Abstract
It is debated how language and praxis are co-represented in the left hemisphere (LH). As voxel-based lesion-symptom mapping in LH stroke patients with aphasia and/or apraxia may contribute to this debate, we here investigated the relationship between language and praxis deficits at the behavioral and lesion levels in 50 sub-acute stroke patients. We hypothesized that language and (meaningful) action are linked via semantic processing in Broca’s region. Behaviorally, half of the patients suffered from co-morbid aphasia and apraxia. While 24 % (n = 12) of all patients exhibited aphasia without apraxia, apraxia without aphasia was rare (n = 2, 4 %). Left inferior frontal, insular, inferior parietal, and superior temporal lesions were specifically associated with deficits in naming, reading, writing, or auditory comprehension. In contrast, lesions affecting the left inferior frontal gyrus, premotor cortex, and the central region as well as the inferior parietal lobe were associated with apraxic deficits (i.e., pantomime, imitation of meaningful and meaningless gestures). Thus, contrary to the predictions of the embodied cognition theory, lesions to sensorimotor and premotor areas were associated with the severity of praxis but not language deficits. Lesions of Brodmann area (BA) 44 led to combined apraxic and aphasic deficits. Data suggest that BA 44 acts as an interface between language and (meaningful) action thereby supporting parcellation schemes (based on connectivity and receptor mapping) which revealed a BA 44 sub-area involved in semantic processing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is a longstanding debate how language and praxis are co-represented in the left hemisphere (LH) of the human brain (Roby-Brami et al. 2012). Currently, the notion of separate albeit partially overlapping LH networks for praxis and language that may be lesioned together by a stroke affecting the territory of the left middle cerebral artery (MCA) is prevailing. However, lesion studies which directly address this issue remain scarce (Kertesz et al. 1984). An alternative account for a joint vasculature-related vulnerability assumes that the association of aphasia and apraxia may result from lesion-induced disturbances of cognitive (sub-) functions critically involved in both language and praxis. Current cognitive models propose that the processing of language (Kümmerer et al. 2013) and action (Tessari et al. 2007) are both supported by a semantic (indirect) and non-semantic (direct) route. This cognitive similarity has inspired theories that assume a close link between language and praxis [e.g., motor theory of speech perception (Pulvermüller and Fadiga 2010), embodied cognition (Rizzolatti and Arbib 1998), evolution of language via gestural communication (Roby-Brami et al. 2012)].
Since language and praxis deficits are frequent sequelae of left-hemispheric stroke with strong impact upon rehabilitation outcome (Donkervoort et al. 2006; Laska et al. 2001) that debate has also important implications: a better understanding of the pathophysiology of aphasia and apraxia is needed to inspire both theories of language and praxis and new therapeutic approaches.
To inform the on-going debate on whether language and praxis share a common neural basis and to elucidate how these two dissociable cognitive functions are represented within the same hemisphere of the human brain, we here employed detailed neuropsychological testing and precise statistical lesion analyses [voxel-based lesion-symptom mapping, VLSM (Bates et al. 2003)] in a clinically representative sample of LH stroke patients (n = 50). Based on previous reports postulating an important role of Broca’s region for the interaction of language and praxis (Binkofski and Buccino 2004; Kühn et al. 2013; Nishitani et al. 2005; Willems and Hagoort 2007), we were especially interested to investigate whether one of the sub-areas characterized in the recently proposed parcellation schemes of Broca’s region (i.e., Brodmann’s areas (BA) 44 and 45) based on connectivity (Clos et al. 2013) and receptor mapping (Amunts et al. 2010) serves as a neural interface of language and praxis. In view of the importance of semantic processing for the interplay between language and (meaningful) action (Mengotti et al. 2013; Saygin et al. 2004), the anterior-ventral sub-area of BA 44, specifically involved in the detection of (supra-modal) meaning (cluster 3 of Clos et al. 2013), constitutes a promising candidate region for this interface.
Methods
Patients
In total, 69 patients, who were admitted to the Department of Neurology of the University Hospital Cologne with the tentative diagnosis of a (ischemic or hemorrhagic) LH stroke, were consecutively recruited in different time intervals during the period from January 2009 to May 2011. These patients fulfilled the inclusion criteria for the neuropsychological testing (i.e., sufficient knowledge of German, age >18 or <90 years, right-handedness, and no other neurological or psychiatric diseases affecting cognitive abilities).
In 3 patients, stroke diagnosis was not confirmed (e.g., sinus vein thrombosis or subarachnoid hemorrhage). Lesion analysis could be performed in 50 of the remaining 66 patients due to additional exclusion criteria specifically related to this procedure: bi-hemispheric lesions according to patient history or imaging data [n = 14; note that patients with right-hemispheric defects greater than 10 mm were considered to suffer from bi-hemispheric lesions, (Weisberg 1988)] or lack of lesion demarcation in clinical imaging despite persisting neurological/neuropsychological deficit (n = 2).
The final patient sample (n = 50; 33 males, 66 %) had a mean age of 65.9 years (SD 13.7, median 71, range 34–87). Five of the 50 patients (10 %) suffered from a hemorrhagic stroke. Ischemic strokes (n = 45) involved the carotid artery territory in 34 cases (76 %, including two combined ACA and MCA infarcts and one anterior borderzone infarction) and the vertebro-basilar artery territory in 8 cases (18 %, mainly affecting the territory of the posterior cerebral artery, PCA). The remaining three cases (7 %) with ischemic strokes presented with unilateral multifocal lesions in both vascular territories. There was one patient who presented with an additional infra-tentorial lesion affecting the pons. Thus, the current sample constitutes a clinically representative sample of LH stroke patients according to the Lausanne stroke registry (Bogousslavsky et al. 1988).
The mean time interval between stroke onset and detailed neuropsychological assessment was 6.53 days (median 5.0, range 2–20, SD 4.72). All patients were examined during the sub-acute stage of their disease (i.e., >24 h post-stroke; Hillis et al. 2002). The study was approved by the local ethics committee.
