Abstract
To date, the delineation of the human visual “motion area” still relies on functional paradigms originally devised to identify monkey area MT. Using fMRI, we have identified putative human area V5/MT+ in normals by modelling the BOLD responses to alternating radially moving and stationary dot patterns. Functional activations were compared with cytoarchitectonic probability maps of its putative correlate area hOc5, which was calculated based upon data from histological sections of ten human post-mortem brains. Bilateral visual cortex activations were seen in the single subject dynamic versus stationary contrasts and in the group random-effects analysis. Comparison of group data with area hOc5 revealed that 19.0%/39.5% of the right/left functional activation was assigned to the right/left hOc5. Conversely, 83.2%/53.5% of the right/left hOc5 was functionally activated. Comparison of functional probability maps (fPM) with area hOc5 showed that 28.6%/18.1% of the fPM was assigned to hOc5. In turn, 84.9%/41.5% of the area hOc5 was covered by the respective fPM. Thus, random-effects data and fPMs yielded similar results. The present study shows for the first time the correspondence between the functionally defined human V5/MT+ and the post-mortem cytoarchitectonic area hOc5.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the primate, a motion-sensitive area in the occipito-temporal visual cortex was the first to be identified both functionally and with respect to its anatomy (Zeki 1974). It was named area V5 or area MT, after its middle temporal location in the owl monkey (Allman and Kaas 1971), and more recently area MT+ indicating that it probably comprises functionally segregated subregions (Dukelow et al. 2001; Huk et al. 2002). In the macaque, it is now well accepted that the preference of V5/MT+ for motion stimuli is rooted in the receptive field properties of retinal M ganglion cells, which project exclusively to neurons of the magnocellular subdivisions of the dorsal lateral geniculate nucleus (Zeki 1993). This magnocelluar pathway has been shown to project to area V5/MT+ (Maunsell et al. 1990). With the advent of non-invasive brain imaging tools like positron emission tomography (PET) (Watson et al. 1993) and functional magnetic resonance imaging (fMRI) (Tootell et al. 1995), human motion processing was investigated more directly. Both techniques confirmed the existence of a bilateral motion-sensitive area V5/MT+ that may coincide with Flechsig’s Feld 16 (Watson et al. 1993; Flechsig 1927).
Functional brain segregation is often assumed to ultimately rely on an anatomical basis, be it as obvious as in the striate appearance of the primary visual cortex or more hidden in distinct cytoarchitectonic differences between brain areas. For example, a close correspondence of functional and architectonic segregation has been shown for the primary visual areas V1 and V2 (Amunts et al. 2000; Wohlschläger et al. 2005). With regard to area V5/MT+, Malikovic et al. (2001) as well as Annese et al. (2005) reported a putative human correlate of V5/MT+ applying observer-independent analyses of cortical cytoarchitecture in human post-mortem brains. Based on cytoarchitectonic probabilistic maps of putative V5/MT+ (Malikovic et al. 2001) the present study investigates whether this area corresponds to its functionally defined counterpart.
For this purpose, the neural mechanisms underlying visual motion processing were located using fMRI in healthy volunteers. In one approach, functional single subject and random effects group activation maps were calculated from the functional t-statistic contrasts for dynamic versus stationary stimulation. In order to resemble the generation of the anatomical probability maps (aPM) more closely, functional probability maps (fPM) were also calculated from the single subject contrasts. Both, random effects group activation maps and fPMs were then quantitatively compared with the aPMs of putative human V5/MT+ obtained from post-mortem brains using a percent overlap measure, in order to test the hypothesis that functionally defined V5/MT+ and the anatomically defined equivalent overlap and hence correspond to each other.
Methods
Subjects
Fourteen healthy subjects aged 18–39 years (nine females and five males, 23±5 years) participated in the functional imaging measurements. Functional data were collected from a mixed group of male and female volunteers to reflect the mixed set of post-mortem brains (five females, five males). All subjects were healthy and without any history of neurological or psychiatric diseases, had normal visual acuity, and were right-handed as assessed by the Edinburgh inventory (Oldfield 1971), with an average laterality quotient of 84%. Subjects gave informed written consent before participating in the study.
