Abstract
Sarcomatoid malignant mesothelioma (SMM) tends to occur in the pleura and is morphologically similar to lung sarcomatoid carcinoma (LSC) and organizing pleuritis (OP). Because SMM often does not express mesothelial markers, it is very difficult to distinguish from LSC and OP. GATA-binding protein 3 (GATA3) is a specific immunohistochemical (IHC) marker of breast and urothelial carcinoma. We routinely find that GATA is expressed in MM; however, GATA3 expression in SMM and its reference value for distinguishing SMM from LSC and OP remain unclear. Here, we used IHC methods to detect the expression of GATA3 and classic mesothelial markers in 17 SMM, 12 LSC, and 7 OP cases. We detected the following expression rates in SMM versus LSC cases: GATA3 (70.6% vs. 16.7%, p = 0.008), calretinin (52.9% vs. 8.3%, p = 0.019), Wilms tumor (WT)-1 (64.7% vs. 0%, p = 0.000), D2-40 (47.1% vs. 16.7%, p = 0.126), CK5/6 (35.3% vs. 25.0%, p = 0.694), and pan-cytokeratin (CKpan) (88.2% vs. 100.0%, p = 0.498). The specificities of calretinin, WT-1, and GATA3 in distinguishing SMM from LSC were 91.7%, 100%, and 83.3%, respectively, and combinations of any two of these three markers exhibited 100% specificity for SMM. Notably, the sensitivity of calretinin+/WT1+ staining for SMM was only 23.5%, which increased to 64.7% after including GATA3. Furthermore, all OP cases showed partial or diffuse expression of CKpan, WT-1, and D2-40 but no GATA3 and calretinin expression. In conclusion, GATA3 is an IHC marker with excellent sensitivity and specificity for SMM, and the combined consideration of GATA3, calretinin, and WT-1 was best for distinguishing SMM from LSC. Moreover, CKpan, WT-1, and D2-40 had no value for distinguishing SMM from OP, and GATA3 and calretinin were the most specific markers for distinguishing these two lesions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sarcomatoid malignant mesothelioma (SMM) commonly occurs in the pleura and needs to be distinguished from various primary or metastatic sarcomas and sarcomatoid carcinomas. In particular, distinguishing SMM from lung sarcomatoid carcinomas (LSCs) involving the pleura is especially challenging [1]. SMM exhibits an insidious onset, and vast majority of patients have advanced disease when they receive treatment. Moreover, its diagnosis is mainly based on small biopsy specimens; therefore, immunohistochemical staining plays a decisive role in SMM diagnosis. However, immunohistochemical markers for mesotheliomas show limited or even no expression in most SMM cases, whereas they can be expressed in LSCs, thus easily leading to misdiagnoses [2, 3]. Guo et al. [4] retrospectively analyzed 92 patients diagnosed with malignant mesothelioma (MM) in Zhejiang, China, and found a misdiagnosis rate of 43.5%, which was mainly due to the abnormal expression of mesothelial markers in nonmesothelioma tumors.
Desmoplastic MM (DMM) mainly comprises a collagenic stroma interspersed with only a few tumor cells and often needs to be distinguished from organizing pleuritis (OP) [1]. Mesothelial markers play an important role in distinguishing the two diseases. However, we have often observed that mesothelial markers are also expressed in spindle cells of OP, which can seriously confuse differential diagnosis. Thus, clarifying the expression of mesothelial markers in OP is an important basis for distinguishing it from DMM. However, to the best of our knowledge, this topic has not been investigated.
GATA-binding protein 3 (GATA3) is a specific marker for breast cancer and bladder cancer and is commonly used in the differential diagnosis of pleural tumors. However, we found that GATA3 was also frequently expressed in MM, especially in cases of SMM that did not express mesothelial markers, and such cases were commonly misdiagnosed. Therefore, we aimed to detect the expression of GATA3 and mesothelial markers in SMM, LSC, and OP to define the reference values for various antibodies and their combinations for the differential diagnosis of SMM, LSC, and OP.
Materials and methods
Patients and tissue samples
Among the MM patients diagnosed at the Ningbo Diagnostic Pathology Center between 2012 and 2020, we selected a total of 17 MM cases based on the clinical course, imaging data, pathologic, and immunohistochemical features. Twelve cases of LSC and 7 cases of OP were included in the control group. This study was approved by the Ethics Committee of the Ningbo Diagnostic Pathology Center.
