Abstract
Diffuse large B-cell lymphoma (DLBCL) is an aggressive and heterogenous group of diseases and the most common subtype of non-Hodgkin lymphoma. In the past decade, there has been an explosion in molecular profiling that has helped to identify subgroups and shared oncogenic driving mechanisms. Since the 2017 World Health Organization (WHO) classification, additional studies investigating these genomic abnormalities and phenotypic findings have been reported. Here we review these findings in DLBCL and address the proposed changes by the 2022 International Consensus Classification.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diffuse large B-cell lymphoma (DLBCL) represents a clinically and biologically heterogeneous group of diseases. Molecular studies have significantly expanded our understanding of these tumors identifying novel subgroups and potential therapeutic targets. DLBCL is the most common lymphoma subtype worldwide accounting for approximately 40% of all non-Hodgkin lymphomas[1]. The 2017 World Health Organization (WHO) classification of lymphoid malignancies recognizes within the group of DLBCL several subtypes characterized by unique clinical and/or pathological features including primary mediastinal large B-cell lymphoma (PMBL), primary DLBCL of the central nervous system (CNS), primary cutaneous DLBCL, leg-type, T-cell/histiocyte-rich large cell lymphoma (TCRBCL), Epstein-Barr virus (EBV) positive DLBCL “not otherwise specified” (NOS) among others. Nevertheless, most cases of DLBCL fall into the category of NOS. This is an aggressive disease and requires immediate treatment. The current standard of care for these patients is multi-agent CHOP in combination with rituximab (R-CHOP). Despite the improvements in outcome, patients still experience relapse and refractory disease; therefore, there is an ongoing effort to tailor therapy based on specific molecular alterations to further improve treatment and prognosis of these patients. The advent of molecular testing has improved the potential for therapeutic targets as well as the classification of this heterogeneous disease. Both the 2022 International Consensus Classification (ICC)[2] and the WHO-HAEM5[3] made recommendations to retain the cell of origin classification for DLBCL, NOS, with the expectation that transition to a molecular genetic classification will be feasible in the near future. These biologic subgroups and other well-defined large B-cell entities (Table 1) will be further discussed in this review.
Diffuse large B-cell lymphoma, NOS
Morphologic features
The diagnosis of DLBCL is usually not challenging from the morphologic standpoint. Lymph nodes or extranodal sites demonstrate a diffuse proliferation of large lymphoid cells that have totally or partially effaced the normal architecture of tissues. Cytological variants of de novo DLBCL such as centroblastic, immunoblastic, and anaplastic have been reported in previous classifications but are not widely used for prognostic or predictive purposes. The anaplastic variant comprises of bizarre pleomorphic nuclei that can have multinucleated forms and abundant cytoplasm. These cases can be CD30-positive and have a sinusoidal distribution that can mimic anaplastic large cell lymphoma of T-cell origin [4].
Immunophenotypically, the neoplastic cells express pan B-cell markers including CD19, CD20, CD22, PAX5, and CD79a. Surface and/or cytoplasmic immunoglobulin (IgM > IgG > IgA) can be demonstrated in up to 75% of the cases. Markers commonly used in the characterization of DLBCL include CD10, BCL6, BCL2, and IRF4/MUM1. Aberrant expression of cytoplasmic CD3 has been documented mainly in extranodal presentations, without the expression of other T-cell markers [5].
Expression of CD5 in de novo DLBCL is seen in approximately 5–10% of cases. The majority of cases exhibit an activated B-cell (ABC) phenotype and have an extranodal distribution [6]. The reported adverse prognosis of these cases is likely related to their ABC-type cell of origin (COO) [7, 8]. The ICC recommends deemphasizing the importance of “DLBCL CD5 + ” because the adverse prognosis is weak and does not seem to reflect a true biological group. Cyclin D1 expression has been detected in approximately 1–2% DLBCL raising the differential diagnosis with blastoid or pleomorphic mantle cell lymphoma (MCL) [9,10,11]. Most of these tumors are SOX11 and CD5 negative and do not carry a CCND1-rearrangement. However, the translocation has also been detected in some of these cases, but they are negative for SOX11 and carry other oncogenic rearrangements, such as BCL2 and/or BCL6, and absence of mutations associated with MCL [10, 12].
Some DLBCL express both MYC and BCL2 by immunohistochemistry (so-called double-protein expressors, DPE) at high levels, without the presence of rearrangements of these genes [13]. DPE without genetic double-hit can be seen in ABC-DLBCL despite nearly all cases of molecular double-hit lymphoma are of GCB-type. Recent analysis shows that cases that are DPE that are GCB-DLBCL do indeed confer a poor overall survival, but this was not seen with ABC-DLBCL from prospective randomized clinical trials [14]. Another study also showed that poor survival associated with DPE was dependent on other factors such as alterations of TP53. But in the context of BN2/Cluster 1-like DLBCL with BCL6 translocations (see below), the DPE cases showed a favorable outcome [15]. This further underlines the complexity and heterogeneity of de novo DLBCL, and the term DPE should not be used synonymously with true double-hit lymphomas. The ICC recommends deemphasizing DPE, since these cases most probably represent the final stage of different biological pathways.
Cell of origin (COO)
One of the key findings in DLBCL is in relation to its COO. Two major molecularly distinct forms of DLBCL, NOS—identified by gene expression profiling (GEP)—have indicated different stages of B-cell differentiation. The first has a profile characteristic of germinal center B-cells (GCB) and the second a profile characteristic of in vitro activation of peripheral blood B-cells (activated B-cell, ABC)[16]. A small subset of DLBCL cases have been identified as “unclassified” and not fitting in either the GCB or ABC category.