Visual stimuli
The stimuli for localizing area V5/MT+ were similar to those used in other fMRI studies (Tootell et al. 1995; Huk et al. 2002). Radially moving or stationary white dots (n=500), each with a Gaussian spatial luminance profile (0.4° absolute width), covered a 17° circular grey aperture of a visual stimulation device (goggles, Silent VisionTM, Avotec, FL, USA). Stimulus sequences were custom programmed using IDL software (Version 6.0 (c) 2003, Research Systems Inc., Boulder, CO, USA). The stimulus presentation was controlled with the PresentationTM software (Version 0.76, http://www.neuro-bs.com). Moving dots travelled at 6.5°/s and coherently changed their movement direction every 1.3 s (radially inward and outward from a central fixation cross). Blocks of dynamic and stationary stimulation lasted for 18 s each. Eight pairs of dynamic-stationary blocks were presented in a row (~5 min) before the subject was allowed a short pause. This paradigm was repeated four times yielding a total of 20 min presentation time for the whole experiment. Subjects were asked to always fixate a high-contrast red fixation cross in the middle of their visual field. Despite the possibility of an enhanced signal-to-noise ratio (Beauchamp et al. 1997; O’Craven et al. 1997), no further attentional factors were included to minimise the contribution of confounds (Zeki et al. 1991). The subjects’ eye movements were qualitatively monitored throughout the measurements by means of an infra-red camera (Avotec Real EyeTM, FL, USA).
Functional MRI
MRI was carried out using a 1.5 T scanner (Siemens Magnetom Sonata, Erlangen, Germany) with a standard head coil for radio-frequency transmission and signal reception. A whole brain T1-weighted anatomical MRI (1×1×1 mm3) was acquired for every subject using the 3D MP-RAGE sequence (Mugler and Brookeman 1990) with parameters: repetition time (TR) =2.2 s, echo time (TE)=3.93 ms, inversion time (TI) =1.2 s, flip angle (FA) =15°, field of view (FOV) =256 mm.
Multislice T2*-weighted echoplanar images (EPI) were obtained from a gradient-echo sequence that employed the following pulse sequence parameters: TR =3.15 s, TE =66 ms, FA =90°, FOV= 200×200 mm2, slice thickness =4 mm, inter-slice gap =0.4 mm, in-plane resolution =3.125×3.125 mm2, matrix size =64×64. Thirty fMRI slices were oriented parallel to the calcarine sulcus and covered the whole brain. Head movements were minimised by the slight pressure of the protective headphones on either side of the head and the goggles’ oculars on the eyebrows. The scanning procedure was performed continuously over the entire experiment including pauses (20 min).
Image processing
Most of the data analysis, including realignment, slice timing, normalisation, and statistical analysis of the EPI time series was performed using the SPM2 software (Wellcome Department of Imaging Neuroscience, London, UK, http://www.fil.ion.ucl.ac.uk/spm) implemented in MATLAB (Mathworks, Inc., Sherborn, MA, USA). The first three fMRI volumes were discarded to avoid transient magnetic saturation effects and to allow for the haemodynamic response to reach steady state. Images were realigned to compensate for the subjects’ head movements during scanning. Slice acquisition timing was interpolated to the acquisition time of the middle slice. The 3D-anatomical data set was coregistered to the mean EPI image and then spatially normalised to the stereotactic space defined by the single-subject template provided by the Montreal Neurological Institute (MNI) (Evans et al. 1992; Collins et al. 1994; Holmes et al. 1998). The normalisation parameters were then applied to the EPI volumes yielding all EPI data with 3×3×3 mm3 voxel size in MNI space. This was crucial as we wanted to compare functional activations with anatomical maps that were also defined in MNI space. Data were subsequently spatially smoothed with an isotropic Gaussian kernel of 8 mm at full-width half-maximum to compensate for normal variations in brain size and gyral patterns.
Subject-specific low-frequency drifts in the BOLD-signal (blood oxygen level dependent) were removed by a high-pass filter with 1/128 Hz cut-off.