Neuropsychological assessment
Based on the models proposing a semantic and a non-semantic route for both language and praxis we used a battery of seven neuropsychological tasks that assessed the integrity of both the semantic and the non-semantic routes (for language: naming, reading, writing, and auditory comprehension; for praxis: pantomime and imitation of meaningful and meaningless gestures). Note that the performance in many language and praxis tasks depends on contributions of both routes [e.g., for naming cf. (Schwartz et al. 2012); for imitation cf. (Rumiati et al. 2005)] rather than one route only. These seven tasks were administered by a clinical neuropsychologist of the University Hospital Cologne and were based on a larger neuropsychological bedside screening tool specifically designed for stroke patients in the sub-acute stage of stroke [Cologne Neuropsychological Screening for Stroke patients, German: Kölner Neuropsychologisches Screening für Schlaganfall-Patienten (KöpSS); Kaesberg et al. 2013b].
The following language tasks were applied:
-
Naming The patient is asked to name a compound noun and an action depicted on a picture. As the third item, the patient is asked to describe with one sentence a scene which is presented as a picture.
-
Reading aloud The patient is asked to read aloud a compound noun, a verb, and a sentence describing an action.
-
Writing The patient is asked to write down a compound noun, a verb, and a sentence describing an action all of which are read by the examiner. The patient is allowed to use her/his preferred hand. It has to be mentioned that the writing task could not be performed in nine patients. In most of these cases, right-sided hemiparesis precluded the proper evaluation of writing performance. Moreover, some patients refused to write with the left hand.
-
Auditory comprehension A compound noun, a verb, and a simple sentence describing an action are read out aloud to the patient. For each of the three items, the patient is asked to choose the matching picture among three different options. The other two pictures show a semantically related and a neutral (unrelated) distractor.
The following praxis tasks were applied:
The praxis tasks were performed with the left, i.e., ipsi-lesional hand.
-
Pantomime The patient is shown six pictures illustrating different objects (ball-point pen, dice, drinking glass, razor, toothbrush, and watering pot). In response to each picture, the patient is asked to pantomime the use of the depicted object. One patient could not be tested as he refused to perform pantomimes with his left, non-dominant hand/arm.
-
Imitation of meaningful gestures Based on four pictures which show a person demonstrating different meaningful gestures (blowing cheeks, sticking out the tongue, urging somebody to be quiet, and the stop-sign gesture), the patient is asked to imitate these four meaningful gestures.
-
Imitation of meaningless gestures The patient has to imitate two meaningless gestures (moving in the lips, holding the open hand close to the ipsi-lateral ear). Again, the gestures are presented as pictures of a person demonstrating these two meaningless gestures.
Each neuropsychological task was explained to the patient using an example item. The score range per task item was 0–2. Therefore, the maximum score for a domain (language, praxis) consisting of 12 items each was 24. Impairment in a given neuropsychological task was defined as scoring less than the maximum score (i.e., failing at one item). Note that patients were allowed to correct their performance in a given trial. Corrected trials were not scored as errors.
Patients in whom at least one of the routes for language or praxis was impaired (i.e., they showed deficient performance in at least one of the language or praxis tasks) were classified as aphasic or apraxic, respectively. This approach of defining cognitive impairment has already been used in previous studies (Dovern et al. 2011; Vossel et al. 2012).
Lesion mapping
Lesion mapping was performed using either MRI (n = 26) or CT (n = 24). If multiple images were available, the image set closest to the date of neuropsychological testing was selected. In addition to the current stroke lesion, microangiopathic lesions in both hemispheres were mapped if they were between 5 and 10 mm in diameter (see also Wahlund et al. 2001).
As in (Schnur et al. 2009), we identified lesion boundaries on a standard Montreal Neurological Institute (MNI) template using the freely available MRIcron software package (http://www.mccauslandcenter.sc.edu/mricro/mricron/install.html; MRIcron, RRID:nif-0000-00122) after reslicing the T1-weighted MNI template brain (ch2.nii) to match the angle of acquisition for each patient’s scan. Then, we matched each slice of the patient’s scan to a slice in the MNI template. Thereafter, SDU manually drew the lesion contour onto the corresponding axial slices of the template (at 5 mm slice distance). As CT scans provide fewer slices than the template, we manually interpolated between CT slices to render the lesion on the respective slices of the template. This manual tracing technique is currently considered to be best suited for exact lesion delineation (Wilke et al. 2011). Lesion mapping was consecutively checked by PHW, a board-certified neurologist. Both examiners were blind to the individual patient’s neuropsychological performance at the time of lesion mapping and had to agree upon the exact lesion extent.
Statistical analyses
All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS®, IBM®) version 22 and the above-mentioned MRIcroN software package. To assess possible correlations between task scores, Spearman non-parametric correlation analyses were performed, since the task scores were not normally distributed (as assessed by the Kolmogorov–Smirnov test). Results are reported at p < 0.05 (Bonferroni-corrected).
Voxel-based lesion-symptom mapping (VLSM) was performed for the individual task scores of the four language and three praxis tasks as well as for the domain scores (language, praxis). For all VLSM analyses, only voxels damaged in at least 5 % of the patients were included. All VLSM results are reported at p < 0.05 [family-wise error (FWE) Bonferroni-corrected for the number of unique lesion patterns].
The anatomical interpretation of the lesion mapping results was performed using the SPM Anatomy toolbox (Eickhoff et al. 2005; http://www.fz-juelich.de/inm/inm-1/DE/Forschung/_docs/SPMAnatomyToolbox/SPMAnatomyToolbox_node.html).
Results
Behavioral data
Fifty-four percent of the patients with sub-acute left-hemispheric stroke suffered from apraxia (n = 27), while 74 % were aphasic (n = 37).
Impairment of specific language functions was found with the following prevalence rates: 34 % (n = 17) for auditory comprehension, 50 % (n = 25) for reading, 52 % (n = 26) for naming, and 66 % (n = 27) for writing (note that only 41 patients could be tested for writing). The severity of specific language impairments reflected by the individual task scores correlated significantly for all language tasks (p < 0.0014). The strongest correlation was revealed between the naming and reading aloud tasks (r = 0.86, Table 1).
Specific praxis deficits were found in 23 (47 %) of the 49 patients tested for pantomimes, in 21 (42 %) of the 50 patients tested for the imitation of meaningful gestures, and in 19 (38 %) of the 50 patients tested for the imitation of meaningless gestures. Within the praxis domain, all task scores correlated significantly with each other (p < 0.0014). The strongest correlation was found between pantomime and the imitation of meaningful gestures (r = 0.79, Table 1).
