-way analysis of variance (ANOVA) revealed that “musician status” had an effect on the BrainAGE scores (F 2,122 = 4.56, P = 0.012). An independent sample t test comparing non-musicians against musicians as a whole group including both professional and amateur musicians revealed that musicians in general exhibited lower BrainAGE scores (M = −4.12, SD = 6.06, N = 87) than non-musicians (t 123 = 2.97, P = 0.004, two-tailed). Together, these two tests suggest a general age-decelerating effect of music-making on the brain.
Distribution of the BrainAGE scores within and across musician status. The distribution of BrainAGE scores is groupwise visualized as violin plots. The horizontal lines depict the individual BrainAGE scores expressed in the unit of years reflecting the difference between the estimated brain age value and the chronological age. Positive scores reflect an age-accelerating brain effect, whereas negative scores reflect an age-decelerating brain effect. The width of the plot shapes indicates the probability density of the scores. The shading transition indicates the median and the box the interquartile range. Two-tailed **P < 0.01. Bonferroni-corrected (α′ < 0.05/3 = 0.017)
Most interesting, the effect of musician status on the BrainAGE scores was more strongly influenced by the group of amateur musicians, as the ANOVA post hoc pairwise comparisons (Bonferroni-adjusted at the level of α = 0.05 for three between-group t tests) revealed a significant difference between amateur musicians and non-musicians (t 81 = 2.95, Bonferroni-adjusted P = 0.014, two-tailed). Professional musicians on the other hand showed a statistical trend of lower BrainAGE scores compared to the non-musicians (t 78 = −2.15, Bonferroni-adjusted P = 0.074, two-tailed), but did not differ from the amateur musicians (t 85 = 0.81, Bonferroni-adjusted P = 1.00, two-tailed).
Intelligence was assessed in IQ scores using the Shipley–Hartford Retreat Scale (Shipley 1940) (professional musicians: M = 118.21, SD = 6.69, 8 missing values; amateur musicians: M = 120.35, SD = 4.69, 12 missing values; and non-musicians: M = 120.35, SD = 4.69, 8 missing values). According to a one-way analysis of covariance with IQ as categorical covariate (categorized as: 85–100, 101–115, and 115–130, missing values), IQ did not have a significant effect on the BrainAGE scores in this cohort (F 2,121 = 0.42, P = 0.520), and the musician-specific effect remained after adjusting for this covariate (F 2,121 = 4.36, P = 0.015).
The relationship between the dose of music-making and BrainAGE scores
Figure 3 shows the relationship between BrainAGE scores and the number of years involved in musical activities for the professional (a) and amateur (b) musician groups. While no significant correlation between these years of music-making and BrainAGE score was found in the amateur musicians (rs45 = −0.068, P = 0.665, two-tailed), the professional musicians showed a small positive correlation (rs42 = 0.323, P = 0.037, two-tailed), explaining 10.43% of the variation in BrainAGE scores. This suggests that with increasing number of years of music-making, the professional musician group might have less of an age-decelerating effect.
Relationship between BrainAGE scores and music engagement. Dots depict individual pairs of BrainAGE score and music engagement shown by a professional musicians and b amateur musicians. BrainAGE scores are expressed in the unit of years reflecting the difference between the estimated brain age value and the chronological age. Positive scores reflect an age-accelerating brain effect, whereas negative scores reflect an age-decelerating brain effect. Music engagement is expressed in number of years reflecting the time involved in musical activities. Straight lines show best linear fits and the R 2 indicates the explaining variance
Discussion
In this study, we determined the impact of long-term instrumental music-making on the mature brain. By means of the BrainAGE approach, we were able to parsimoniously capture MR signal differences corresponding to age differences without the biases of cross-sectional cohort effects and panel attrition that a longitudinal design potentially would have entailed. The BrainAGE approach has been successfully applied to examine neurodevelopmental effects in children and adolescents (Franke et al. 2012), to predict progression in Alzheimer’s disease (Franke and Gaser 2012; Gaser et al. 2013; Löwe et al. 2016), to determine brain-aging effects in psychiatric and non-psychiatric diseases (Franke et al. 2013; Koutsouleris et al. 2014), and to examine hormonal influences and lifestyle factors (Franke et al. 2014, 2015; Luders et al. 2016).