The use of GEP for COO determination has not been routinely introduced in the clinical setting. Several immunohistochemical algorithms have been developed trying to reproduce the molecular subclassification in routine diagnostic tissues, the most widely used being the Hans algorithm, which distinguishes GCB from non-GCB DLBCL [17]. However, the prognostic impact of these IHC algorithms are limited. Recent trials in DLBCL using COO classification per patient selection with incorporation of targeted agents have yielded disappointing results [18, 19]. A possible explanation for the negative findings is that the Hans algorithm may not provide an accurate DLBCL subtype classification because it shows only moderate concordance with GEP. This has been recently demonstrated especially in cases having abnormal co-expression of CD10 and MUM1, whereby the Hans algorithm is by default assigned to the GCB group but by GEP, 30–50% correspond to ABC-type DLBCL [20]. In addition, co-expression of CD10 and MUM1 may occur in large cell lymphomas with plasmablastic differentiation [21]. Other algorithms such as Tally and Choi methods have shown higher concordance with GEP compared to the Hans algorithm [22, 23]. However, there are technical difficulties with GCET1, FOXP1, and LMO2 that have hindered adoption of these algorithms [22]. Several assays are available that are robust and clinically validated to determine COO from FFPE tissue [24, 25]. Nevertheless, even with GEP, the COO classification has shown to be insufficient to capture the complex heterogeneity of DLBCL, NOS, and moving forward, a combination with recently reported molecular classifications may provide better stratification of the disease [26]. The ICC [2] recommends retaining the COO classification at the present time with the expectation that transition to a more precise molecular genetic classification integrating the sequencing analysis of these tumors will be feasible in the near future. A similar statement is made in the WHO-HAEM5 [3]. It is generally agreed that a technical harmonization between the different proposed genetic classifications and bioinformatic aspects supported by clinical trial is needed before it can be incorporated in daily routine diagnostics.
Molecular classification of de novo DLBCL
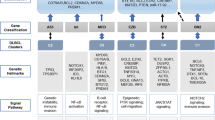
Recent molecular and cytogenetic profiling from multiple large multi-platform genomic studies showed 4–7 molecular subgroups with overlapping features [27,28,29,30] (Table 2). Chapuy et al.[28] and Wright et al. [27] divided DLBCL into at least 4 genetic clusters with certain groups strongly enriched by COO subgroup. For example, Chapuy’s cluster 3 has overlap with Wright’s EZB with these cases having frequent abnormalities in BCL2 and EZH2. Another group with similarities seen in both studies are cluster 2 and A53 showing aneuploidy and frequent TP53 abnormalities. However, other than the TP53 abnormalities, there are few recurrent abnormalities that are seen within this group. Also, Lacy et al. [30]—by using a targeted sequencing “only” approach—described 5 molecular subtypes with overlapping groupings similar to the other studies in 3 particular groups. Cases with MYD88 mutations (Cluster 5, MCD) have a strong association with ABC-type DLBCL and contain the majority of primary CNS lymphomas, primary testicular lymphoma, and are associated with a poor prognosis. Cases with a BCL2 translocation (Cluster 3, EZB) are strongly associated with a GCB-type DLBCL and have a mutational profile that overlaps with follicular lymphoma. These cases have a favorable prognosis but may also include cases of double-hit lymphoma with adverse outcome. Cases with a NOTCH2 mutation (Cluster 1, BN2) do not show an obvious association with COO and have mutational similarities to marginal zone lymphoma. Of note, in the study by Chapuy et al. [28], all cases of DLBCL were obligated to be classified whereas only 65% of the cases were classified in the Wright et al. [27] study. Although the studies show discrepancies, there are at least some encouraging findings that these 3 studies showed overlapping molecular subgroups of biological importance. These findings are at least a step forward in the right direction of developing a robust molecular taxonomy that could be used for clinical benefit. The publicly available LymphGen algorithm (https://llmpp.nih.gov/lymphgen/index.php) allows classification of DLBCL based on their genomic profile. Nevertheless, still ~ 35% of DLBCL are not classifiable with the current molecular method, and there is still no ability to triage cases of DLBCL into genomic subgroups prospectively for clinical trial purposes.
Tumor microenvironment
Recent technological advances with multispectral immunofluorescence and mass spectrometry have provided insight into the complex interaction of tumor cells and the microenvironment. In DLBCL, the majority of the cellular component are sheets of neoplastic B-cells, but T-cells and macrophages are present and play a role in supporting these tumor cells. Recent studies looking at the tumor microenvironment (TME) in DLBCL have shown that increased CD4 T-cells or overall T-cell content have improved survival compared to cases that are devoid of T-cells (so-called cold tumors)[31, 32]. In addition, PD-L1 expression in the tumor cells and TME may identify cases that portend a poor survival, but this may be a useful biomarker to target not only the tumor cells but also the TME that has high expression [31, 33]. Another recent study illustrated using transcriptome deconvolution and single-cell RNA sequencing of various cell states within the TME forming “ecosystems.” Cases with a TME expressing high CD4 T-cells and TFH phenotype showed an improved survival [32], while cases that showed a high CD8 T-cell content did not show a prognostic significance with R-CHOP but showed a specific benefit with bortezomib [32]. Overall, the TME of DLBCL appears to be important from the biologic and prognostic standpoint and potentially from the therapeutic aspect but more complex as various subtypes may exist even within defined genomic subtypes [32].
High-grade B-cell lymphomas with MYC and BCL2 and/or BCL6 rearrangements
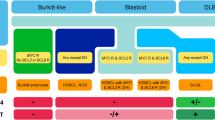
The ICC recognizes two groups of high-grade B-cell lymphomas (HGBCL) with double genetic alterations, one with MYC and BCL2 rearrangement, and a provisional entity of HGBCL with MYC and BCL6 rearrangement [2]. Those are categories defined genetically, which morphologically encompasses cases with blastoid or intermediate cytology between Burkitt lymphoma (BL) and DLBCL with approximately half of the cases displaying a DLBCL, NOS morphology [1, 34] (Fig. 1). The ICC also recognizes a third category of HGBCL without these double-hit alterations but with blastoid or intermediate cytology. Further information regarding HGBCL and double-hit lymphoma are discussed in the specific manuscript in this issue by King et al. [35], but the fact that half of these cases have a DLBCL morphology deserves considering its differential diagnosis in this manuscript and emphasizes the need for FISH studies using a sensitive probes to exclude these double-hit lymphomas.