Regarding the association of aphasia and apraxia, the most common constellation was combined aphasia and apraxia which was found in 50 % (n = 25) of the patients. Approximately two-thirds (67 %, n = 25) of all aphasic patients (n = 37) were also apraxic. Conversely, 93 % of all apraxic patients additionally suffered from aphasia. This predominance of aphasic deficits after LH stroke is in accordance with clinical experience and empirical studies (De Ajuriaguerra et al. 1960; De Renzi et al. 1980). Aphasia without apraxia was present in 24 % (n = 12) of all cases, while we only found two patients (4 %) suffering from apraxia who showed no symptoms of aphasia. Eleven patients (22 %) did not show any language or praxis impairment. Note that the writing task score was not available for two of these 11 patients, who performed above cut-off in all the other six tasks. Fisher’s exact test revealed that the prevalence of aphasia was not independent of that of apraxia (p < 0.05). Furthermore, the domain scores for language and praxis correlated significantly with each other (p < 0.0014, Table 1).
Moreover, the between-domain correlations (i.e., correlations between the scores of the language and the praxis tasks) were significant for almost all pairings with the exception of the following two: between the scores of the writing and imitation of meaningless gestures tasks (r = 0.40, p = 0.011), and also between the language domain score and the score for the imitation of meaningless gestures task (r = 0.48, p = 0.002; see Table 1). Furthermore, the performance scores of all language tasks and the language domain score correlated best with the pantomime score.
Lesion analysis
General distribution of the lesions
The lesion overlay plot (n = 50) is shown in Fig. 1. Consistent with the fact that the current sample constituted a clinically representative sample of LH stroke patients, the highest lesion overlap was observed within the territory of the middle cerebral artery (MCA), especially in the insula and adjacent subcortical areas, i.e., areas of the core MCA territory. In contrast, (anterior) frontal and parietal regions were affected to a lesser degree. Note that anterior (inferior frontal) and posterior (temporo-parietal) peri-sylvian brain regions were affected to a similar degree.
Lesions associated with severity of aphasia and specific aphasic deficits
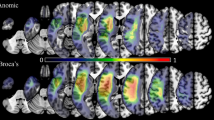
The severity of aphasia was reflected in the language domain score, which was the sum of the four language task scores. VLSM analysis based on this language domain score revealed that severity of aphasia was mainly associated with lesions involving the left inferior frontal gyrus (IFG), anterior insula, and superior temporal gyrus [STG, extending into the supramarginal gyrus (SMG); Fig. 2a; red as well as purple and yellow areas].
Lesion correlates of combined aphasic and apraxic deficits (VLSM). a Summary of VLSM results for the language domain score (red, n = 41), the praxis domain score in all available patients (dark blue, n = 49) and the praxis domain score in aphasic patients only (light blue, n = 36). The overlap of the VLSM results for the language and praxis domains in all available patients is shown in purple. The common lesion site revealed by the VLSM for the language domain (n = 41) and the VLSM for the praxis domain in aphasic patients only (n = 36) is depicted in yellow (note that the yellow marked common lesion site is part of the overlap lesion marked in purple). According to the Anatomy toolbox (Eickhoff et al. 2005), this common lesion marked in yellow was mainly located in Brodmann area 44 (i.e., the opercular part of the inferior frontal gyrus, IFG) as part of Broca’s region. All results are FWE Bonferroni-corrected for the number of unique lesion patterns, p < 0.05: z > 4.06 for aphasic deficits (i.e., language domain score, red, n = 41), z > 4.22 for apraxic deficits (i.e., praxis domain score in all available patients, dark blue, n = 49), and z > 4.16 for apraxic deficits in aphasia (i.e., praxis domain score in aphasic patients only, light blue, n = 36). MNI-z-coordinates from −7 to +58 are shown. b Relationship of the common lesion site for aphasic and apraxic deficits (marked yellow) with the anterior-ventral sub-area of BA 44 involved in semantic processing (blue, cluster 3 of Clos et al. 2013). Note that the BA 44 sub-area encompasses the specific lesion site for combined aphasia and apraxia in its full extent (axial slices at MNI-z-coordinates 8, 13, and 18 mm)
With respect to the single language tasks, further VLSM analyses showed that impairment in the naming and reading aloud tasks was associated with similar lesions in the left inferior frontal and superior temporal gyri (again extending into the SMG). However, the lesions associated with deficits in reading aloud were more extensive and encompassed also the dorsal portion of the IFG (Fig. 3a, b). At p < 0.05, FWE corrected for the number of unique lesion patterns, VLSM analysis for the writing task yielded a few significant voxels mainly within the rolandic operculum and precentral gyrus (Fig. 3c). Deficits in auditory comprehension were also mainly associated with damage to the left IFG and STG with additional involvement of the middle frontal gyrus and a more extended involvement of the STG (Fig. 3d).
Lesion correlates of deficits in the language tasks (VLSM). a Naming task (n = 50), b reading aloud task (n = 50), c writing task (n = 41), d auditory comprehension task (n = 50). All results are FWE Bonferroni-corrected for the number of unique lesion patterns; z > 4.22 for the naming, reading aloud and auditory comprehension tasks, z > 4.06 for the writing task. Only voxels damaged in at least 5 % of the patients were analyzed. MNI-z-coordinates from −7 to +58 are shown. Red circles delineate single significant voxels found for the writing task
Lesions associated with severity of apraxia and specific apraxic deficits
Voxel-based lesion-symptom mapping analysis based on the praxis domain score (i.e., the sum of the three praxis task scores) revealed that the severity of apraxia was associated with lesions of the left IFG, premotor cortex and the pre- and postcentral regions (Fig. 2a; dark blue as well as purple and yellow areas). VLSM analyses based on the individual praxis task scores showed a predominant association of impaired pantomime of object use with lesions of the left IFG, premotor and (pre-)central regions as well as the inferior parietal lobe (IPL, more precisely: SMG; Fig. 4a). Deficient imitation of meaningful gestures was related to lesions of the left IFG extending into the middle frontal gyrus, premotor cortex and peri-central regions (Fig. 4b). At this stringent threshold, only one significant voxel was found within the left superior parietal lobe. Deficits in imitating meaningless gestures were marginally associated with a few lesion voxels in the left IFG (triangular part only), but were strongly associated with lesions of the (post)-central regions (Fig. 4c). Overall, compared to pantomime impairments deficits in (meaningless) gesture imitation were more strongly related to lesions affecting the postcentral gyrus of the parietal lobe (Fig. 4a-c). Moreover, in patients with deficits in imitating meaningless gestures there was no association with lesions of the opercular part of the left IFG or of the premotor cortex (BA 6).