In line with past research (Bugos et al. 2007; Hanna-Pladdy and MacKay 2011; Verghese et al. 2003; White-Schwoch et al. 2013; Hanna-Pladdy and Gajewski 2012), our data confirm that making music can modulate the effect of aging on the brain. The continuous and long-term engagement in cognitively demanding sensorimotor and frequently multimodal (i.e., sight, sound, and somatosensory information) activities modulates and enhances neural and synaptic activity as well as regional cerebral blood flow and metabolism (Ames 2000; Mintun et al. 2001; Attwell et al. 2010; Devor et al. 2003; Jonasson et al. 2016), all having the potential to maintain and increase brain health. The evidence that supports these physiological processes is drawn from studies focusing on neural functioning and cognitive vitality in relation with increased and long-term physical and mental activities (Jonasson et al. 2016; Prakash et al. 2015; Kramer et al. 1999; Swain et al. 2003; Zheng and Purves 1995). If particular sensorimotor and/or multimodal activities are learned and practiced over a long period of time, the result might be microstructural changes in brain gray and white matters that can be measured on a macrostructural level such as an increase in myelination and complexity of relevant white matter tracts, an increase in glial cells and capillary density, an increase in the number of dendritic spines, and possibly even new brain cells, leading to an increase in gray matter volume and density (Black et al. 1990; Kleim et al. 1996; Johansson 2004; van Praag et al. 1999; Anderson et al. 2002) which can be visually demonstrated as changes in gray and white matter signals on MR images (Golestani et al. 2002; Draganski et al. 2004; Scholz et al. 2009; Wan et al. 2014). Several studies have made the claim that this gain in brain structure constitutes a “reserve” function that buffers against brain pathology and age-related atrophy (Cheng 2016; Katzman 1993; Katzman et al. 1989; Stern 2006). Not only intelligence, education, occupational attainment but also lifestyle and leisure activities such as making music and dancing contribute to this “reserve”, as longitudinal and postmortem studies have linked these activities to a reduction of risk of age-related cognitive decline (Verghese et al. 2006) and dementia (Verghese et al. 2003; Scarmeas et al. 2001) or to a delayed (Hall et al. 2009) or reduced manifestation of symptoms in patients with dementia (Scarmeas et al. 2001; Katzman et al. 1989). A large body of cross-sectional and longitudinal studies suggests that the long-term engagement in music-making activities leads to structural differences and changes in various parts of the brain, such as major fiber tracts [i.e., corpus callosum (Hyde et al. 2009; Steele et al. 2013), arcuate fasciculus (Halwani et al. 2011), and corticospinal tract (Rüber et al. 2015; Bengtsson et al. 2005)] and networks of particular cortical (i.e., motor and prefrontal cortex, sensory motor, and auditory regions) and cerebellar regions (Gaser and Schlaug 2003; Altenmüller and Schlaug 2015; Amunts et al. 1997). The frontal lobe is considered particularly relevant in regard to the potential age-decelerating function of music-making, since data show that it is strongly affected by any degeneration process relatively early on, while data also show that the frontal lobe in general plays an important role in core functions underlying music-making activities, namely, the planning, preparation, and controlled execution of fine motor actions (Gaser and Schlaug 2003; Amunts et al. 1997) (i.e., primary, premotor, and supplementary areas), auditory-motor interactions (Gaser and Schlaug 2003; Jäncke and Shah 2002; Maess et al. 2001) (i.e., inferior frontal gyri and premotor regions), and most importantly executive functions (Jäncke 2013; Gaser and Schlaug 2003; Sluming et al. 2002, 2007; Bugos et al. 2007; Bialystok and DePape 2009) (i.e., dorsolateral prefrontal cortex). The specific and long-term engagement of these regions during music-making activities might stimulate and maintain neurovascular support for metabolic demands of synaptic and neural activities, enhance synaptic plasticity and glial activity, and might lead to a richer connectivity profile protecting this vulnerable part of the brain from aging-related structural changes. Similar effects have been described in animal experiments (Zheng and Purves 1995; van Praag et al. 1999; Markham and Greenough 2004).