High-grade B-cell lymphoma with MYC, BCL2, and BCL6 rearrangement. A) Sheets of large, atypical cells with irregular nuclear contours, and open chromatin. In this case, the “triple-hit” lymphoma has a centroblastic appearance and is positive for B) CD20, C) CD10, and D) shows a high proliferation index of nearly 100% with Ki-67
Large B-cell lymphoma with 11q aberration
The 2017 WHO classification recognized a provisional entity called Burkitt-like lymphoma with 11q aberration, which pathologically and by GEP, overlapped with Burkitt lymphomas (BL) but lacked MYC rearrangement and instead harbors a telomeric 11q loss combined with 11q proximal gain. These tumors are more frequently seen in children and young adults presenting with localized (stage I/II) nodal disease [36]. Recent studies have shown that these tumors may not have the monomorphic appearance as seen in BL, and cytology may vary from medium- to large-sized cells (Fig. 2). In contrast to BL, the tumors have a “starry sky” pattern with coarser phagocytosed apoptotic bodies [37]. These lesions typically have a phenotype similar to BL with expression of CD10, BCL6, and lack BCL2 by IHC. Although not widely used, LMO2 is expressed in 50% of these tumors, whereas it is usually negative in BL [36, 38]. Genetic studies have shown these cases have recurrent mutations in BTG2, DDX3X, ETX1, NFRKB, EP300, and GNA13 but lack typical mutations seen in BL, such as ID3, TCF3, or CCND3 [36, 39] supporting the idea that these tumors are closer to GCB DLBCL rather than Burkitt lymphoma. The outcome of these patients is favorable after treatment. The Clinical Advisory Committee (CAC) conference supported the need to change the term Burkitt-like in this tumor but debated whether the most appropriate term could be “high-grade B-cell lymphoma” or “large B-cell lymphoma” with 11q abnormalities. Finally, the latter was preferred due to the mutational profile similar to other GCB-type DLBCL, and the favorable prognosis of these tumors significantly differs from the other HGBCL categories in this classification [36, 40]. The WHO-HAEM5 has designated these cases as “high-grade B-cell lymphoma with 11q aberrations”[3]. The ICC considered maintaining this category as a provisional entity recognizing the need of additional studies. To further understand these tumors, it is important to identify them using copy number array or FISH analysis with the 11q probe in DLBCL/HGBCL that have a GCB phenotype, negative for BCL2 by IHC, high proliferation index with Ki-67 (> 90%), and lack MYC rearrangement, particularly in young patients [37]. Copy number analysis by array may not be feasible at most institutions, and the FISH probes for 11q should adequately identify these cases [36, 37]. Still an open question is whether cases with only 11q loss may be acceptable within the spectrum of the disease, and additional studies are warranted to answer these questions.
T-cell/histiocyte-rich large B-cell lymphoma (TCRBCL)
TCRBCL is an aggressive B-cell lymphoma that usually affects middle-aged males with advanced stage disease and frequent involvement of the liver and spleen. Most tumors arise de novo, but some cases may correspond to progression of nodular lymphocyte predominant Hodgkin lymphoma (NLPHL). The CAC conference considered the close biological relationship of these two entities and the difference of NLPHL with classic Hodgkin lymphoma. For these reasons, the CAC accepted the change of the term NLPHL to nodular lymphocyte predominant B-cell lymphoma (NLPBL) (see further discussion by Tousseyn et al. in this issue) [41]. Histologically, TCRBCL shows scattered large, atypical cells which may be reminiscent of Lymphocyte Predominant (LP) large cells, centroblast-like, or Hodgkin-like nuclei (Fig. 3). The background shows numerous small reactive T-cells and extensive histiocytes which may be epithelioid. The immunophenotype of the cells is similar to LP cells with intact B-cell program but is typically negative for IgD which can be seen in a subset of cases of NLPBL [42]. Genomic studies have identified recurrently mutated genes in JUNB, DUSP2, SGK1, and SOCS1 [43].
T-cell/histiocyte-rich large B-cell lymphoma. A) The large tumor cells are scattered and somewhat inconspicuous in the rich background of small T-cells and histiocytes. B) CD20 immunostain highlights these scattered large tumor cells C) as well as Oct2. D) There are numerous CD8-positive T-cells that are the predominant T-cell population
Primary mediastinal large B-cell lymphoma (PMBL)
PMBL is an uncommon subtype of NHL and accounts for approximately 10% of DLBCLs. The characteristic presentation is a mediastinal mass in young adults with a higher prevalence in women. Histologically, the tumors show sheets of large, atypical cells, often with clear cytoplasm in a background of fine fibrillary fibrosis (Fig. 4). The tumor cells have an intact B-cell program with variable expression of CD30 and CD23. Molecular studies show that PMBL is a distinct entity but has a close relationship with CHL. Constitutive activation of NF kappa B and JAK/STAT pathways has been recognized as a hallmark of this entity [44]. Recurrent alterations with the class II transactivator, CIITA [45, 46] and structural and copy number gains in the 9p24.1 locus (CD274 and PDCD1LG2) have been important in the pathogenesis and are a potential target for checkpoint blockade. Mutations in SOCS1, GNA13, and STAT6 are frequent in PMBL, further supporting the relatedness of this disease with CHL [47]. The distinction between DLBCL and PMBL is not always obvious but important as the management and prognosis differs. Dose-adjusted EPOCH-R is currently the treatment of choice for PMBL [48]. GEP utilizing the Lymph3Cx (LLMPP) provides a reliable method for separating DLBCL from PMBL [49].
Mediastinal gray zone lymphoma (MGZL) has morphologic and immunophenotypic features that are intermediate between PMBL and CHL. Both PMBL and CHL are believed to be derived from a thymic B-cell. MGZL is hypothesized to be intermediate between CHL and PMBL. It was previously described in the 2017 WHO as provisional entity as B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and CHL [1, 50]. MGZL is further discussed in other sections of this issue [41].
Extranodal large B-cell lymphomas of ABC subtype
A number of DLBCL originate in extranodal sites with particular clinical features related to the topographic site of origin. Molecular and genetic studies have identified shared features among many of these categories including an ABC-phenotype and a mutational profile of the MCD/Cluster 5 subgroup with mainly MYD88L265P and/or CD79B mutations, which activate the B-cell receptor and toll-like receptor pathways and increase NF kappa B activity [27, 28]. These tumors include primary DLBCL of the CNS (PCNSL), primary DLBCL of the testis, primary cutaneous DLBCL, leg-type, intravascular large B-cell lymphoma, and other less well-defined categories such as primary breast or adrenal DLBCL. The ICC discussed extensively whether all these tumors should be included under a common umbrella termed “Extranodal lymphoma ABC/non-GCB type” but the final consensus was that there are still unresolved aspects, such as the molecular heterogeneity of some tumors in these locations, that require further studies. It is postulated that recognition of the specific entities will be better captured by upcoming molecular categorization integrated with more conventional information [2].