Lesion correlates of deficits in the praxis tasks (VLSM). a Pantomime task (n = 49), b imitation of meaningful gestures (n = 50), c Imitation of meaningless gestures (n = 50). All results are FWE Bonferroni-corrected for the number of unique lesion patterns; z > 4.22 for all praxis tasks. Only voxels damaged in at least 5 % of the patients were analyzed. MNI-z-coordinates from +3 to +68 are shown. The red circle delineates the only significant voxel found for the imitation of meaningful gestures in the left superior parietal lobe
Lesions associated with combined aphasic and apraxic deficits
The lesion sites common to both VLSM-analyses of the language and praxis domain scores represent the areas involved in the interaction of language and praxis in the left hemisphere (Fig. 2a, purple areas). Restricting the VLSM for the praxis domain to the aphasic patients only (i.e., examining the lesion sites associated with increasing severity of apraxia within the sample of aphasic patients only, Fig. 2a, light blue areas) should reveal the areas that are necessary for a proper interaction between the language and praxis systems rather than those areas that are generally involved in that interaction. This analysis could be performed in 36 of the 37 aphasic patients for whom complete praxis task scores were available and revealed a common lesion site in the opercular part of the left IFG (Fig. 2, yellow areas), which mainly corresponded to Brodmann area (BA) 44 according to the Anatomy toolbox (Eickhoff et al. 2005). Note that this common lesion site for combined aphasia and apraxia in the opercular part of the left IFG was mainly driven by the pantomime task and the imitation of meaningful gestures task, since deficits in imitating meaningless gestures were not significantly associated with lesions of the opercular part of the left IFG (see Fig. 4c).
Broca’s region consists of BA 44 and 45. However, receptor mapping (Amunts et al. 2010) and connectivity-based parcellation of Broca’s region (Clos et al. 2013) recently suggested that BA 44 can be further subdivided into BA 44v and BA 44d, which in turn consist of different functionally defined clusters (BA 44d: clusters 1 and 2; BA 44v: clusters 3 and 4; Clos et al. 2013). Therefore, we examined the relationship of the above reported common lesion site within BA 44 (reflecting the specific lesion site for combined aphasia and apraxia) with the four BA 44 clusters of Clos et al. (2013). The NIfTI-files of these clusters (normalized to MNI space) are freely available at: http://www.fz-juelich.de/inm/inm-1/DE/Forschung/Brain_Network_Modeling/Brain_Network_Modeling_node.html.
There was no overlap with the BA 44 clusters 1, 2, and 4. However, there was a substantial anatomical overlap between the common lesion site for combined aphasia and apraxia in the current study (yellow areas in Fig. 2a, b) and the anterior-ventral BA 44 cluster 3 of Clos et al. (2013; blue areas, Fig. 2b). Moreover, the BA 44 cluster 3 encompassed the specific lesion site for combined aphasia and apraxia in its full extension (see axial slices at MNI-z-coordinates 8, 13, and 18 mm, Fig. 2b).
The correspondence between cytoarchitectonically defined Broca’s region [i.e., BA 44 (red) and BA 45 (green)] and the specific lesion correlate of combined aphasic and apraxic deficits derived from the VLSM analyses (yellow) is further illustrated in Fig. 5 depicting a lateral view on the rendered left hemisphere of the MNI template brain provided by MRIcron. Note that the (semantic) cluster 3 of BA 44 (blue, Clos et al. 2013) forms—by definition—the anterior-ventral border of BA 44, i.e., this cluster borders on BA 45.
Relationship of the specific lesion correlate of combined aphasic and apraxic deficits with the cytoarchitectonically defined Brodmann areas 44 and 45. Lateral view on the rendered left hemisphere of the MNI template brain provided by MRIcron depicting cytoarchitectonically defined Broca’s region based on the maximum probability maps (MPMs) of Brodmann areas (BA) 44 (red) and 45 (green, Amunts et al. 1999, 2004). Depiction of the correspondence between the (macro-anatomical) landmarks of Broca’s region (i.e., pars opercularis and triangularis of the inferior frontal gyrus, IFG) and the specific lesion correlate of combined aphasic and apraxic deficits derived from the VLSM analyses (yellow), the anterior-ventral cluster 3 of Brodmann area (BA) 44 based on connectivity analyses (blue, Clos et al. 2013) as well as the MPMs of BA 44 (red) and BA 45 (green) based on cytoarchitecture (Amunts et al. 1999, 2004). Note that the cluster 3 of BA 44 forms—by definition—the anterior-ventral border of BA 44, i.e., this cluster borders on BA 45 (compare a and b)
Discussion
Characterizing aphasic and apraxic symptoms as deficits of the semantic and/or non-semantic route of language and praxis processing in a clinically representative sample of 50 patients with sub-acute left hemisphere (LH) stroke, we here showed by statistical lesion analyses that concurrent aphasia and apraxia are specifically associated with lesions affecting the left inferior frontal gyrus (IFG), in particular the connectivity-based anterior-ventral sub-area of Brodmann area (BA) 44 (cluster 3 of Clos et al. 2013). As this cluster is specifically involved in extracting meaning from sensory information and semantic processing, this finding strongly suggests that language and (meaningful) action interact via supra-modal semantic processing taking place in BA 44 of Broca’s region. This notion is corroborated by the current finding that the common lesion site for combined aphasia and apraxia in BA 44 was—for praxis—mainly driven by deficits in pantomiming and imitating meaningful gestures.