Statistical analysis
The data were then analysed by defining a model for each subject containing the expected BOLD signal at any voxel. Therefore, regressors for the different stimulus conditions were defined using boxcar reference vectors for dynamic, stationary, and pause conditions, each convolved with a canonical haemodynamic response function (HRF). In addition to that a set of regressors convolved with the time derivative of the canonical HRF were included. Also, the six parameters obtained from the realignment procedure (describing translations and rotations of the head) were added to the design matrix. The estimated model parameters for dynamic and stationary regressors were linearly contrasted and yielded a t-statistic (further transformed to a Z-statistic) for each and every voxel. These t-statistics constitute a subject-specific statistical parametric map. Significant activation was assessed initially on a voxel-by-voxel basis. Corrections for multiple comparisons over the whole brain were implemented by using family-wise error correction (Friston et al. 1996). Areas were considered as significantly activated only if they passed a threshold of P corr <0.01 and extended over ten or more voxels.
Single subject t-statistic contrasts for dynamic versus stationary stimulation were entered into a random-effects analysis comparing the mean activation to the variability in activations from subject to subject (P corr <0.05, no extent threshold). Thus, inference about significant activations could be generalised to the population from which the subjects were selected. Besides this exploratory approach using a whole-brain correction for multiple comparisons advantage was taken of the a priori knowledge about the expected location of area V5/MT+ from other fMRI studies (Table 2, bottom row). The maximum Euclidean displacement of any centre of V5/MT+ activation in the literature data from the mean location of the literature data set was 14 mm. Adding an extra millimetre for the estimated half-width of the underlying point spread of activation, a small-volume correction was performed for multiple comparisons within a sphere of 15 mm radius around the mean location of the respective V5/MT+ centres reported in other functional imaging studies. Voxels that exceeded the small-volume corrected P-value (P corr <0.05) in the group random effects analysis and lay within the search volume were then compared with the anatomical data.
As a different approach for analysing significant activations on the group level, we propose to superimpose thresholded single-subject functional t-maps yielding functional probability maps (fPM). Single-subject functional t-maps were thresholded at P corr <0.01 (voxel extent threshold =10) and all significant voxels set to unity, non-significant voxels to zero. Such “binarised” t-maps were then added over all subjects on a voxel-by-voxel basis. Stereotactic voxels that were significantly activated in many subjects were regarded as more likely to belong to a certain functionally segregated area compared with voxels that were activated in only few subjects. As 14 subjects were included in this analysis, the fPMs took values ranging from 0 to 14. The calculation of fPMs thus resembles the procedure used for the generation of anatomical PMs, and thereby, facilitates a comparison of both data modalities.
Anatomical probability maps
Observer-independent cytoarchitectonic mapping was performed on histological sections stained for cell bodies of ten human post-mortem brains (Schleicher et al. 1999). This technique revealed an area in occipito-temporal visual cortex, hOc5, which showed a broad layer III, a high cell density in layers II/III, and a low cell density in layer V (Malikovic et al. 2001). After 3D reconstruction and registration to the single-subject reference space, an aPM of area hOc5 was calculated by superimposing the individual area hOc5 maps. The aPM was then thresholded at 40%, since we found in other brain regions that this threshold reflects the border of an isolated area (i.e. an area, to which neighbouring areas have not been mapped, yet), such as area hOc5 most precisely (Eickhoff et al. 2005). In addition to the map of area hOc5, aPMs of Brodman areas 17 and 18 were applied for topographical interpretation of functional activations (Amunts et al. 2000).
Comparison of functional and anatomical maps
Both the functional random-effects maps and the fPMs were resampled to the isotropic 1×1×1 mm3 resolution of the aPMs. This facilitated the quantitative comparison of functional and anatomical maps using a custom-programmed MATLAB toolbox (Anatomy v0.3, Eickhoff et al. 2005). Like the aPMs, fPMs were thresholded at 40%. The amount of overlap is given as the percentage of the target area. For example, assume a functionally defined area is twice as large as its anatomically defined counterpart. If 40% of the functionally defined volume lies within the anatomically defined counterpart, 80% of the anatomically defined region is activated. Thus percent-overlap values strongly depend on the cluster sizes and hence on the threshold criteria employed for delineating the functionally and anatomically defined areas. Apart from the area-based comparison, the probability for a specific functional voxel being located in an anatomically defined region was also calculated by reporting the aPM value at this coordinate and (in brackets) the range of aPM values for the surrounding voxels. Also, the weighted centres of gravity of functional and anatomical PMs were compared.