Our findings further suggest that making music has a stronger age-decelerating effect when it is not performed as a main profession, but as a leisure or extracurricular activity, possibly enriching a person’s life with multisensory, motor, and socio-affective experiences in addition to other main activities that an amateur musician would engage in. Apparently, the beneficial effects of making music do not infinitely increase with the intensity of practicing musical skills. Brain plasticity may also be maladaptive and have negative consequences when it is driven by extensively performed and highly specialized repetitive sensorimotor activities for many hours per day as well as during stressful public performance periods, resulting in a less enriched environment and sometimes even leading to negative health effects such as overuse syndrome and focal dystonia (Lim et al. 2001). Although both musician groups (professional and amateur musicians) showed the age-decelerating effects, surprisingly professional musicians had slightly less of an effect than amateur musicians, revealing a slightly positive relationship between the time engaged in music-making activities and BrainAGE scores. This somewhat diminishes the overall age-decelerating effect of music-making and it could be due to the burden of a professional music career when dose and intensity of one activity are significantly increased and stress levels might go up as well considering the increasing demands of performing highly skilled activities at very high levels. Chronic stress has been shown to accelerate the atrophying process in the brain, since it increases the glucocorticoid level that damages neurons especially in the hippocampus and the prefrontal cortex in the long term, as shown in animals and humans with stress-related disorders (Radley et al. 2015). The highly competitive situation professional musicians are usually exposed to promotes a stressful lifestyle with dysfunctional manifestations as reflected in higher prevalence rates of anxiety, depression, and sleep disturbance (Vaag et al. 2016a, b). Furthermore, other lifestyle differences between professional musicians and amateur musicians that were not quantitatively assessed in our questionnaires (e.g., weekly alcohol consumption) could have accounted for some of the subtle differences between these two musician groups, but would still not explain the differences between both musician groups and the non-musician group. We have to also consider that professional musicians might not experience music-making activities anymore as an enrichment when compared to amateur musicians for whom music-making might just be a fun and multisensory activity to engage in addition to their main professional activity. Evidence from several rodent studies relating environmental enrichment with increased gene expression of neurotrophical factors, neuro- and gliogenesis, increased synaptic density, dendritic branching, new synapse formation in several cortical and subcortical brain areas, reduction in cerebral β-amyloid level and amyloid deposits, and finally increased size and weight of an animal’s brain (Mora et al. 2007; Lazarov et al. 2005; Kempermann et al. 1997; Hamilton and Kolb 2005; Kolb et al. 2003b; van Praag et al. 2000; Anderson 2011) demonstrate the capacity of enrichment as a functional and structural brain plasticity enhancer. Furthermore, environmental enrichment has been shown to suppress the rats’ behavioral and neurochemical response (i.e., dopamine release in the prefrontal cortex) to acute stress (Segovia et al. 2008) and, in line with our findings, provides a buffer against an aging process in the rodent’s brain, preserving cortical thickness (Diamond et al. 1985), dendritic branching and spines (Kolb et al. 2003a), and neuro- and gliogenesis (Kempermann et al. 1998; Soffié et al. 1999). In contrast to professional musicians, amateur musicians perform music mostly as a leisure activity, constituting an enrichment activity outside their main profession and thus allowing them to exploit to a greater extent the enrichment-dependent plasticity effect of music-making.
Conclusions
Making music is a widespread leisure and professional activity often done throughout someone’s lifetime. We determined the discrepancy between a person’s chronological age and an index of their “real brain age”, termed “brain age”, estimated from magnetic resonance images using a machine-learning algorithm in subjects that differed in their lifetime dose of music-making activities. Separating musicians into a group of amateur and professional musicians, we were able to show that both groups of musicians had BrainAGE scores that suggested younger brains than those of a matched group of non-musicians; amateur musicians had the youngest appearing brains. The “age-decelerating” effect in professional musicians was slightly less prominent and showed an inverse relationship with the dose of music-making. At a professional level, intense music-making activities could also be associated with a stress-related interference and possibly create a less enriched environment than that of amateur musicians possibly somewhat diminishing the otherwise positive effect of instrumental music-making. Our findings may have important implication for Public Health and ultimately Brain Health given that a larger portion of the population have the potential to engage in making music at a leisure/extracurricular level, rather than at a professional level.