Prevalence of aphasic and apraxic deficits and the combination thereof
Patients were classified as aphasic (74 %, n = 37) or apraxic (54 %, n = 27) when at least one of the routes for language or praxis was impaired (i.e., they showed deficient performance in at least one of the neuropsychological tasks for a given domain, see also Dovern et al. 2011; Vossel et al. 2012). The frequency of co-morbid aphasia and apraxia in the current sample of LH stroke patients was 50 % (n = 25). We also observed dissociations between aphasia and apraxia: two patients (4 %) presented with apraxia in the absence of aphasia while almost a quarter of the patients (n = 12, 24 %) showed aphasia without apraxia. Taken together, previous studies (Papagno et al. 1993) and our current data suggest that in LH stroke the co-occurrence of aphasia and apraxia is the most common constellation. Also, the severity of both syndromes as assessed by language and praxis domain scores correlated significantly.
Lesion correlates of aphasic and apraxic deficits and the combination thereof
Deficits in the individual language and praxis tasks were predominantly associated with anterior and posterior lesions [for language: (1) anterior: IFG and anterior insula, and (2) posterior: superior temporal and supramarginal gyri; for praxis: (1) anterior: IFG, premotor and precentral gyrus, and (2) posterior: postcentral gyrus and supramarginal gyrus]. These lesion patterns suggest that processing in either domain (language, praxis) depends on the integrity of the semantic and non-semantic routes that comprise both anterior and posterior brain regions (Binkofski and Buxbaum 2013; Kümmerer et al. 2013; Saur et al. 2008; Tessari et al. 2007).
According to the embodied cognition theory, lesions to motor areas, like primary motor and premotor cortex, should result in deficits of processing action-related language. In stark contrast, in the current sample of sub-acute stroke patients the severity of praxis but not language deficits was associated with sensorimotor and premotor lesions (see Fig. 2). Therefore, the current data do not support the view that the processing of action-related language critically depends on the integrity of the sensorimotor and premotor regions.
By statistical lesion analysis for combined aphasic and apraxic deficits, we here identified the areas that are necessary for the interaction between language and (meaningful) action. This analysis yielded a lesion site located within the IFG that mainly corresponded to the connectivity-based anterior-ventral sub-area (cluster 3 of Clos et al. 2013) of BA 44 (Eickhoff et al. 2005). Therefore, the current data strongly suggest that BA 44 (or more precisely the anterior-ventral sub-area of BA 44) is critically involved in the interaction of the motor and language systems (Binkofski and Buccino 2004). Thus, the current clinico-anatomical study (based on structural lesion mapping, Bates et al. 2003) provides support for the functional significance of the recently developed connectivity-based parcellation method (derived from functional imaging, Eickhoff et al. 2011) for a better understanding of the pathophysiology of clinically important cognitive syndromes in neurological patients.
In addition to its prominent role in the human mirror neuron system (Rizzolatti and Craighero 2004), BA 44 subserves specific praxis functions (Caspers et al. 2010), e.g., action recognition (Buccino et al. 2004), action encoding (Fazio et al. 2009), or gesture imitation (Heiser et al. 2003). On the other hand, several language functions have traditionally been attributed to BA 44 as part of Broca’s region, e.g., syntactic processing (Heim et al. 2006), verbal fluency (Amunts et al. 2004), and lexical decision making (Heim et al. 2007). Accordingly, our finding that praxis and language processing interact in BA 44 is fully consistent with previous functional imaging studies (Kühn et al. 2013; Nishitani et al. 2005).
A recent study in a similar sized (n = 57) cohort of chronic LH stroke patients (Mengotti et al. 2013), which focused on the differential effects of lesions to the semantic (indirect) and non-semantic (direct) route for gesture imitation, demonstrated that lesions to the angular gyrus are associated with deficits in imitating meaningless gestures only (Weiss et al. 2001), while supramarginal gyrus lesions are associated with deficits in meaningful gesture imitation and linguistic impairments. In that study also IFG-lesions were associated with aphasic (i.e., naming and repetition) and apraxic (i.e., imitating meaningful gestures) deficits (page 2611 and Fig. 3; see also Goldenberg and Spatt 2009). Furthermore, a study of patients suffering from primary progressive aphasia revealed that the left anterior inferior parietal cortex (including the supramarginal gyrus) is a cortical relay station of the semantic pathway connecting the superior temporal cortex and the IFG (Nelissen et al. 2010). Therefore, language and praxis may interact in BA 44 as part of the IFG, since BA 44 serves as a convergence zone for the semantic and non-semantic routes of language and praxis (Friederici et al. 2006).
Alternatively, the previously demonstrated involvement of BA 44 in motor (Rumiati and Tessari 2002) and verbal (Rogalsky et al. 2008) working memory (WM) may—at least in part—account for the observed interaction of praxis and language in this area, since WM may constitute a cognitive (sub-)function critically involved in both language and praxis that is compromised by lesions of BA 44. This notion is supported by our behavioral finding that the severity of aphasic deficits (as assessed by the language domain score) predominantly correlated with pantomime performance (Table 1), which is known to rely on WM (Bartolo et al. 2003). Intact WM functioning is also a prerequisite for processing complex hierarchically structured sequences which is necessary for both language and praxis (Higuchi et al. 2009). Interestingly, BA 44 of the IFG is involved in processing complex grammar (Friederici et al. 2006) as well as in complex action sequences during (pantomiming) tool use (Goldenberg et al. 2007; Goldenberg and Spatt 2009) supporting claims of an evolutionary link between language and tool use (Roby-Brami et al. 2012).
Finally, the role of the anterior-ventral sub-area of IFG’s BA 44 (cluster 3 of Clos et al. 2013) in extracting meaning from sensory information and in semantic processing may predestine this area as the site of interaction between language and praxis: pantomime recognition deficits in aphasic patients (Saygin et al. 2004) and deficient recognition of meaningful (transitive and intransitive) gestures (Pazzaglia et al. 2008) are associated with left IFG lesions. Consistent with the current findings functional imaging and connectivity analysis revealed that the left IFG integrates language (speech) and action (pantomime) with respect to semantics (Willems et al. 2009). This semantic integration and unification function (Hagoort 2005) may well predispose BA 44, and more specifically its anterior-ventral sub-area, as the neural site of interaction between language and (meaningful) action (van Schie et al. 2006).