Finally, the applicability of the hOc5 map for selecting functional imaging voxels for region-of-interest (ROI) analyses was tested. Bandpassed single-subject BOLD signal time courses (1/12–1/72 Hz) were averaged over fMRI voxels lying within the aPMs for V1, V2, and hOc5, respectively. Error bars indicate the standard deviations for BOLD signals of voxels within the respective ROI. Stereotactic coordinates are given in the anatomical MNI (aMNI) space, which has an origin that is 4 mm anterior and 5 mm ventral to the origin of the MNI space.
Results
All results presented here are based on the first half of each subject’s data set (10 min recording time each), only. The second half of the data was not included in the analysis since after 10 min of fMRI measurement some subjects started to show signs of fatigue as could be observed via the eye movement control video camera. However, 10 min of data registration was sufficient to detect strong and reliable functional activations.
Dynamic versus stationary functional contrasts (P corr <0.01) showed bilateral activations of primary and secondary visual and the occipito-temporal cortical areas in all but subject 7036. However, subject 7036 exhibited similar activations at P corr <0.05. Figure 1 illustrates the aPMs’ power in predicting these functional activations in individual subjects. Single subject functional t-maps (green, P corr<0.01, extent threshold of ten voxels) and aPMs (shades of grey) were both overlayed on transversal sections of the reference brain through the centre of gravity of the left hOc5.
Illustration of the predicting power of aPMs for functional activations in individual subjects. Dynamic versus stationary visual stimulation contrast (green P corr<0.01, extent threshold of ten voxels) and aPMs (shades of grey), both overlayed on transversal sections of the reference brain through the centre of gravity of the left hOc5. Inset numbers are subject identifiers. Subject 7036 exhibited no activations at this threshold, subject 7058 showed activations not seen in this section (both subjects not shown here)
For the group, significant bilateral activations were also seen in the predicted region of V5/MT+ in the random-effects analysis (small-volume correction, see Methods). Figure 2 demonstrates the overlap of the functional group analysis t-maps (yellow) and aPMs (white). A quantitative comparison revealed that 19.0% (39.5%) of the right (left) functional activation was assigned to the right (left) hOc5. Conversely, 83.2% (53.5%) of the right (left) hOc5 was functionally activated (Table 1, bottom). Centres of gravities for the small volume corrected activation clusters are given in Table 2 (second row). The centre of gravity of the small-volume corrected cluster in the right V5/MT+ (aMNI = [47, −73, 8]) was assigned to area hOc5 with 40% probability (30–50%). The centre of gravity of the small-volume corrected cluster in the left V5/MT+ (aMNI = [−44, −76, 10]) was assigned to area hOc5 with 40% probability (20–40%).
Overlap of functional group analysis t-maps (yellow small-volume correction, blue outline whole-brain correction) and anatomical PMs (white). Close-ups (left column) and whole-brain views (middle column) of the V5/MT+ region at the coordinates of the centre of gravity of the anatomical left hOc5 (aMNI = [−43, −73, 10]). Right column Close-ups of the V5/MT+ region at the coordinates of the centre of gravity of the anatomical right hOc5 (aMNI = [49, −70, 11])
After a whole-brain instead of the small-volume correction for multiple comparisons (family-wise error rate Pcorr < 0.05) the significantly activated area in the right occipito-temporal cortex shrank to 556 mm3 (blue outlined cluster in Fig. 2). The left group activation did not survive this threshold. A proportion of 65.8% of the remaining right occipito-temporal cluster was assigned to anatomically defined right hOc5 and 47.9% of the right hOc5 was functionally activated at this t-threshold (Table 1, bottom). The voxel with maximum Z-value in the right V5/MT+ (Z =5.36, aMNI = [51, −70, 11]) was assigned to hOc5 with 40% probability (20–70%).