References
Altenmüller E, Schlaug G (2015) Apollo’s gift: new aspects of neurologic music therapy. Prog Brain Res 217:237–252
Ames A (2000) CNS energy metabolism as related to function. Brain Res Rev 34(1):42–68
Amunts K, Schlaug G, Jäncke L, Steinmetz H, Schleicher A, Dabringhaus A, Zilles K (1997) Motor cortex and hand motor skills: structural compliance in the human brain. Hum Brain Mapp 5(3):206–215
Anderson BJ (2011) Plasticity of gray matter volume: the cellular and synaptic plasticity that underlies volumetric change. Dev Psychobiol 53(5):456–465
Anderson BJ, Eckburg PB, Relucio KI (2002) Alterations in the thickness of motor cortical subregions after motor-skill learning and exercise. Learn Mem 9(1):1–9. doi:10.1101/lm.43402
Ashburner J, Friston KJ (2005) Unified segmentation. Neuroimage 26(3):839–851
Attwell D, Buchan AM, Charpak S, Lauritzen M, MacVicar BA, Newman EA (2010) Glial and neuronal control of brain blood flow. Nature 468(7321):232–243
Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullén F (2005) Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci 8(9):1148–1150
Bialystok E, DePape A (2009) Musical expertise, bilingualism, and executive functioning. J Exp Psychol Hum Percept Perform 35(2):565
Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT (1990) Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci 87(14):5568–5572
Bugos JA, Perlstein WM, McCrae CS, Brophy TS, Bedenbaugh PH (2007) Individualized piano instruction enhances executive functioning and working memory in older adults. Aging Ment Health 11(4):464–471
Chan AS, Ho Y, Cheung M (1998) Music training improves verbal memory. Nature 396(6707):128
Cheng S (2016) Cognitive reserve and the prevention of dementia: the role of physical and cognitive activities. Curr Psychiatry Rep 18(9):85
Davidson RJ, McEwen BS (2012) Social influences on neuroplasticity: stress and interventions to promote well-being. Nat Neurosci 15(5):689–695
Devor A, Dunn AK, Andermann ML, Ulbert I, Boas DA, Dale AM (2003) Coupling of total hemoglobin concentration, oxygenation, and neural activity in rat somatosensory cortex. Neuron 39(2):353–359
Diamond MC, Johnson RE, Protti AM, Ott C, Kajisa L (1985) Plasticity in the 904-day-old male rat cerebral cortex. Exp Neurol 87(2):309–317
Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A (2004) Neuroplasticity: changes in grey matter induced by training. Nature 427(6972):311–312
Forgeard M, Winner E, Norton A, Schlaug G (2008) Practicing a musical instrument in childhood is associated with enhanced verbal ability and nonverbal reasoning. PLoS One 3(10):e3566. doi:10.1371/journal.pone.0003566
François C, Grau-Sánchez J, Duarte E, Rodriguez-Fornells A (2015) Musical training as an alternative and effective method for neuro-education and neuro-rehabilitation. Front Psychol 6:475
Franke K, Gaser C (2012) Longitudinal changes in individual BrainAGE in healthy aging, mild cognitive impairment, and Alzheimer’s disease. Geropsych J Gerontopsychol Geriatr Psychiatry 25(4):235
Franke K, Ziegler G, Klöppel S, Gaser C, Alzheimer’s Disease Neuroimaging Initiative (2010) Estimating the age of healthy subjects from T1-weighted MRI scans using kernel methods: exploring the influence of various parameters. Neuroimage 50(3):883–892
Franke K, Luders E, May A, Wilke M, Gaser C (2012) Brain maturation: predicting individual BrainAGE in children and adolescents using structural MRI. Neuroimage 63(3):1305–1312
Franke K, Gaser C, Manor B, Novak V (2013) Advanced BrainAGE in older adults with type 2 diabetes mellitus. Front Aging Neurosci 5:90
Franke K, Ristow M, Gaser C (2014) Gender-specific impact of personal health parameters on individual brain aging in cognitively unimpaired elderly. Front Aging Neurosci 6:94
Franke K, Hagemann G, Schleussner E, Gaser C (2015) Changes of individual BrainAGE during the course of the menstrual cycle. Neuroimage 115:1–6
Gaser C, Schlaug G (2003) Brain structures differ between musicians and non-musicians. J Neurosci 23(27):9240–9245