It should be noted that in the language system BA 45 rather than BA 44 is considered the site of semantic processing. Moreover, the classical semantic language pathway is thought to connect temporal areas (e.g., STG) with BA 45 (and 47) of the IFG via a ventral association tract (Saur et al. 2008), thus not involving the IPL. However, for praxis the semantic (indirect) route processing meaningful actions may well involve the (anterior) IPL (Mengotti et al. 2013; Nelissen et al. 2010). Based on the current findings, one might argue that BA 44 is involved in supra-modal semantic processing (common to both language and praxis) and thereby acts as the interaction site between language and (meaningful) action, while other areas (e.g., for language: BA 45; for praxis: IPL) are involved in modality-specific semantic processing.
In contrast to previous studies in chronic LH stroke patients (Dovern et al. 2011; Goldenberg and Karnath 2006; Mengotti et al. 2013), imitation deficits were not strongly associated with parietal lesions in the current study of sub-acute LH stroke patients. It is unlikely that this discrepancy results from differences in sample size, since the current sample size (n = 50) was well comparable to that of previous studies on imitation deficits (Dovern et al. 2011: n = 48; Goldenberg and Karnath 2006: n = 44; Mengotti et al. 2013: n = 57) or other apraxic deficits in LH stroke (Goldenberg et al. 2007; Goldenberg and Spatt 2009; Kalénine et al. 2010; Randerath et al. 2010). Rather, the different times post stroke are likely to have contributed to this apparent discrepancy. As mentioned before, our study constitutes the first investigating the interaction between language and (meaningful) action in the early sub-acute, rather than the chronic phase of LH stroke. In the current clinically representative sample of sub-acute LH stroke patients, the highest lesion overlap was observed in core regions of the MCA territory, while (anterior) frontal and parietal regions were affected to a lesser degree (Fig. 1). On the other hand, samples of chronic LH stroke patients usually show a greater involvement of parietal cortex (Dovern et al. 2011; Goldenberg and Spatt 2009; Kalénine et al. 2010; Randerath et al. 2010)—most likely caused by the fact that studies in chronic patients (often realized in the rehabilitation setting) tend to enroll those patients who still suffer from (cognitive) deficits in their chronic phase of stroke due to lesions in the anterior and posterior association areas. Unfortunately, some studies in chronic LH stroke patients do not provide a lesion overlay plot of all enrolled patients (Mengotti et al. 2013). It should be noted that the main finding of our study (i.e., that language and (meaningful) action interact in the opercular part of left IFG) cannot solely be explained by the lesion distribution of the current sample of sub-acute LH stroke patients, since anterior (e.g., IFG) and posterior (e.g., STG) peri-sylvian brain regions were affected to a similar degree. Furthermore, the here reported sub-region of Brodmann area 44 which acts as an interface between language and (meaningful) action (yellow region in Fig. 2) is located outside the maximal lesion overlap (i.e., the brain regions lesioned in more than 10 stroke patients, see yellow, red and purple areas in Fig. 1). Finally, due to the underlying statistical procedures, VLSM is relatively insensitive to the confounding effects of lesion distribution (Bates et al. 2003; Kimberg et al. 2007).
The present study constitutes a new approach in studying brain lesion-behavior relationships. Instead of focusing on one cognitive deficit at a time, the concurrent investigation of two cognitive functions (here: mutually represented in the same hemisphere) offers a new perspective on structure–function relationships in the human brain (Willems and Hagoort 2009). As a consequence of this and in view of the current sample of sub-acute LH stroke patients, the duration of the neuropsychological testing had to be adjusted. Therefore, the current (bed-side) testing of the two cognitive functions (language, praxis) involved fewer items than traditional tests tapping one cognitive domain only. Nevertheless, the seven neuropsychological tasks covered many aspects of language and praxis in a comprehensive and valid way. Moreover, despite the relatively low number of items in each task, we were able to find significant lesion-behavior relationships (FWE Bonferroni-corrected for the number of unique lesion patterns) for all seven (praxis and language) tasks (see Figs. 3, 4) which correspond well with previous findings (Fridriksson et al. 2014; Goldenberg et al. 2007; Tessari et al. 2007). Thus, data suggest that we were able to overcome this putative limitation [most likely as a result of the statistical power due to our sample size (n = 50) and the lesion mapping method employed (VLSM)]. Furthermore, this short, but comprehensive neuropsychological assessment allowed investigating—for the first time—the neural correlates of aphasic and apraxic deficits in the sub-acute stroke patients who often exhibit a markedly reduced resilience (Kaesberg et al. 2013a).
The concurrent analysis of language and praxis functions in a large sample of sub-acute LH stroke patients revealed (in addition to confirming the known domain-specific lesion sites) that lesions of the left IFG, in particular of the connectivity-based anterior-ventral sub-area of BA 44, lead to combined apraxic and aphasic deficits. In addition to their clinical implications, our findings thereby point to an organizational principle by which the human brain represents multiple cognitive functions within a hemisphere and underline the functional relevance of cortical parcellation schemes based on functional connectivity and receptor mapping (Weiss et al. 2013).