In a second approach, the fPMs were compared with their anatomical equivalents (Fig. 3). Quantitative data of brain volumes and percent-overlaps are given in Table 1. The right fPM had a volume of 2,267 mm3, 28.6% of which were assigned to anatomically defined right hOc5. In turn, most (84.9%) of the anatomically defined right hOc5 was found to be functionally activated. The location of the right fPM’s maximum was identical with the most significant voxel in the random effects group analysis (aMNI=[51, −70, 11]).
Overlap of functional (red) and anatomical (white) PMs. Close-ups (left column) and whole-brain views (middle column) of the V5/MT+ PMs at the coordinates of the centre of gravity of the anatomical left hOc5 (aMNI = [−43, −73, 10]). Right column close-ups of the V5/MT+ PMs at the coordinates of the centre of gravity of the anatomical right hOc5 (aMNI = [49, −70, 11]). Cross-hairs mark the centres of gravity of the aPMs (black) and fPMs (dark red)
The left fPM had a smaller volume (1,528 mm3) than the right fPM. A proportion of 18.1% of the left fPM was assigned to the anatomical left hOc5. A fraction of 41.5% of the anatomically defined left hOc5 was found to be functionally activated, that is, covered by the left fPM. The maximum of the left fPM (aMNI=[−48, −76, 17]) was assigned to anatomical hOc5 with 30% probability (20–40%). Interestingly, the left PM volume was smaller than the right PM volume for both anatomical and functional data: the left aPM (622 mm3) was 87% of the size of the right aPM (719 mm3) and the left fPM (1,528 mm3) was 67% of the size of the right fPM (2,267 mm3). Figure 3 visualises the overlap between functional (red) and anatomical PMs (white). The centres of gravity for anatomical and functional PMs were similar as indicated by the superimposed cross-hairs in Fig. 3.
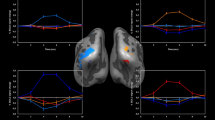
Figure 4 demonstrates the applicability of the aPMs for hOc5 in selecting functional imaging voxels for ROI analyses. Bandpassed BOLD signal time courses (subject 6974, see Methods) were averaged over fMRI voxels lying within the aPMs for V1, V2, and V5/MT+, respectively. Obviously, the ROI defined by the aPMs for hOc5 captures voxels that are strongly modulated by the alternating dynamic-stationary stimulation paradigm, that is, voxels that are motion-sensitive. However, a weaker stimulus-driven modulation of V1/V2 ROIs is also visible and is the basis for the relatively strong V1/V2 activation in the single-subject contrasts.
Discussion
We report here a functionally identified motion-sensitive visual area and its anatomical correlate as derived from microscopic cytoarchitectonic studies of post-mortem brains. A comparison was made of the functionally and anatomically defined areas and a significant spatial overlap was found to exist.
Functional activation in the dynamic versus stationary contrast
In addition to bilateral V5/MT+, visual areas V1 and V2 were more active during dynamic than during stationary stimulus presentation (Fig. 1). This is not surprising given that the stationary visual stimulus was more or less stabilised on the retina due to the fixation task. Such stimuli tend to fade away as the quick eye-movements that otherwise constantly refresh the input to the visual system are diminished or absent (Yarbus 1967). Contrary to that, dynamic stimuli bear potentially more information, thus recruit more neurons, and thus should produce a stronger BOLD signal. An alternative explanation for augmented neural activity in V1 and V2 during visual motion processing is provided by studies showing a top–down enhancement of neural activity in early visual processing areas (Fink et al. 1996; Kastner et al. 1998): it is indeed possible that the motion stimuli captured more attention than the stationary stimuli resulting in increased top-down modulation of the neural processes underlying motion processing.