Gaser C, Franke K, Klöppel S, Koutsouleris N, Sauer H (2013) BrainAGE in mild cognitive impaired patients: predicting the conversion to Alzheimer’s disease. PLoS One 8(6):e67346
Golestani N, Paus T, Zatorre RJ (2002) Anatomical correlates of learning novel speech sounds. Neuron 35(5):997–1010
Hall CB, Lipton RB, Sliwinski M, Katz MJ, Derby CA, Verghese J (2009) Cognitive activities delay onset of memory decline in persons who develop dementia. Neurology 73(5):356–361. doi:10.1212/WNL.0b013e3181b04ae3
Hallam S (2010) The power of music: its impact on the intellectual, social and personal development of children and young people. Int J Music Educ 28(3):269–289
Halwani GF, Loui P, Rüber T, Schlaug G (2011) Effects of practice and experience on the arcuate fasciculus: comparing singers, instrumentalists, and non-musicians. Front Psychol 2:39–47
Hamilton DA, Kolb B (2005) Differential effects of nicotine and complex housing on subsequent experience-dependent structural plasticity in the nucleus accumbens. Behav Neurosci 119(2):355
Hanna-Pladdy B, Gajewski B (2012) Recent and past musical activity predicts cognitive aging variability: direct comparison with general lifestyle activities. Training-induced cognitive and neural plasticity. Front Hum Neurosci 6:198
Hanna-Pladdy B, MacKay A (2011) The relation between instrumental musical activity and cognitive aging. Neuropsychology 25(3):378
Herholz SC, Zatorre RJ (2012) Musical training as a framework for brain plasticity: behavior, function, and structure. Neuron 76(3):486–502
Ho Y, Cheung M, Chan AS (2003) Music training improves verbal but not visual memory: cross-sectional and longitudinal explorations in children. Neuropsychology 17(3):439
Hyde KL, Lerch J, Norton A, Forgeard M, Winner E, Evans AC, Schlaug G (2009) Musical training shapes structural brain development. J Neurosci 29(10):3019–3025
Jancke L (2009a) Music drives brain plasticity. F1000 Biol Rep 1:78. doi:10.3410/B1-78
Jancke L (2009b) The plastic human brain. Restor Neurol Neurosci 27(5):521–538. doi:10.3233/RNN-2009-0519
Jäncke L (2013) Music making and the aging brain. Zeitschrift für Neuropsychologie 24(2):113–121. doi:10.1024/1016-264X/a000095
Jäncke L, Shah NJ (2002) Does dichotic listening probe temporal lobe functions? Neurology 58(5):736–743
Johansson BB (2004) Functional and cellular effects of environmental enrichment after experimental brain infarcts. Restor Neurol Neurosci 22(3–5):163–174
Jonasson LS, Nyberg L, Kramer AF, Lundquist A, Riklund K, Boraxbekk CJ (2016) Aerobic exercise intervention, cognitive performance, and brain structure: results from the physical influences on brain in aging (PHIBRA) study. Front Aging Neurosci 8:336
Katzman R (1993) Education and the prevalence of dementia and Alzheimer’s disease. Neurology 43(1):13–20
Katzman R, Aronson M, Fuld P, Kawas C, Brown T, Morgenstern H, Frishman W, Gidez L, Eder H, Ooi WL (1989) Development of dementing illnesses in an 80-year-old volunteer cohort. Ann Neurol 25(4):317–324
Kempermann G, Kuhn HG, Gage FH (1997) More hippocampal neurons in adult mice living in an enriched environment. Nature 386(6624):493–495
Kempermann G, Kuhn HG, Gage FH (1998) Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci 18(9):3206–3212
Kleim JA, Lussnig E, Schwarz ER, Comery TA, Greenough WT (1996) Synaptogenesis and Fos expression in the motor cortex of the adult rat after motor skill learning. J Neurosci 16(14):4529–4535
Kolb B, Gibb R, Gorny G (2003a) Experience-dependent changes in dendritic arbor and spine density in neocortex vary qualitatively with age and sex. Neurobiol Learn Mem 79(1):1–10
Kolb B, Gorny G, Söderpalm AHV, Robinson TE (2003b) Environmental complexity has different effects on the structure of neurons in the prefrontal cortex versus the parietal cortex or nucleus accumbens. Synapse 48(3):149–153
Koutsouleris N, Davatzikos C, Borgwardt S, Gaser C, Bottlender R, Frodl T, Falkai P, Riecher-Rössler A, Möller H, Reiser M (2014) Accelerated brain aging in schizophrenia and beyond: a neuroanatomical marker of psychiatric disorders. Schizophr Bull 40(5):1140–1153
Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, Chason J, Vakil E, Bardell L, Boileau RA, Colcombe A (1999) Ageing, fitness and neurocognitive function. Nature 400(6743):418–419. doi:10.1038/22682
Lazarov O, Robinson J, Tang Y, Hairston IS, Korade-Mirnics Z, Lee VM, Hersh LB, Sapolsky RM, Mirnics K, Sisodia SS (2005) Environmental enrichment reduces Aβ levels and amyloid deposition in transgenic mice. Cell 120(5):701–713
Lim VK, Altenmüller E, Bradshaw JL (2001) Focal dystonia: current theories. Hum Mov Sci 20(6):875–914
Löwe LC, Gaser C, Franke K, Alzheimer’s Disease Neuroimaging Initiative (2016) The effect of the APOE genotype on individual BrainAGE in normal aging, mild cognitive impairment, and Alzheimer’s disease. PLoS One 11(7):e0157514
Luders E, Cherbuin N, Gaser C (2016) Estimating brain age using high-resolution pattern recognition: younger brains in long-term meditation practitioners. Neuroimage 134:508–513
Maess B, Koelsch S, Gunter TC, Friederici AD (2001) Musical syntax is processed in Broca’s area: an MEG study. Nat Neurosci 4(5):540–545
Marcus DS, Wang TH, Parker J, Csernansky JG, Morris JC, Buckner RL (2007) Open Access Series of Imaging Studies (OASIS): cross-sectional MRI data in young, middle aged, nondemented, and demented older adults. J Cogn Neurosci 19(9):1498–1507
Markham JA, Greenough WT (2004) Experience-driven brain plasticity: beyond the synapse. Neuron Glia Biol 1(4):351–363. doi:10.1017/s1740925x05000219
Mintun MA, Lundstrom BN, Snyder AZ, Vlassenko AG, Shulman GL, Raichle ME (2001) Blood flow and oxygen delivery to human brain during functional activity: theoretical modeling and experimental data. Proc Natl Acad Sci 98(12):6859–6864
Mora F, Segovia G, del Arco A (2007) Aging, plasticity and environmental enrichment: structural changes and neurotransmitter dynamics in several areas of the brain. Brain Res Rev 55(1):78–88
Moreno S, Marques C, Santos A, Santos M, Castro SL, Besson M (2009) Musical training influences linguistic abilities in 8-year-old children: more evidence for brain plasticity. Cereb Cortex 19(3):712–723
Münte TF, Altenmüller E, Jäncke L (2002) The musician’s brain as a model of neuroplasticity. Nat Rev Neurosci 3(6):473–478
Prakash RS, Voss MW, Erickson KI, Kramer AF (2015) Physical activity and cognitive vitality. Annu Rev Psychol 66:769–797
Radley J, Morilak D, Viau V, Campeau S (2015) Chronic stress and brain plasticity: mechanisms underlying adaptive and maladaptive changes and implications for stress-related CNS disorders. Neurosci Biobehav Rev 58:79–91
Rüber T, Lindenberg R, Schlaug G (2015) Differential adaptation of descending motor tracts in musicians. Cereb Cortex 25(6):1490–1498
Scarmeas N, Levy G, Tang M, Manly J, Stern Y (2001) Influence of leisure activity on the incidence of Alzheimer’s disease. Neurology 57(12):2236–2242
Schellenberg EG (2004) Music lessons enhance IQ. Psychol Sci 15(8):511–514
Schlaug G (2001) The brain of musicians. Ann N Y Acad Sci 930(1):281–299
Schlaug G (2015) Chapter 3—musicians and music making as a model for the study of brain plasticity. In: Altenmüller E, Finger S, Boller F (eds) Progress in brain research: music, neurology, and neuroscience: evolution, the musical brain, medical conditions, and therapies, vol 217. Elsevier, Amsterdam, pp 37–55
Schneider P, Scherg M, Dosch HG, Specht HJ, Gutschalk A, Rupp A (2002) Morphology of Heschl’s gyrus reflects enhanced activation in the auditory cortex of musicians. Nat Neurosci 5(7):688–694. doi:10.1038/nn871
Schölkopf B, Smola AJ (2002) Learning with kernels: support vector machines, regularization, optimization, and beyond. MIT Press, Cambridge
Scholz J, Klein MC, Behrens TEJ, Johansen-Berg H (2009) Training induces changes in white-matter architecture. Nat Neurosci 12(11):1370–1371
Segovia G, del Arco A, de Blas M, Garrido P, Mora F (2008) Effects of an enriched environment on the release of dopamine in the prefrontal cortex produced by stress and on working memory during aging in the awake rat. Behav Brain Res 187(2):304–311