Immunohistochemistry
IHC of sections from formalin fixed paraffin-embedded tissues was performed using the EnVision two-step system (Agilent Technologies, Glostrup, Denmark). All IHC labeling was performed with a Benchmark ULTRA automated immunohistochemical station (Ventana, Roche Diagnostics, Switzerland), labeled antibodies included those recognizing GATA3 (clone EP368, 1:500, ZSGB-BIO, China), calretinin (clone CAL6, prediluted, Leica Biosystems, Germany), Wilms tumor (WT)-1 (clone 6F-H2, prediluted, DAKO, Denmark), D2-40 (clone D2-40, prediluted, DAKO, Denmark ), cytokeratin (CK)5/6 (clone OT11C, 1:500, ZSGB-BIO, China), and pan-cytokeratin (CKpan) (clone AE1/AE3, 1:300, ZSGB-BIO, China). The IHC results verified that nuclear calretinin (nucleoplasmic), WT-1, and GATA3 staining were positive, whereas the other markers showed localized staining in the cell membrane or cytoplasm. Staining intensity was scored as follows: 0, no staining; 1+, weakly positive (light yellow); 2+, moderately positive (yellow); and 3+, strongly positive (brownish yellow). The specimens were then scored according to the percentage of positive cells as follows: 0, < 1%; 1+, 1% to 10%; 2+, 11% to 50%; and 3+, > 50%. Finally, the staining intensity and percentage scores were summed and divided into categories: negative or indeterminate staining of individual cells (0–2), partial expression (3–4), and diffuse expression (5–6), with scores ≥ 3 considered positive.
Statistical analysis
Data were statistically analyzed using SPSS (v.20.0; IBM Corp., Armonk, NY, USA). Between-group comparisons of the positivity rates for the markers were performed using the chi-squared test (Fisher’s exact probability test), with p < 0.05 considered statistically significant. The sensitivity and specificity of the individual antibodies and their combinations for SMM were calculated as follows:
where TP is the number of true positives, TN is the number of true negatives, FP is the number of false positives, and FN is the number of false negatives.
Results
Patients’ characteristics
A total of 17 MM patients were selected in this study, including 13 females and 4 males with a median age of 62 years (range from 13 to 78 years). There were 15 SMM (12 spindle cell type and 3 DMM) and 2 biphasic MM (sarcomatoid area accounting for at least 80%). Tumors arose from the pleura in 16 patients and the peritoneum in one patient. All cases showed diffuse pleural/peritoneal thickening with multiple nodules. All the cases of pleural SMM underwent biopsy for pathologic diagnosis, and one case of peritoneal SMM underwent surgery. Given that the spindle cell type was almost common type of SMM, we selected 12 cases of LSC with mainly spindle cell carcinoma components. All the cases arose from lung parenchyma and were treated with surgery. All cases of OP exhibited varying degrees of pleural thickening, and neoplastic lesions were excluded according to the clinical course.
Expression levels of markers for SMM and LSC
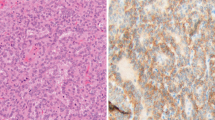
Microscopically, both SMM and LSC cases showed diffuse proliferation pattern of atypical spindle cells (Fig. 1A, D). GATA3 was expressed in 12 cases (70.6%) of SMM, including 7 cases (41.2%) showing diffuse expression (Fig. 1B), whereas 2 cases (16.7%) of LSC showed partial expression (Fig. 1E). Calretinin was expressed in 9 cases (52.9%) of SMM, including 6 cases (35.4%) showing diffuse expression (Fig. 1C), whereas 1 case (8.3%) of LSC showed partial expression (Fig. 1F). WT-1 was expressed in 11 cases (64.7%) of SMM, including 5 cases (29.4%) showing diffuse staining, whereas 11 of 12 LSC cases showed diffuse cytoplasmic staining, and none nuclear expression (Fig. 1G). There were no significant differences between SMM and LSC with respect to D2-40 (47.1% vs. 16.7%, p = 0.126), CK5/6 (35.3% vs. 25.0%, p = 0.694), and CKpan (88.2% vs. 100.0%, p = 0.498) staining (Table 1).