Significant random-effects group activations were found in the right but not the left V5/MT+ when a whole-brain correction for multiple comparisons was performed (P corr <0.05, Figure 2). However, using data from other fMRI and PET studies concerned with area V5/MT+ (Table 2) we were able to confirm bilateral activation of area V5/MT+ at a t-threshold corrected for the respective small volumes (spheres of 15 mm radius, see Methods). Nonetheless, activation clusters were greater in the right hemisphere than in the left hemisphere (Fig. 2). This asymmetry can be explained in several ways: first, stronger right-hemispheric activation in single subjects could amount to larger clusters of significant voxels. However, the most significant V5/MT+ voxels were as often found in the left as in the right hemispheres (Table 3), which leaves this explanation inconclusive. Second, greater inter-subject variability of the V5/MT+ activation clusters in the left than in the right hemispheres could result in a reduced overlap of activation clusters in the left compared with the right hemisphere. As an estimate for the inter-subject variability, we calculated the Euclidean distance between each subject’s most significant voxel in V5/MT+ and the mean location of the most significant voxel of all subjects (Table 2, third row and Table 3). Distances were 9.5±7.6 mm and 8.7±3.9 mm (mean ± standard deviation) for the left and right V5/MT+, respectively. Thus, inter-subject variability was more pronounced in the left V5/MT+, but did not differ significantly between the hemispheres. Given that single subject left V5/MT+ activations were neither weaker in terms of peak t-values (Table 3) nor more variant in their localization between subjects we would like to suggest that V5/MT+ activations were physiologically spatially more confined to the left than the right hemisphere. In fact, as shown by the analysis of the fPMs, the left functional V5/MT+ was about 67% of the size of the right functional V5/MT+ (Table 1).
The greater confinement of the left V5/MT+ activation could be based on the well-established right hemispheric dominance for visual attention (Heilman and Van den Abell 1980; Marshall and Fink 2001). According to Heilman and Van den Abell, the left hemisphere attends to contralateral stimuli whereas the right hemisphere attends to contralateral and ipsilateral stimuli. Thus, in healthy volunteers fullfield stimuli should provide stronger input to the right than to the left hemisphere and may thus lead to larger right hemispheric activations. This line of argument is supported by the observation of marked structural asymmetries between the hemispheres (Toga and Thompson 2003) and the occipital lobe in particular (Amunts et al. 2000; Zilles et al. 1996). A right hemispheric predominance of V5/MT+ activation has also been reported by Dupont et al. (1993, 1994), while others have not been able to observe differences between left and right hemispheric V5/MT+ activations (Dumoulin et al. 2000).
Functional PMs
The centres of gravities for the small-volume corrected group activation clusters were similar to those of the functional and anatomical PMs (Table 2, second row). The small deviation of the right centre of gravity can be explained by a postero-medial cluster extension at this t-threshold (Fig. 2).
The use of functional PMs enabled us to compare similar approaches to data analysis (probability maps) rather than statistical t-maps versus probability maps. Interestingly, the left PM volume was smaller than the right PM volume for both, anatomical and functional data (87% and 67%, respectively), suggesting that fPMs and aPMs are indeed comparable measures for the same cortical entity.
A substantial overlap of fPMs and aPMs was observed: 28.6% of the right fPM was assigned to the corresponding right aPM and 84.9% of the right aPM was found to be functionally activated, that is, overlapped with the right fPM. Likewise, in the left hemisphere 18.1% of the left fPM was assigned to the corresponding left aPM and 41.5% of the left aPM was found to be functionally activated (Fig. 3). We suggest that the lesser degree of overlap in the left V5/MT+ may at least in part be due to the more confined left V5/MT+ activations as pointed out previously.
How does the location of V5/MT+ fPMs relate to the literature data?
The mean location of the right V5/MT+ in 12 functional studies listed in Table 2 (aMNI = [43, −73, 5]) was 10 mm away from the centre of gravity of the right fPM reported in this study (aMNI = [51, −72, 10]). The mean location of the left V5/MT+ in the same studies (aMNI = [−44, −76, 2], N=11) was 12 mm away from the centre of gravity of the left fPM in our data (aMNI = [−45, −76, 14]). The differences between the literature data and the results presented here are likely to result from inaccuracies produced by the transformation of coordinates between the different reference coordinate systems used in the respective studies. Most of the data listed in the literature on the locations of V5/MT+ are presented in Talairach space (Talairach and Tournoux 1988). Calculations of Talairach from aMNI coordinates and vice versa are based on affine or non-linear transformations that are inherently problematic and erroneous (Brett 2002). In contrast to that, the V5/MT+ coordinates from our functional and anatomical data match very well since the coordinates of the functionally and anatomically delineated areas were compared in the same native aMNI space.