PCNSL and primary DLBCL of the testis are recognized in the ICC as two different, although closely related entities characterized by unique clinical and molecular features, probably related to their topographic origin in immune privileged areas. The majority of PCNSL (Fig. 5) and primary DLBCL of testis have an ABC-like phenotype, which share frequent mutations involving MYD88 and CD79B [51, 52]. Many of the cases show co-occurrence of these 2 mutations. These tumors display a common molecular feature as seen in Cluster 5 and MCD [27, 28]. Because of the immune privileged sites and paucity of antigen presenting cells, mutations leading to constitutional activation of these signaling cascades may play a role due to lack of stimuli [51]. Recent evaluation of the tumor microenvironment in PCNSL shows that PD1 is highly expressed in the tumor infiltrating lymphocytes as well as PD-L1 in the tumor associated macrophages [53]. A retrospective analysis of PCNSL and primary DLBCL of the testis showed these cases had frequent copy number alterations related to PD-L1 and PD-L2 (9p24.1) and associated protein expression, which may be justification for anti-PD1 immunotherapy[54, 55]. Therefore, primary DLBCL of the testis is now considered a distinct entity both in the ICC and WHO-HAEM5; in the latter, it is listed under the umbrella term “Primary large B-cell lymphoma of immune-privileged sites” and also includes “Primary large B-cell lymphoma of the vitreoretinal”[2, 3].
Intravascular large B-cell lymphoma is a rare form of DLBCL characterized by neoplastic large cells confined to the lumen of blood vessels and capillaries, although in some cases the neoplastic cells can infiltrate beyond the vessel walls and form extravascular tumor clusters or even tumor masses [56]. These tumors carry a MYD88L265P mutation in approximately 40% of the cases, with a subset having a CD79B mutation (26%) [57]. Primary cutaneous DLBCL, leg-type is a rare subtype of DLBCL that preferentially involves the legs and is more common in older patients. A recent study by Pham-Ledard et al. found that these tumors showed frequent mutations with MYD88 (59%) and had a poor overall survival as compared to wild-type cases [58]. Primary breast DLBCL also has frequent MYD88 (59%) and CD79B (33%) mutations with a high number of cases expressing CD5 [59].
Large B-cell lymphomas of other extranodal sites
In addition to the ABC-DLBCL in the extranodal sites described above, large B-cell lymphomas in other locations such as bone and ovary are predominantly of GCB-subtype [60, 61]. Particularly, primary bone lymphomas show a GCB COO by GEP and have frequent mutations in B2M, TNFRSF14, IRF8, and EZH2 [60]. Both B2M and TNFRSF14 mutations likely are important for the tumor cells to evade immune surveillance. EZH2 is commonly mutated in GCB DLBCL and follicular lymphoma and usually co-occurs with the BCL2 translocation. However, even though EZH2 was frequently mutated in these cases, only one case showed a BCL2 translocation [60]. Primary bone DLBCL also appears to show a good prognosis when treated with immunochemotherapy [60, 62]. Primary DLBCL of the ovary has been reported as being of GCB-subtype, carrying translocations of BCL2, BCL6, or MYC only in occasional cases. Additional studies will be necessary to determine if tumors in these locations represent distinct entities.
HHV-8 and EBV-negative primary effusion-based lymphoma
Primary effusion lymphoma (PEL) present as a high-grade malignant effusion in body cavities was defined by the 2017 WHO classification [1] as a tumor associated with KSHV/HHV-8, and most cases occur in adult males with AIDS. PELs associated with HIV infection are mostly positive for EBV. There are rare effusion-based lymphomas lacking HHV-8. Although these cases have some overlap with typical PEL, the term PEL should be restricted for cases with HHV8 positivity.
The ICC recognizes HHV-8 and EBV-negative primary effusion-based lymphoma as a new provisional entity characterized by unifying features including presentation in elderly patients, HIV-negative, and medical conditions leading to fluid overload [2]. These cases can be seen worldwide, but more than half of them have been reported in Japan [63]. The WHO-HAEM5 also recognizes a similar entity termed “fluid overload-associated large B-cell lymphoma” but, contrary to the ICC, accepts EBV-positivity in a subset of cases (up to 30%). The ICC felt that EBV-positive cases should be excluded from this category as these are generally aggressive and/or associated with immunosuppression and would be best categorized as EBV-positive DLBCL, NOS, or as polymorphic EBV + B-cell LPD [64]. The pathogenesis of these tumors might be related to chronic serosal stimulation. Most cases show centroblastic or immunoblastic morphology and typically express pan-B-cell markers (e.g., CD20 and CD19) and lack CD138 as compared to typical PEL. Many of these cases show a favorable prognosis with systemic chemotherapy, and even some patients may have spontaneous regression or cure with drainage alone [63, 65, 66]. Caution should be taken with these cases as this category should not include cases of tissue based DLBCL with associated effusion, and careful clinical and radiographic evaluation is necessary. Patients with unknown clinical history of fluid overload should be phrased descriptively with the emphasis that clinical correlation is needed as this distinction is important due to the management and prognosis. Further studies are warranted to clarify whether these cases represent a distinctive entity and obtain consensus in the criteria that define this entity.
Large B-cell lymphomas with terminal B-cell differentiation
ALK-positive diffuse large B-cell lymphoma
ALK-positive DLBCL is a rare and aggressive subtype characterized by large B-cells expressing ALK, mainly presenting in young immunocompetent patients. Morphologically, the nodes show a diffuse infiltrate of monomorphic large immunoblast-like cells. Some cases can show plasmablastic morphology and multinucleated neoplastic giant cells may be present (Fig. 6). The tumor cells express plasma cell markers such as CD138 and BLIMP1 while they are negative for the pan-B-cell markers CD20, CD79a, and PAX5. MUM1, EMA, and STAT3 are positive, but CD30 is negative [67, 68]. The tumor cells have a restricted granular cytoplasmic staining with ALK protein which is indicative of the CLTC::ALK fusion, which is the most frequent translocation, t(2;17)(p23;q35). These tumors lack EBV, HHV-8, and pan T-cell associated markers as well as MYC rearrangements [68]. ALK inhibitors have been used in conjunction with chemotherapy and has shown mixed responses [69, 70].
Plasmablastic lymphoma (PBL)
PBL is an aggressive B-cell neoplasm, mainly occurring in immunodeficient patients, characterized by sheets of large, atypical cells with an immunoblastic or plasmablastic morphology with expression of plasma cell markers and commonly associated with EBV, especially in HIV-positive patients (Fig. 7). Approximately 50% of cases have a MYC rearrangement, more commonly seen in the EBV-positive cases. Most cases present in extranodal sites, mainly in oral and upper respiratory mucosa and gastrointestinal tract. Recent studies have shown a particular mutational profile different from DLBCL and multiple myeloma, frequently involving genes of the MAPK and JAK/STAT pathways and TP53 [71, 72]. EBV-negative PBL shows higher mutational and copy number abnormalities with more frequent mutations in TP53, CARD11, and MYC. In contrast, EBV-positive PBLs tend to have more mutations related to STAT3 [71]. Interestingly, a similar pattern of mutations was seen in HIV-associated DLBCL related to EBV status [73].