H&E and IHC staining in sarcomatoid malignant mesothelioma (A–C) and lung sarcomatoid carcinoma (D–G). A H&E staining of sarcomatoid malignant mesothelioma showed atypical spindle cells arranged in a fascicular pattern. B Tumor cells showed diffuse nuclear staining for GATA3. C Nucleoplasmic staining for calretinin. D H&E staining of lung sarcomatoid carcinoma showed atypical spindle and/or polygonal cells proliferating in the lung parenchyma. E GATA3 staining was strongly positive in lymphocytes and negative in tumor cells. F Tumor cells showed focal nucleoplasmic staining for calretinin. G Diffuse cytoplasmic staining, but not nuclear staining, was seen for WT-1
Sensitivity and specificity of marker combinations to differentiate SMM from LSC
The levels of calretinin, WT-1, and GATA3 in SMM and LSC differed significantly (p < 0.05). The sensitivities of calretinin, WT-1, and GATA3 for SMM were 52.9%, 64.7%, and 70.6%, respectively, and their specificities for distinguishing SMM from LSC were 91.7%, 100%, and 83.3%, respectively. The specificity of any two of these three markers showing simultaneous positivity for SMM reached 100%. The sensitivity of calretinin+/WT-1+ staining for SMM was 23.5%, which increased to 64.7% after including GATA3 (Table 2).
Marker levels in OP
Microscopically, all OP cases exhibited dense irregular proliferation of slender or fibroblastic like spindle cells (Fig. 2A). All the cases were negative for GATA3 and calretinin, including with weak expression of GATA3 (2 cases) and calretinin (2 cases) in very small number of cells (Fig. 2B,C). All the cases of OP showed partial or diffuse expression of WT-1, D2-40, and CKpan, and CK5/6 was not expressed in any cases (Fig. 2D–F). Furthermore, there were significant differences in GATA3 and calretinin staining (p < 0.05), but not between WT-1 and CK5/6 staining (p > 0.05), between SMM and OP cases. D2-40 was expressed in 8 cases (47.1%) of SMM, whereas all the cases (100%) of LSC showed partial or diffuse expression (Table 1). The detail immunohistochemical results in the supplementary material 1.
H&E staining and immunostaining in organizing pleuritis. A H&E staining showed dense proliferation of spindle cells, and mitotic figures were observed. B GATA3 staining was strongly positive in lymphocytes and negative in spindle cells. C Spindle cells showed diffuse cytoplasmic staining for calretinin, with indeterminate nuclear staining in a very small number cells. D Diffuse nuclear staining was seen for WT-1. E Diffuse cytoplasmic staining was seen for D2-40 F and CKpan
Discussion
Differentiating SMM from LSC is challenging as the histomorphological features are extremely similar. Marchevsdy et al. [5] proposed an evidence-based criteria for the differential diagnosis between SMM and LSC. As they noted, the two can be identified by mesothelial and epithelial markers (claudin 4, TTF-1, and Ber-EP4) in some cases; however, in fact, a number of SMM and LSC cases express cytokeratin only [1]. Pleura is the almost common site of SMM, and unique growth patterns, such as diffuse thickening of the pleura and multiple pleural nodules, are significant reference features for MM diagnosis [1, 5]. However, a small number of lung cancers, metastatic carcinomas, and sarcomas can also present “pseudomesotheliomatous” growth on chest computed tomography findings [6].
As a specific marker for breast cancer and bladder cancer, GATA3 is often used for the differential diagnosis of pleural tumors [7]; however, we have observed that GATA3 is often expressed in MM. A large study of 2500 samples conducted by Miettinen et al. [8] found that in addition to breast and bladder cancers, a high percentage of skin and skin adnexal tumors (81–100%), pancreatic ductal carcinomas (37%), germ cell tumors (40–100%), kidney cancers (2–51%), and MMs (58%) expressed GATA-3. Lymphocytes are a good internal control for GATA3 expression, and the tumor cells of SMM usually stain less intensely than lymphocytes. In the present study, we found that 70.6% of SMM cases expressed GATA3, and approximately 40% of those cases showed diffuse expression. Berg et al. [9] reported that the sensitivity of GATA3 for SMM was 100% but also suggested that the absence of GATA3 expression did not support a diagnosis of SMM. Nevertheless, in the present study, we found that 29.4% of SMM cases did not express GATA3, whereas 16.7% of LSC cases did (with 20–30% GATA3+ cells). The specificity of GATA3 expression for distinguishing SMM from LSC was 83.3%, which suggested that GATA3 cannot be used independently to distinguish the two diseases. Recently, Prabhakaran et al. [10] examined GATA3 expression in 149 cases of MM, including 40 cases of SMM. They found that 72% of SMM cases expressed GATA3, which is consistent with our results.