Abbreviations
- ACA:
-
Anterior cerebral artery
- BA:
-
Brodmann area
- FWE:
-
Family-wise error
- IFG:
-
Inferior frontal gyrus
- IPL:
-
Inferior parietal lobe
- LH:
-
Left hemisphere
- MCA:
-
Middle cerebral artery
- MNI:
-
Montreal neurological institute
- MPM:
-
Maximum probability map
- PCA:
-
Posterior cerebral artery
- SMG:
-
Supramarginal gyrus
- SPM:
-
Statistical parametric mapping
- SPSS:
-
Statistical package for the social sciences
- STG:
-
Superior temporal gyrus
- VLSM:
-
Voxel-based lesion-symptom mapping
- WM:
-
Working memory
References
Amunts K, Schleicher A, Bürgel U, Mohlberg H, Uylings HBM, Zilles K (1999) Broca’s region revisited: cytoarchitecture and intersubject variability. J Comp Neurol 412:319–341
Amunts K, Weiss PH, Mohlberg H, Pieperhoff P, Eickhoff S, Gurd J, Marshall JC, Shah NJ, Fink GR, Zilles K (2004) Analysis of neural mechanisms underlying verbal fluency in cytoarchitectonically defined stereotaxic space: the roles of Brodmann areas 44 and 45. Neuroimage 22:42–56
Amunts K, Lenzen M, Friederici AD, Schleicher A, Morosan P, Palomero-Gallagher N, Zilles K (2010) Broca’s region: novel organizational principles and multiple receptor mapping. PLoS Biol 8:e1000489
Bartolo A, Cubelli R, Della Sala S, Drei S (2003) Pantomimes are special gestures which rely on working memory. Brain Cogn 53:483–494
Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, Dronkers NF (2003) Voxel-based lesion-symptom mapping. Nat Neurosci 6:448–450
Binkofski F, Buccino G (2004) Motor functions of the Broca’s region. Brain Lang 89:362–369
Binkofski F, Buxbaum LJ (2013) Two action systems in the human brain. Brain Lang 127:222–229
Bogousslavsky J, van Melle G, Regli F (1988) The Lausanne stroke registry: analysis of 1000 consecutive patients with first stroke. Stroke 19:1083–1092
Buccino G, Binkofski F, Riggio L (2004) The mirror neuron system and action recognition. Brain Lang 89:370–376
Caspers S, Zilles K, Laird AR, Eickhoff SB (2010) ALE meta-analysis of action observation and imitation in the human brain. Neuroimage 50:1148–1167
Clos M, Amunts K, Laird AR, Fox PT, Eickhoff SB (2013) Tackling the multifunctional nature of Broca’s region meta-analytically: co-activation-based parcellation of area 44. Neuroimage 83:174–188
De Ajuriaguerra J, Hecaen H, Angelergues R (1960) Les apraxies. Varietes cliniques et lateralisation lesionelle. Revue Neurologique 102:566–594
De Renzi E, Motti F, Nichelli P (1980) Imitating gestures. A quantitative approach to ideomotor apraxia. Arch Neurol 37:6–10
Donkervoort M, Dekker J, Deelman BG (2006) The course of apraxia and ADL functioning in left hemisphere stroke patients treated in rehabilitation centres and nursing homes. Clin Rehabil 20:1085–1093
Dovern A, Fink GR, Saliger J, Karbe H, Koch I, Weiss PH (2011) Apraxia impairs intentional retrieval of incidentally acquired motor knowledge. J Neurosci 31:8102–8108
Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K (2005) A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25:1325–1335
Eickhoff SB, Bzdok D, Laird AR, Roski C, Caspers S, Zilles K, Fox PT (2011) Co-activation patterns distinguish cortical modules, their connectivity and functional differentiation. Neuroimage 57:938–949
Fazio P, Cantagallo A, Craighero L, D’Ausilio A, Roy AC, Pozzo T, Calzolari F, Granieri E, Fadiga L (2009) Encoding of human action in Broca’ area. Brain 132:1980–1988
Fridriksson J, Fillmore P, Guo D, Rorden C (2014) Chronic Broca’s aphasia is caused by damage to Broca’s and Wernicke’s areas. Cereb Cortex. doi:10.1093/cercor/bhu152
Friederici AD, Bahlmann J, Heim S, Schubotz RI, Anwander A (2006) The brain differentiates human and non-human grammars: functional localization and structural connectivity. Proc Nat Acad Sci 103:2458–2463
Goldenberg G, Karnath H-O (2006) The neural basis of imitation is body part specific. J Neurosci 26:6282–6287
Goldenberg G, Spatt J (2009) The neural basis of tool use. Brain 132:1645–1655
Goldenberg G, Hermsdörfer J, Glindemann R, Rorden C, Karnath H-O (2007) Pantomime of tool use depends on integrity of left inferior frontal cortex. Cereb Cortex 17:2769–2776
Hagoort P (2005) On Broca, brain, and binding: a new framework. Trends Cogni Sci 9:416–423
Heim S, Eickhoff SB, Opitz B, Friederici AD (2006) BA 44 in Broca’s area supports syntactic gender decisions in language production. Neuroreport 17:1097–1101
Heim S, Eickhoff SB, Ischebeck AK, Supp G, Amunts K (2007) Modality-independent involvement of the left BA 44 during lexical decision making. Brain Struct Funct 212:95–106
Heiser M, Iacoboni M, Maeda F, Marcus J, Mazziotta JC (2003) The essential role of Broca’s area in imitation. Eur J Neurosci 17:1123–1128
Higuchi S, Chaminade T, Imamizu H, Kawato M (2009) Shared neural correlates for language and tool use in Broca’s area. Neuroreport 20:1376–1381
Hillis AE, Wityk RJ, Barker PB, Beauchamp NJ, Gailloud P, Murphy K, Cooper O, Metter EJ (2002) Subcortical aphasia and neglect in acute stroke: the role of cortical hypoperfusion. Brain 125:1094–1104
Kaesberg S, Fink GR, Kalbe E (2013a) Neuropsychological assessment EARLY after stroke: an overview of diagnostic instruments available in German and introduction of a new screening tool. Fortschr Neurol Psychiatr 81:482–492
Kaesberg S, Kalbe E, Finis J, Kessler J, Fink GR (2013b) Kölner neuropsychologisches screening für Schlaganfall-Patienten (KöpSS). Hogrefe Verlag, Göttingen
Kalénine S, Buxbaum LJ, Coslett HB (2010) Critical brain regions for action recognition: lesion symptom mapping in left hemisphere stroke. Brain 133:3269–3280
Kertesz A, Ferro JM, Shewan CM (1984) Apraxia and aphasia: the functional-anatomical basis for their dissociation. Neurology 34:40–47
Kimberg DY, Coslett HB, Schwartz MF (2007) Power in voxel-based lesion-symptom mapping. J Cogn Neurosci 19:1067–1080