Plasmablastic lymphoma. A) Large cells many with one central nucleolus and abundant cytoplasm. B) Higher magnification with Giemsa stain that highlights the blastic chromatin, large central nucleolus, and moderate basophilic cytoplasm. C) CD20 stain is negative in the tumor cells. D) MUM1 is positive in the majority of the tumor cells. E) MYC stain is strongly positive in the majority of the tumor cells suggesting a MYC translocation. F) The cells are CD10 positive. G) Epstein-Barr encoding small RNA (EBER) in situ hybridization is positive in the tumor cells
HHV8-positive diffuse large B-cell lymphoma, not otherwise specified
HHV-8 is a gamma herpesvirus involved in the development of various lymphoid neoplasm such as PEL, multicentric Castleman disease (MCD), germinotropic lymphoproliferative disorder (GLPD), and HHV-8-positive diffuse large B-cell lymphoma, NOS (HHV8 + DLBCL). HHV8 + DLBCL is rare and often arises in the setting of MCD. Aggregates of plasmablasts can be present in MCD and are positive for viral IL-6, IgM, and lambda light chain. These plasmablast aggregates do not efface the underlying architecture. In contrast, HHV8 + DLBCL cells form sheets and destroy the underlying normal architecture [74]. These tumor cells resemble plasmablasts or immunoblasts and have variable staining with B-cell markers, such as CD20, and are positive for MUM1 but typically negative for CD138. Also, in contrast to the plasmablast aggregates seen in MCD, HHV8 + DLBCL shows clonal immunoglobulin gene rearrangements. Outcomes in HHV8 + DLBCL are very poor especially in persons living with HIV/AIDS (PLWH).
Primary effusion lymphoma (PEL)
PEL is also a rare and aggressive B-cell neoplasm defined by the presence of HHV-8 typically occurring in the pleura, peritoneum, and pericardium. Most cases are also EBV-positive; however, rare EBV-negative cases exist (typically elderly individuals without HIV)[75, 76]. Solid lesions of PEL (so-called extracavitary PEL) arise in lymph nodes or extranodal sites such as skin and the GI tract. PEL most frequently occurs in PLWH especially in patients with low CD4 T-cell counts but is also seen in immunocompetent elderly men from HHV-8-endemic areas or other immunodeficiency states such as solid organ transplant [77]. The cells are large and pleomorphic resembling immunoblasts and plasmablasts, and HRS cells can be seen (Fig. 8). These tumor cells typically lack B- and T-cell markers but are positive for plasma cell markers and are usually positive for CD30 and EMA [74]. The prognosis of PEL remains poor. PEL can be seen in conjunction with multicentric Castleman disease and have a negative impact on survival [78]. Extracavitary PEL in elderly, HIV-negative individuals are usually EBV-negative. The differential diagnosis with HHV-8-positive DLBCL might be difficult. The latter should be favored in EBV-negative cases with cytoplasmic IgM, lambda restriction, and/or association with multicentric Castleman disease.
Conclusion
The recent growth of genomic information in lymphoid neoplasms has provided new insights to the driving mechanisms and pathogenesis of DLBCL. These studies have expanded the view of DLBCL as a heterogenous and complex group of diseases which prognosis and response to treatment are dependent on multiple factors such as clinical features, genomic subtypes, and interplay of the TME. Further clinical trials and novel therapies incorporating these new biomarkers will prove challenging but should provide impact in the management and guide treatment for this aggressive disease in the near future.
Data Availability
There is no data generated from this article.
References
Swerdlow S, Campo, E, Harris, NL, Jaffe, ES, Pileri, SA, Stein, H, Thiele, J, Arber, DA, Hasserjian, RP, Le Beau, MM, Orazi, A, and Siebert, R (2017) WHO classification of tumours of haematopoietic and lymphoid tissues. International Agency for Research on Cancer, Lyon, France, pp
Campo E, Jaffe ES, Cook JR et al (2022) The International Consensus Classification of Mature Lymphoid Neoplasms: a report from the Clinical Advisory Committee. Blood 140:1229–1253. https://doi.org/10.1182/blood.2022015851
Alaggio R, Amador C, Anagnostopoulos I et al (2022) The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours Lymphoid Neoplasms. Leukemia 36:1720–1748
Lai R, Medeiros LJ, Dabbagh L et al (2000) Sinusoidal CD30-positive large B-cell lymphoma: a morphologic mimic of anaplastic large cell lymphoma. Mod Pathol 13:223–228. https://doi.org/10.1038/modpathol.3880041
Oliveira JL, Grogg KL, Macon WR et al (2012) Clinicopathologic features of B-cell lineage neoplasms with aberrant expression of CD3: a study of 21 cases. Am J Surg Pathol 36:1364–1370. https://doi.org/10.1097/PAS.0b013e31825e63a9
Durani U, Ansell SM (2021) CD5+ diffuse large B-cell lymphoma: a narrative review. Leuk Lymphoma 62:3078–3086. https://doi.org/10.1080/10428194.2021.1953010
Hu B, Nastoupil LJ, Loghavi S et al (2020) De novo CD5+ diffuse large B-cell lymphoma, NOS: clinical characteristics and outcomes in rituximab era. Leuk Lymphoma 61:328–336. https://doi.org/10.1080/10428194.2019.1663418
Tzankov A, Leu N, Muenst S et al (2015) Multiparameter analysis of homogeneously R-CHOP-treated diffuse large B cell lymphomas identifies CD5 and FOXP1 as relevant prognostic biomarkers: report of the prospective SAKK 38/07 study. J Hematol Oncol 8:70. https://doi.org/10.1186/s13045-015-0168-7
Hsiao SC, Cortada IR, Colomo L et al (2012) SOX11 is useful in differentiating cyclin D1-positive diffuse large B-cell lymphoma from mantle cell lymphoma. Histopathology 61:685–693. https://doi.org/10.1111/j.1365-2559.2012.04260.x