In conclusion, random-effects data as well as fPMs yield similar results. Due to the method of their generation, fPMs more closely resemble the aPMs. The convergence of both data modalities shows that the functionally defined human V5/MT+ correlates with the cytoarchitectonic area hOc5 obtained from post-mortem brains. This suggests that aPMs for hOc5 can be employed to characterise functional activations of the motion-sensitive area V5/MT+ in group analyses. However, due to the substantial inter-subject variability in the location of area V5/MT+, functional data is recommended for the localisation of V5/MT+ in the individual brain.
References
Allman JM, Kaas JH (1971) A representation of the visual field in the caudal third of the middle temporal gyrus of the owl monkey (Aotus trivirgatus). Brain Res 31(1):85–105
Amunts K, Malikovic A, Mohlberg H, Schormann T, Zilles K (2000) Brodman’s areas 17 and 18 brought into stereotaxic space. Where and how variable? NeuroImage 11:66–84
Annese J, Gazzaniga MS, Toga AW (2005) Localization of the human cortical visual area MT based on computer aided histological analysis. Cerebral Cortex 15(7):1044–1053
Beauchamp MS, Cox RW, DeYoe EA (1997) Graded effects of spatial and featural attention on human area MT and associated motion processing areas. J Neurophysiol 78:516–520
Brett M (2002) http://www.mrc-cbu.cam.ac.uk/Imaging/Common/mnispace.shtml
Collins DL, Neelin P, Peters TM, Evans AC (1994) Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr 18(2):192–205
Dukelow SP, De Souza JFX, Culham JC, van den Berg AV, Menon RS, Vilis T (2001) Distinguishing subregions of human MT+ complex using visual field and pursuit eye movements. J Neurophysiol 86:1991–2000
Dumoulin SO, Bittar RG, Kabani NJ, Baker CL Jr, Le Goualher G, Pike GB, Evans AC (2000) A new anatomical landmark for reliable identification of human area V5/MT: a quantitative analysis of sulcal patterning. Cereb Cortex 10:454–463
Dupont P, Orban GA, Vogels R, Bormans G, Nuyts J, Schiepers C, De Roo M, Mortelmans L (1993) Different perceptual tasks performed with the same visual stimulus attribute activate different regions of the human brain: a positron emission tomography study. PNAS 90:10927–10931
Dupont P, Orban GA, de Bruyn B, Verbruggen A, Mortelmans L (1994) Many areas in the human brain respond to visual motion. J Neurophysiol 72:1420–1424
Dupont P, de Bruyn B, Vandenberghe R, Rosier A-M, Michiels J, Marchal G, Mortelmans L, Orban GA (1997) The kinetic occipital region in human visual cortex. Cereb Cortex 7:283–292
Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K (2005) A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage 25:1325–1335
Evans AC, Marrett S, Neelin P, Collins L, Worsley K, Dai W, Milot S, Meyer E, Bub D (1992) Anatomical mapping of functional activation in stereotactic coordinate space. NeuroImage 1:43–53
Fink GR, Halligan PW, Marshall JC, Frith CD, Frackowiak RS, Dolan RJ (1996) Where in the brain does visual attention select the forest and the trees? Nature 382:626–628
Flechsig P (1927) Meine myelogenetische Hirnlehre mit biographischer Einleitung. Springer, Berlin
Friston KJ, Holmes A, Poline J-B, Price CJ, Frith CD (1996) Detecting activations in PET and fMRI: Levels of inference and power. NeuroImage 4:223–235
Goebel R, Khorram-Sefat D, Muckli L, Hacker H, Singer W (1998) The constructive nature of vision: direct evidence from fMRI studies of apparent motion and motion imagery. Eur J Neurosci 10:1563–1573
Hasnain MK, Fox PT, Woldorff MG (1998) Intersubject variability of functional areas in the human visual cortex. Hum Brain Map 6:301–315
Heilman KM, Van den Abell T (1980) Right hemisphere dominance for attention: The mechanism underlying hemispheric asymmetries of inattention (neglect). Neurology 30:327–330
Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC (1998) Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr 22:324–333