CKpan is an important marker for distinguishing SMM from sarcomas; however, previous studies have reported that 7% to 30% of SMMs are negative for CKpan [2, 11]. It is challenging to distinguish CKpan-negative SMM cases from sarcomas, especially synovial sarcomas, because most synovial sarcomas express CK and even express calretinin (52–79%) and GATA3 (20%) [8, 12, 13]. In the present study, we found that 11.8% of the SMM cases were negative for CKpan. For these cases, after extensive IHC analysis and molecular testing combined with consideration of imaging data and clinical experience, we fully excluded other sarcomas and eventually reached a diagnosis of SMM.
The expression rate of calretinin is significantly lower in SMM (31–77.8%) than in epithelioid MM, and the pattern of expression is mostly focal [2, 11]. Calretinin localizes to the nucleoplasm in MM but shows nonspecific cytoplasmic staining in various tumors [14]. Kushitani et al. [14] found that 66.7% of LSC cases expressed calretinin, although their description indicated that most cases showed staining in the cytoplasm of tumor cells. Lucas et al. [2] reported that 60% of LSC cases expressed calretinin; however, a detailed evaluation of their figures revealed that calretinin was mainly localized to the cytoplasm of tumor cells, and there was indeterminate focal staining or an absence of clear staining of the nucleus. The criterion for positive staining in the present study was definite nuclear staining, and we determined calretinin expression rates in SMM and LSC cases of 52.9% and 8.3%, respectively, and the difference between the two groups was significant. Additionally, the specificity of calretinin for distinguishing SMM from LSC reached 91.7%.
Previous studies have reported that the WT-1+ rate in SMM ranges from 10 to 41.7% [2, 3]. Kushitani et al. [14] reported that 44.4% of LSC and 47.0% of sarcomas expressed WT-1; however, their findings indicated that WT-1 was expressed in the cytoplasm of tumor cells in all cases. We considered obvious nuclear staining as a positive result and found a 64.7% positivity rate for WT-1 expression in SMMs. Among the 12 LSC cases, 11 showed diffuse cytoplasmic positivity with no staining of nuclei. Furthermore, the specificity for WT-1 in distinguishing SMM from LSC reached 100%.
D2-40 is mostly localized to the cell membrane in epithelioid MMs and to the cytoplasm in SMMs [15]. However, we have often observed nonspecific D2-40 expression in the fibrous stroma; therefore, D2-40 localization in SMM can be difficult to discriminate and potentially lead to erroneous conclusions [13]. In the present study, we found that D2-40 expression did not differ significantly between SMMs (47.1%) and LSCs (16.7%), suggesting its limited value for distinguishing the two diseases. Additionally, we found that the CK5/6+ rate was low in SMMs, and CK5/6 was also expressed in some LSC cases, suggesting its limited value for distinguishing the two diseases.
The results showed that combined positivity for calretinin, WT-1, and GATA3 was optimal for distinguishing SMM from LSC, suggesting that analysis of biopsy specimens tested with antibodies targeting these three markers can effectively prevent misdiagnosis. The specificity associated with simultaneous positive staining for ≥2 of these antibodies in SMM cases was 100%. Moreover, the sensitivity of calretinin+/WT-1+ staining for SMM (23.5%) was low but increased to 64.7% after including GATA3.
Because DMM comprises mainly a significantly collagenic stroma with only a few interspersed tumor cells, it is difficult to distinguish DMM from OP [16]. OP is often accompanied by pleural thickening along with densely proliferating spindle cells and can present with obvious cell atypia and mitotic figures. The presence of an invasive growth pattern is key to distinguishing these two lesions; however, growth patterns usually cannot be evaluated in small biopsy specimens. Therefore, mesothelial markers have important utility for distinguishing the two diseases. We routinely find that spindle cells in OP express mesothelial markers, such as WT-1; however, to the best of our knowledge, this has not been investigated to date. In all 7 cases of OP in the present study, the spindle cells showed partial or diffuse expression for CKpan, WT-1, and D2-40 and simultaneous expression of two to three of these markers. Therefore, staining using antibodies targeting these markers could potentially lead to misdiagnosis. We found that one of the three DMM cases (33.3%) expressed calretinin, and three of the seven OP cases showed cytoplasmic staining. Therefore, calretinin is a good marker for distinguishing DMM from OP when definite nuclear staining is positive. Additionally, we found that GATA3 could effectively distinguish DMM cases from OP cases. However, the tumor cells in DMM are small; therefore, careful assessment should be performed to distinguish them from squeezed lymphocytes when evaluating GATA3. In the present study, all the DMM cases showed nuclear staining in most tumor cells for GATA3; however, all the OP cases were negative. This result suggests that diffuse GATA3 nuclear staining supports a diagnosis of DMM. Recently, Prabhakaran et al. [10] also examined GATA3 expression in 10 cases of fibrous pleuritis and found that all cases were negative. However, due to the small number of OP cases, further clarification with more cases is needed.