Cheng J, Hashem MA, Barabe F et al (2021) CCND1 genomic rearrangement as a secondary event in high grade B-cell lymphoma. Hemasphere 5:e505. https://doi.org/10.1097/HS9.0000000000000505
Ok CY, Xu-Monette ZY, Tzankov A et al (2014) Prevalence and clinical implications of cyclin D1 expression in diffuse large B-cell lymphoma (DLBCL) treated with immunochemotherapy: a report from the International DLBCL Rituximab-CHOP Consortium Program. Cancer 120:1818–1829. https://doi.org/10.1002/cncr.28664
Koduru PR, Chen W, Garcia R et al (2015) Acquisition of a t(11;14)(q13;q32) in clonal evolution in a follicular lymphoma with a t(14;18)(q32;q21) and t(3;22)(q27;q11.2). Cancer Genet 208:303–309. https://doi.org/10.1016/j.cancergen.2015.03.007
Horn H, Ziepert M, Becher C et al (2013) MYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B-cell lymphoma. Blood 121:2253–2263. https://doi.org/10.1182/blood-2012-06-435842
Staiger AM, Ziepert M, Horn H et al (2017) Clinical impact of the cell-of-origin classification and the MYC/ BCL2 dual expresser status in diffuse large B-cell lymphoma treated within prospective clinical trials of the German high-grade non-Hodgkin’s lymphoma study group. J Clin Oncol 35:2515–2526. https://doi.org/10.1200/JCO.2016.70.3660
Meriranta L, Pasanen A, Alkodsi A et al (2020) Molecular background delineates outcome of double protein expressor diffuse large B-cell lymphoma. Blood Adv 4:3742–3753. https://doi.org/10.1182/bloodadvances.2020001727
Alizadeh AA, Eisen MB, Davis RE et al (2000) Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403:503–511. https://doi.org/10.1038/35000501
Hans CP, Weisenburger DD, Greiner TC et al (2004) Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 103:275–282. https://doi.org/10.1182/blood-2003-05-1545
Leonard JP, Kolibaba KS, Reeves JA et al (2017) Randomized phase II study of R-CHOP with or without bortezomib in previously untreated patients with non-germinal center B-cell-like diffuse large B-cell lymphoma. J Clin Oncol 35:3538–3546. https://doi.org/10.1200/JCO.2017.73.2784
Nowakowski GS, Chiappella A, Gascoyne RD et al (2021) ROBUST: a phase III study of lenalidomide plus R-CHOP versus placebo plus R-CHOP in previously untreated patients with ABC-type diffuse large B-cell lymphoma. J Clin Oncol 39:1317–1328. https://doi.org/10.1200/JCO.20.01366
Frauenfeld L, Castrejon-de-Anta N, Ramis-Zaldivar JE et al (2022) Diffuse large B-cell lymphomas in adults with aberrant coexpression of CD10, BCL6, and MUM1 are enriched in IRF4 rearrangements. Blood Adv 6:2361–2372. https://doi.org/10.1182/bloodadvances.2021006034
Colomo L, Loong F, Rives S et al (2004) Diffuse large B-cell lymphomas with plasmablastic differentiation represent a heterogeneous group of disease entities. Am J Surg Pathol 28:736–747. https://doi.org/10.1097/01.pas.0000126781.87158.e3
Meyer PN, Fu K, Greiner TC et al (2011) Immunohistochemical methods for predicting cell of origin and survival in patients with diffuse large B-cell lymphoma treated with rituximab. J Clin Oncol 29:200–207. https://doi.org/10.1200/JCO.2010.30.0368
Choi WW, Weisenburger DD, Greiner TC et al (2009) A new immunostain algorithm classifies diffuse large B-cell lymphoma into molecular subtypes with high accuracy. Clin Cancer Res 15:5494–5502. https://doi.org/10.1158/1078-0432.CCR-09-0113
Ahmed S, Glover P, Taylor J et al (2021) Comparative analysis of gene expression platforms for cell-of-origin classification of diffuse large B-cell lymphoma shows high concordance. Br J Haematol 192:599–604. https://doi.org/10.1111/bjh.17246
Scott DW, Wright GW, Williams PM et al (2014) Determining cell-of-origin subtypes of diffuse large B-cell lymphoma using gene expression in formalin-fixed paraffin-embedded tissue. Blood 123:1214–1217. https://doi.org/10.1182/blood-2013-11-536433
Wilson WH, Wright GW, Huang DW et al (2021) Effect of ibrutinib with R-CHOP chemotherapy in genetic subtypes of DLBCL. Cancer Cell 39(1643–1653):e1643. https://doi.org/10.1016/j.ccell.2021.10.006
Wright GW, Huang DW, Phelan JD et al (2020) A probabilistic classification tool for genetic subtypes of diffuse large B cell lymphoma with therapeutic implications. Cancer Cell 37(551–568):e514. https://doi.org/10.1016/j.ccell.2020.03.015
Chapuy B, Stewart C, Dunford AJ et al (2018) Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med 24:679–690. https://doi.org/10.1038/s41591-018-0016-8
Schmitz R, Wright GW, Huang DW et al (2018) Genetics and pathogenesis of diffuse large B-cell lymphoma. N Engl J Med 378:1396–1407. https://doi.org/10.1056/NEJMoa1801445
Lacy SE, Barrans SL, Beer PA et al (2020) Targeted sequencing in DLBCL, molecular subtypes, and outcomes: a Haematological Malignancy Research Network report. Blood 135:1759–1771. https://doi.org/10.1182/blood.2019003535
Autio M, Leivonen SK, Bruck O et al (2022) Clinical impact of immune cells and their spatial interactions in diffuse large B-cell lymphoma microenvironment. Clin Cancer Res 28:781–792. https://doi.org/10.1158/1078-0432.CCR-21-3140
Steen CB, Luca BA, Esfahani MS et al (2021) The landscape of tumor cell states and ecosystems in diffuse large B cell lymphoma. Cancer Cell 39(1422–1437):e1410. https://doi.org/10.1016/j.ccell.2021.08.011
Godfrey J, Tumuluru S, Bao R et al (2019) PD-L1 gene alterations identify a subset of diffuse large B-cell lymphoma harboring a T-cell-inflamed phenotype. Blood 133:2279–2290. https://doi.org/10.1182/blood-2018-10-879015
Scott DW, King RL, Staiger AM et al (2018) High-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements with diffuse large B-cell lymphoma morphology. Blood 131:2060–2064. https://doi.org/10.1182/blood-2017-12-820605