Huk AC, Dougherty RF, Heeger DJ (2002) Retinotopy and functional subdivision of human areas MT and MST. J Neurosci 22:7195–7205
Kastner S, De Weerd P, Desimone R, Ungerleider LG (1998) Mechanisms of directed attention in the human extrastriate cortex as revealed by functional MRI. Science 282:108–111
Malikovic A, Amunts K, Schleicher A, Mohlberg H, Palomero-Gallagher N, Schormann T, Zilles K (2001) Cytoarchitecture and stereotactic location of a preoccipital area in the region of V5/MT. NeuroImage 13:S909
Marshall JC, Fink GR (2001) Spatial cognition: where we were and where we are. Neuroimage 14:2–7
Maunsell JHR, Nealey TA, DePriest DD (1990) Magnocellular and parvocellular contributions to responses in the middle temporal visual area (MT) of the macaque monkey. J Neurosci 10:3323–3334
Mugler JP 3rd, Brookeman JR (1990) Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP RAGE). Magn Reson Med 15(1):152–157
O’Craven KM, Rosen BR, Kwong KK, Treisman A, Savoy RL (1997) Voluntary attention modulates fMRI activity in human MT-MST. Neuron 18:591–598
Oldfield RC (1971) The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9:97–113
Rees G, Friston K, Koch C (2000) A direct quantitative relationship between the functional properties of human and macaque V5. Nature Neuroscience 3(7):716–723
Schleicher A, Amunts K, Geyer S, Morosan P, Zilles K (1999) Observer-independent method for microstructural parcellation of cerebral cortex. A quantitative approach to cytoarchitectonics. NeuroImage 9:165–177
Smith AT, Greenlee MW, Singh KD, Kraemer FM, Hennig J (1998) The processing of first- and second-order motion in human visual cortex assessed by functional magnetic resonance imaging fMRI. J Neurosci 18:3816–3830
Sunaert S, van Hecke P, Marchal G, Orban GA (1999) Motion-responsive regions of the human brain. Exp Brain Res 127:355–370
Talairach J, Tournoux P (1988) Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system—an approach to cerebral imaging. Thieme, New York
Toga AW, Thompson PM (2003) Mapping brain asymmetry. Nat Rev Neurosci 4:37–48
Tootell RBH, Reppas JB, Kwong KK, Malach R, Born RT, Brady TJ, Rosen BR, Belliveau JW (1995) Functional analysis of human MT and related visual cortical areas using magnetic resonance imaging. J Neurosci 15(4):3215–3230
Watson JDG, Myers R, Frackowiak RSJ, Hajnal JV, Woods RP, Mazziotta JC, Shipp S, Zeki S (1993) Area V5 of the human brain: evidence from a combined study using Positron Emission Tomography and Magnetic Resonance Imaging. Cereb Cortex 3:79–94
Wohlschläger AM, Specht K, Lie C, Wohlschläger A, Bente K, Pietrzyk U, Stöcker T, Zilles K, Amunts K, Fink GR (2005) Linking retinotopic fMRI mapping and anatomical probability maps of human occipital areas V1 and V2. NeuroImage 26(1):73–82
Yarbus AL (1967) Eye movements and vision. Plenum Press, New York
Zeki S (1974) Functional organization of a visual area in the posterior bank of the superior temporal sulcus of the rhesus monkey. J Physiol 236:549–573
Zeki S, Watson JDG, Lueck CJ, Friston KJ, Kennard C, Frackowiak RSJ (1991) A direct demonstration of functional specialization in human visual cortex. J Neurosci 11(3):641–649
Zeki S (1993) A Vision of the Brain. Blackwell Scientific Publications, Oxford
Zilles K, Dabringhaus A, Geyer S, Amunts K, Qu M, Schleicher A, Gilissen E, Schlaug G, Steinmetz H (1996) Structural asymmetries in the human forebrain and the forebrain of non-human primates and rats. Neurosci Biobehav Rev 20(4):593–605
Acknowledgements
We are grateful to our colleagues from the MR and Cognitive Neurology group for their assistance. Gereon R. Fink is supported by the Deutsche Forschungsgemeinschaft (DFG KPO-112, TP1 and TP8).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wilms, M., Eickhoff, S.B., Specht, K. et al. Human V5/MT+: comparison of functional and cytoarchitectonic data. Anat Embryol 210, 485–495 (2005). https://doi.org/10.1007/s00429-005-0064-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-005-0064-y