Kühn S, Brass M, Gallinat J (2013) Imitation and speech: commonalities within Broca’s area. Brain Struct Funct 218:1419–1427
Kümmerer D, Hartwigsen G, Kellmeyer P, Glauche V, Mader I, Klöppel S, Suchan J, Karnath H-O, Weiller C, Saur D (2013) Damage to ventral and dorsal language pathways in acute aphasia. Brain 136:619–629
Laska AC, Hellblom A, Murray V, Kahan T, von Arbin M (2001) Aphasia in acute stroke and relation to outcome. J Intern Med 249:413–422
Mengotti P, Corradi-Dell’Acqua C, Negri GA, Ukmar M, Pesavento V, Rumiati RI (2013) Selective imitation impairments differentially interact with language processing. Brain 136:2602–2618
Nelissen N, Pazzaglia M, Vandenbulcke M, Sunaert S, Fannes K, Dupont P, Aglioti S, Vandenberghe R (2010) Gesture discrimination in primary progressive aphasia: the intersection between gesture and language processing pathways. J Neurosci 30:6334–6341
Nishitani N, Schürmann M, Amunts K, Hari R (2005) Broca’s region: from action to language. Physiology 20:60–69
Papagno C, Della Sala S, Basso A (1993) Ideomotor apraxia without aphasia and aphasia without apraxia: the anatomical support for a double dissociation. J Neurol Neurosurg Psychiatry 56:286–289
Pazzaglia M, Smania N, Corato E, Aglioti SM (2008) Neural underpinnings of gesture discrimination in patients with limb apraxia. J Neurosci 28:3030–3041
Pulvermüller F, Fadiga L (2010) Active perception: sensorimotor circuits as a cortical basis for language. Nat Rev Neurosci 11:351–360
Randerath J, Goldenberg G, Spijkers W, Li Y, Hermsdörfer J (2010) Different left brain regions are essential for grasping a tool compared with its subsequent use. Neuroimage 53:171–180
Rizzolatti G, Arbib MA (1998) Language within our grasp. Trends in Neuroscience 21:188–194
Rizzolatti G, Craighero L (2004) The mirror-neuron system. Annual Review. Neurosciences 27:169–192
Roby-Brami A, Hermsdörfer J, Roy AC, Jacobs S (2012) A neuropsychological perspective on the link between language and praxis in modern humans. Philos Trans Royal Soc London B 367:144–160
Rogalsky C, Matchin W, Hickok G (2008) Broca’s area, sentence comprehension, and working memory: an fMRI study. Front Hum Neurosci. doi:10.3389/neuro.09.014.2008
Rumiati RI, Tessari A (2002) Imitation of novel and well-known actions. The role of short-term memory. Exp Brain Res 142:425–433
Rumiati RI, Weiss PH, Tessari A, Assmus A, Zilles K, Herzog H, Fink GR (2005) Common and differential neural mechanisms supporting imitation of meaningful and meaningless actions. J Cogn Neurosci 17:1420–1431
Saur D, Kreher BW, Schnell S, Kümmerer D, Kellmeyer P, Vry M-S, Umarova RM, Musso M, Glauche V, Abel S, Huber W, Rijntjes M, Hennig J, Weiller C (2008) Ventral and dorsal pathways for language. Proc Nat Acad Sci 105:18035–18040
Saygin AP, Wilson SM, Dronkers NF, Bates E (2004) Action comprehension in aphasia: linguistic and non-linguistic deficits and their lesion correlates. Neuropsychologia 42:1788–1804
Schnur T, Schwartz MF, Kimberg DY, Hirshorn E, Coslett HB, Thompson-Schill SL (2009) Localizing interference during naming: convergent neuroimaging and neuropsychological evidence for the function of Broca’s area. Proc Nat Acad Sci 106:322–327
Schwartz MF, Faseyitan O, Kim J, Coslett HB (2012) The dorsal stream contribution to phonological retrieval in object naming. Brain 135:3799–3814
Tessari A, Canessa N, Ukmar M, Rumiati RI (2007) Neuropsychological evidence for a strategic control of multiple routes in imitation. Brain 130:1111–1126
van Schie HT, Toni I, Bekkering H (2006) Comparable mechanisms for action and language: neural systems behind intentions, goals, and means. Cortex 42:495–498
Vossel S, Weiss PH, Eschenbeck P, Saliger J, Karbe H, Fink GR (2012) The neural basis of anosognosia for spatial neglect after stroke. Stroke 43:1954–1956
Wahlund LO, Barkhof F, Fazekas F, Bronge L, Augustin M, Sjögren M, Wallin A, Ader H, Leys D, Pantoni L, Pasquier F (2001) A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke 32:1318–1322
Weisberg LA (1988) Diagnostic classification of stroke, especially lacunes. Stroke 19:1071–1073
Weiss PH, Dohle C, Binkofski F, Schnitzler A, Freund H, Hefter H (2001) Motor impairment in patients with parietal lesions: disturbances of meaningless arm movement sequences. Neuropsychologia 39:397–405
Weiss PH, Achilles E, Moos K, Hesse MD, Sparing R, Fink GR (2013) Transcranial direct current stimulation (tDCS) of left parietal cortex facilitates gesture processing in healthy subjects. J Neurosci 33:19205–19211
Wilke M, de Haan B, Juenger H, Karnath H-O (2011) Manual, semi-automated, and automated delineation of chronic brain lesions: a comparison of methods. Neuroimage 56:2038–2046
Willems RM, Hagoort P (2007) Neural evidence for the interplay between language, gesture and action: a review. Brain Lang 101:278–289
Willems RM, Hagoort P (2009) Broca’s region: battles are not won by ignoring half of the facts. Trends Cogn Sci 13:101
Willems RM, Özyürek A, Hagoort P (2009) Differential roles for left inferior frontal and superior temporal cortex in multimodal integration of action and language. Neuroimage 47:1992–2004
Acknowledgments
The authors would like to thank their colleagues of the Cognitive Neuroscience division (INM-3), especially Dr. Anna Dovern. Support from the Marga and Walter Boll Stiftung to GRF is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
P. H. Weiss and S. D. Ubben contributed equally to this work and thus share the first authorship.
Rights and permissions
About this article
Cite this article
Weiss, P.H., Ubben, S.D., Kaesberg, S. et al. Where language meets meaningful action: a combined behavior and lesion analysis of aphasia and apraxia. Brain Struct Funct 221, 563–576 (2016). https://doi.org/10.1007/s00429-014-0925-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-014-0925-3