King RL, Hsi ED, Chan WC et al (2022) Diagnostic approaches and future directions in Burkitt lymphoma and high-grade B-cell lymphoma. Virchows Arch. https://doi.org/10.1007/s00428-022-03404-6
Gonzalez-Farre B, Ramis-Zaldivar JE, Salmeron-Villalobos J et al (2019) Burkitt-like lymphoma with 11q aberration: a germinal center-derived lymphoma genetically unrelated to Burkitt lymphoma. Haematologica 104:1822–1829. https://doi.org/10.3324/haematol.2018.207928
Horn H, Kalmbach S, Wagener R et al (2021) A diagnostic approach to the identification of Burkitt-like lymphoma with 11q aberration in aggressive B-cell lymphomas. Am J Surg Pathol 45:356–364. https://doi.org/10.1097/PAS.0000000000001613
Colomo L, Vazquez I, Papaleo N et al (2017) LMO2-negative expression predicts the presence of MYC translocations in aggressive B-cell lymphomas. Am J Surg Pathol 41:877–886. https://doi.org/10.1097/PAS.0000000000000839
Wagener R, Seufert J, Raimondi F et al (2019) The mutational landscape of Burkitt-like lymphoma with 11q aberration is distinct from that of Burkitt lymphoma. Blood 133:962–966. https://doi.org/10.1182/blood-2018-07-864025
Gebauer N, Witte HM, Merz H et al (2021) Aggressive B-cell lymphoma cases with 11q aberration patterns indicate a spectrum beyond Burkitt-like lymphoma. Blood Adv 5:5220–5225. https://doi.org/10.1182/bloodadvances.2021004635
Tousseyn TA, King RL, Fend F et al (2022) Evolution in the definition and diagnosis of the Hodgkin lymphomas and related entities. Virchows Arch. https://doi.org/10.1007/s00428-022-03427-z
Prakash S, Fountaine T, Raffeld M et al (2006) IgD positive L&H cells identify a unique subset of nodular lymphocyte predominant Hodgkin lymphoma. Am J Surg Pathol 30:585–592. https://doi.org/10.1097/01.pas.0000194741.87798.45
Schuhmacher B, Bein J, Rausch T et al (2019) JUNB, DUSP2, SGK1, SOCS1 and CREBBP are frequently mutated in T-cell/histiocyte-rich large B-cell lymphoma. Haematologica 104:330–337. https://doi.org/10.3324/haematol.2018.203224
Rosenwald A, Wright G, Leroy K et al (2003) Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med 198:851–862. https://doi.org/10.1084/jem.20031074
Mottok A, Woolcock B, Chan FC et al (2015) Genomic alterations in CIITA are frequent in primary mediastinal large B cell lymphoma and are associated with diminished MHC class II expression. Cell Rep 13:1418–1431. https://doi.org/10.1016/j.celrep.2015.10.008
Steidl C, Shah SP, Woolcock BW et al (2011) MHC class II transactivator CIITA is a recurrent gene fusion partner in lymphoid cancers. Nature 471:377–381. https://doi.org/10.1038/nature09754
Mottok A, Hung SS, Chavez EA et al (2019) Integrative genomic analysis identifies key pathogenic mechanisms in primary mediastinal large B-cell lymphoma. Blood 134:802–813. https://doi.org/10.1182/blood.2019001126
Dunleavy K, Pittaluga S, Maeda LS et al (2013) Dose-adjusted EPOCH-rituximab therapy in primary mediastinal B-cell lymphoma. N Engl J Med 368:1408–1416. https://doi.org/10.1056/NEJMoa1214561
Mottok A, Wright G, Rosenwald A et al (2018) Molecular classification of primary mediastinal large B-cell lymphoma using routinely available tissue specimens. Blood 132:2401–2405. https://doi.org/10.1182/blood-2018-05-851154
Sarkozy C, Hung SS, Chavez EA et al (2021) Mutational landscape of gray zone lymphoma. Blood 137:1765–1776. https://doi.org/10.1182/blood.2020007507
Nakamura T, Tateishi K, Niwa T et al (2016) Recurrent mutations of CD79B and MYD88 are the hallmark of primary central nervous system lymphomas. Neuropathol Appl Neurobiol 42:279–290. https://doi.org/10.1111/nan.12259
Kraan W, van Keimpema M, Horlings HM et al (2014) High prevalence of oncogenic MYD88 and CD79B mutations in primary testicular diffuse large B-cell lymphoma. Leukemia 28:719–720. https://doi.org/10.1038/leu.2013.348
Alame M, Pirel M, Costes-Martineau V et al (2020) Characterisation of tumour microenvironment and immune checkpoints in primary central nervous system diffuse large B cell lymphomas. Virchows Arch 476:891–902. https://doi.org/10.1007/s00428-019-02695-6
Chapuy B, Roemer MG, Stewart C et al (2016) Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood 127:869–881. https://doi.org/10.1182/blood-2015-10-673236
Nayak L, Iwamoto FM, LaCasce A et al (2017) PD-1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma. Blood 129:3071–3073. https://doi.org/10.1182/blood-2017-01-764209
Gonzalez-Farre B, Ramis-Zaldivvar J, Castrejon de Anta N, et al. (2022) Intravascular large B-cell lymphoma genomic profile is characterized by alterations in genes regulating NF- κB and immune checkpoint. American Journal of Surgical Pathology
Schrader AMR, Jansen PM, Willemze R et al (2018) High prevalence of MYD88 and CD79B mutations in intravascular large B-cell lymphoma. Blood 131:2086–2089. https://doi.org/10.1182/blood-2017-12-822817
Pham-Ledard A, Beylot-Barry M, Barbe C et al (2014) High frequency and clinical prognostic value of MYD88 L265P mutation in primary cutaneous diffuse large B-cell lymphoma, leg-type. JAMA Dermatol 150:1173–1179. https://doi.org/10.1001/jamadermatol.2014.821
Taniguchi K, Takata K, Chuang SS et al (2016) Frequent MYD88 L265P and CD79B mutations in primary breast diffuse large B-cell lymphoma. Am J Surg Pathol 40:324–334. https://doi.org/10.1097/PAS.0000000000000592
de Groen RAL, van Eijk R, Bohringer S et al (2021) Frequent mutated B2M, EZH2, IRF8, and TNFRSF14 in primary bone diffuse large B-cell lymphoma reflect a GCB phenotype. Blood Adv 5:3760–3775. https://doi.org/10.1182/bloodadvances.2021005215