Seither-Preisler A, Parncutt R, Schneider P (2014) Size and synchronization of auditory cortex promotes musical, literacy, and attentional skills in children. J Neurosci 34(33):10937–10949. doi:10.1523/JNEUROSCI.5315-13.2014
Shipley WC (1940) A self-administering scale for measuring intellectual impairment and deterioration. J Psychol 9(2):371–377
Sluming V, Barrick T, Howard M, Cezayirli E, Mayes A, Roberts N (2002) Voxel-based morphometry reveals increased gray matter density in Broca’s area in male symphony orchestra musicians. Neuroimage 17(3):1613–1622
Sluming V, Brooks J, Howard M, Downes JJ, Roberts N (2007) Broca’s area supports enhanced visuospatial cognition in orchestral musicians. J Neurosci 27(14):3799–3806
Soffié M, Hahn K, Terao E, Eclancher F (1999) Behavioural and glial changes in old rats following environmental enrichment. Behav Brain Res 101(1):37–49
Steele CJ, Bailey JA, Zatorre RJ, Penhune VB (2013) Early musical training and white-matter plasticity in the corpus callosum: evidence for a sensitive period. J Neurosci 33(3):1282–1290
Stern Y (2006) Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord 20(2):112–117
Swain RA, Harris AB, Wiener EC, Dutka MV, Morris HD, Theien BE, Konda S, Engberg K, Lauterbur PC, Greenough WT (2003) Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience 117(4):1037–1046
Tierney AT, Krizman J, Kraus N (2015) Music training alters the course of adolescent auditory development. Proc Natl Acad Sci. doi:10.1073/pnas.1505114112
Tipping ME (2001) Sparse Bayesian learning and the relevance vector machine. J Mach Learn Res 1(Jun):211–244
Vaag J, Bjørngaard JH, Bjerkeset O (2016a) Symptoms of anxiety and depression among Norwegian musicians compared to the general workforce. Psychol Music 44(2):234–248
Vaag J, Saksvik-Lehouillier I, Bjørngaard JH, Bjerkeset O (2016b) Sleep difficulties and insomnia symptoms in norwegian musicians compared to the general population and workforce. Behav Sleep Med 14(3):325–342
van Praag H, Christie BR, Sejnowski TJ, Gage FH (1999) Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci 96(23):13427–13431. doi:10.1073/pnas.96.23.13427
van Praag H, Kempermann G, Gage FH (2000) Neural consequences of environmental enrichment. Nat Rev Neurosci 1(3):191–198
Verghese J, Lipton RB, Katz MJ, Hall CB, Derby CA, Kuslansky G, Ambrose AF, Sliwinski M, Buschke H (2003) Leisure activities and the risk of dementia in the elderly. N Engl J Med 348(25):2508–2516
Verghese J, LeValley A, Derby C, Kuslansky G, Katz M, Hall C, Buschke H, Lipton RB (2006) Leisure activities and the risk of amnestic mild cognitive impairment in the elderly. Neurology 66(6):821–827
Wan CY, Schlaug G (2010) Music making as a tool for promoting brain plasticity across the life span. Neuroscientist 16(5):566–577
Wan CY, Zheng X, Marchina S, Norton A, Schlaug G (2014) Intensive therapy induces contralateral white matter changes in chronic stroke patients with Broca’s aphasia. Brain Lang 136:1–7
White-Schwoch T, Carr KW, Anderson S, Strait DL, Kraus N (2013) Older adults benefit from music training early in life: biological evidence for long-term training-driven plasticity. J Neurosci 33(45):17667–17674. doi:10.1523/JNEUROSCI.2560-13.2013
Wise RA (2004) Dopamine, learning and motivation. Nat Rev Neurosci 5(6):483–494. doi:10.1038/nrn1406
Zheng D, Purves D (1995) Effects of increased neural activity on brain growth. Proc Natl Acad Sci 92(6):1802–1806
Acknowledgements
The authors acknowledge support from the National Science Foundation (BCS-0132508), the NIH/NIDCD (RO1-DC009823), and the Swiss National Science Foundation (P1ZHP1_158642, P2ZHP1_168587) to carry out this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
The study was approved by the local ethics committee and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All participants gave written informed consent prior to their inclusion in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Rogenmoser, L., Kernbach, J., Schlaug, G. et al. Keeping brains young with making music. Brain Struct Funct 223, 297–305 (2018). https://doi.org/10.1007/s00429-017-1491-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-017-1491-2