Sun J, Zhang J, Ling Q et al (2015) Primary diffuse large B-cell lymphoma of the ovary is of a germinal centre B-cell-like phenotype. Virchows Arch 466:93–100. https://doi.org/10.1007/s00428-014-1682-7
Subik MK, Herr M, Hutchison RE et al (2014) A highly curable lymphoma occurs preferentially in the proximal tibia of young patients. Mod Pathol 27:1430–1437. https://doi.org/10.1038/modpathol.2014.51
Alexanian S, Said J, Lones M et al (2013) KSHV/HHV8-negative effusion-based lymphoma, a distinct entity associated with fluid overload states. Am J Surg Pathol 37:241–249. https://doi.org/10.1097/PAS.0b013e318267fabc
Gisriel SD, Yuan J, Braunberger RC et al (2022) Human herpesvirus 8-negative effusion-based large B-cell lymphoma: a distinct entity with unique clinicopathologic characteristics. Mod Pathol 35:1411–1422. https://doi.org/10.1038/s41379-022-01091-x
Kaji D, Ota Y, Sato Y et al (2020) Primary human herpesvirus 8-negative effusion-based lymphoma: a large B-cell lymphoma with favorable prognosis. Blood Adv 4:4442–4450. https://doi.org/10.1182/bloodadvances.2020002293
Kubota T, Sasaki Y, Shiozawa E et al (2018) Age and CD20 expression are significant prognostic factors in human herpes virus-8-negative effusion-based lymphoma. Am J Surg Pathol 42:1607–1616. https://doi.org/10.1097/PAS.0000000000001168
Laurent C, Do C, Gascoyne RD et al (2009) Anaplastic lymphoma kinase-positive diffuse large B-cell lymphoma: a rare clinicopathologic entity with poor prognosis. J Clin Oncol 27:4211–4216. https://doi.org/10.1200/JCO.2008.21.5020
Valera A, Colomo L, Martinez A et al (2013) ALK-positive large B-cell lymphomas express a terminal B-cell differentiation program and activated STAT3 but lack MYC rearrangements. Mod Pathol 26:1329–1337. https://doi.org/10.1038/modpathol.2013.73
Cerchietti L, Damm-Welk C, Vater I et al (2011) Inhibition of anaplastic lymphoma kinase (ALK) activity provides a therapeutic approach for CLTC-ALK-positive human diffuse large B cell lymphomas. Plos One 6:e18436. https://doi.org/10.1371/journal.pone.0018436
GambacortiPasserini C, Farina F, Stasia A et al (2014) Crizotinib in advanced, chemoresistant anaplastic lymphoma kinase-positive lymphoma patients. J Natl Cancer Inst 106:djt378. https://doi.org/10.1093/jnci/djt378
Ramis-Zaldivar JE, Gonzalez-Farre B, Nicolae A et al (2021) MAPK and JAK-STAT pathways dysregulation in plasmablastic lymphoma. Haematologica 106:2682–2693. https://doi.org/10.3324/haematol.2020.271957
Garcia-Reyero J, Martinez Magunacelaya N, Gonzalez de Villambrosia S et al (2021) Genetic lesions in MYC and STAT3 drive oncogenic transcription factor overexpression in plasmablastic lymphoma. Haematologica 106:1120–1128. https://doi.org/10.3324/haematol.2020.251579
Chapman JR, Bouska AC, Zhang W et al (2021) EBV-positive HIV-associated diffuse large B cell lymphomas are characterized by JAK/STAT (STAT3) pathway mutations and unique clinicopathologic features. Br J Haematol 194:870–878. https://doi.org/10.1111/bjh.17708
Chadburn A, Said J, Gratzinger D et al (2017) HHV8/KSHV-positive lymphoproliferative disorders and the spectrum of plasmablastic and plasma cell neoplasms: 2015 SH/EAHP workshop report—part 3. Am J Clin Pathol 147:171–187. https://doi.org/10.1093/ajcp/aqw218
Song JY, Jaffe ES (2013) HHV-8-positive but EBV-negative primary effusion lymphoma. Blood 122:3712. https://doi.org/10.1182/blood-2013-07-515882
Teruya-Feldstein J, Zauber P, Setsuda JE et al (1998) Expression of human herpesvirus-8 oncogene and cytokine homologues in an HIV-seronegative patient with multicentric Castleman’s disease and primary effusion lymphoma. Lab Invest 78:1637–1642
Cesarman E, Chadburn A, Rubinstein PG (2022) KSHV/HHV8-mediated hematologic diseases. Blood 139:1013–1025. https://doi.org/10.1182/blood.2020005470
Ramaswami R, Lurain K, Polizzotto MN et al (2021) Characteristics and outcomes of KSHV-associated multicentric Castleman disease with or without other KSHV diseases. Blood Adv 5:1660–1670. https://doi.org/10.1182/bloodadvances.2020004058
Morin RD, Arthur SE, Hodson DJ (2022) Molecular profiling in diffuse large B-cell lymphoma: why so many types of subtypes? Br J Haematol 196:814–829. https://doi.org/10.1111/bjh.17811
Author information
Authors and Affiliations
Contributions
JYS drafted the manuscript. JYS, SD, LQM, SP, MAP, and EC edited the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Compliance with all ethical standards was undertaken for this work. No research involving human or animals was performed. No informed consent was required.
Conflict of interest
JYS, SD, LQM, SP do not have any conflicts to disclose. MAP has the following disclosures: Millenium/Takeda: Advisory Board, Lecture Fees, Research Funding; Celgene: Advisory Board; Gilead: Advisory Board; Research funding; Jansen: Advisory Board; Lecture Fees; Nanostring: Advisory Board; Kyowa Kirin: Advisory Board; Kura: Research Funding. EC has been a consultant for Takeda, NanoString, and Illumina; has received honoraria from Janssen, EUSPharma, Takeda and Roche for speaking at educational activities; and is an inventor on a Lymphoma and Leukemia Molecular Profiling Project patent “Method for subtyping lymphoma subtypes by means of expression profiling” (PCT/US2014/64161) and on a bioinformatic pipeline “IgCaller” not related to this project.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, J.Y., Dirnhofer, S., Piris, M.A. et al. Diffuse large B-cell lymphomas, not otherwise specified, and emerging entities. Virchows Arch 482, 179–192 (2023). https://doi.org/10.1007/s00428-022-03466-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-022-03466-6