We found that GATA3 effectively distinguished SMM from LSC cases; however, the expression of GATA3 in other sarcomas or sarcomatoid carcinomas is currently unknown due to the paucity of relevant studies. The reported positivity rate of GATA3 in bladder sarcomatoid carcinoma is 31%; therefore, metastatic sarcomatoid carcinoma, especially from the bladder and breast, needs to be excluded in GATA3+ cases [17]. Additionally, Miettinen et al. [8] found that some sarcomas expressed GATA3 (clone L50-823) but at generally low rates (0–20%), whereas Haraguchi et al. [18] found high rates of GATA3 (clone D13C9) expression in various sarcomas, which signified poor prognosis.
Recently, a few researches have shown that BRACA1-associated protein 1 (BAP1) is a useful marker for differential diagnosis of MM. BAP1 is typically used to differentiate MM from reactive mesothelial cells or other malignancy [4, 19]. However, BAP1 loss is more frequent in epithelioid and biphasic mesothelioma (32–82%) compared with the sarcomatoid subtype (0–12%); therefore, the value of BAP1 in the differential diagnosis of SMM is limited [4, 10].
In conclusion, we identified GATA3 as an excellent IHC marker for SMM, and combining GATA3 with calretinin and WT-1 produced the best method for distinguishing SMM from LSC. Moreover, CKpan, WT-1, and D2-40 had no utility for distinguishing SMM from OP, while GATA3 and calretinin were the most specific markers for distinguishing these two diseases.
Change history
12 March 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00428-021-03072-y
References
Roggli V, Churg A, Chirieac LR, Galateau-Salle F, Borczuk A, Dacic S, Hammar S, Husain AN, Inai K, Ladanyi M, Marchevsky AM, Naidich D, Ordonez NG, Rice DC, Sheaff MT, Travis WD, van Meerbeeck J (2015) Sarcomatoid, desmoplastic, and biphasic mesothelioma. In: Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG (eds) WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. World Health Organization Classification of Tumours 4th Edition, Lyon, pp 165–168
Lucas DR, Pass HI, Madan SK, Adsay NV, Wali A, Tabaczka P, Lonardo F (2003) Sarcomatoid mesothelioma and its histological mimics: a comparative immunohistochemical study. Histopathology 42:270–279. https://doi.org/10.1046/j.1365-2559.2003.01583.x
Takeshima Y, Amatya VJ, Kushitani K, Kaneko M, Inai K (2009) Value of immunohistochemistry in the differential diagnosis of pleural sarcomatoid mesothelioma from lung sarcomatoid carcinoma. Histopathology 54:667–676. https://doi.org/10.1111/j.1365-2559.2009.03298.x
Guo Z, Carbone M, Zhang X, Su D, Sun W, Lou J, Gao Z, Shao D, Chen J, Zhang G, Hu J, Chen K, Wang F, Pass HI, Yu H, Napolitano A, Yang H, Mao W (2017) Improving the accuracy of mesothelioma diagnosis in china. J Thorac Oncol 12:714–723. https://doi.org/10.1016/j.jtho.2016.12.006
Marchevsky AM, LeStang N, Hiroshima K, Pelosi G, Pelosi G, Attanoos R, Churg A, Chirieac L, Dacic S, Husain A, Khoor A, Klebe S, Lantuejoul S, Roggli V, Vignaud JM, Weynand B, Sauter J, Henderson D, Nabeshima K, Francoise GS (2017) The differential diagnosis between pleural sarcomatoid mesothelioma and spindle cell/pleomorphic (sarcomatoid) carcinomas of the lung: evidence based guidelines from the International Mesothelioma Panel and the MESOPATH National Reference Center. Hum Pathol 67:160e8
Attanoos RL, Gibbs AR (2003) ‘Pseudomesotheliomatous’ carcinomas of the pleura: a 10-year analysis of cases from the environmental lung disease research group, Cardiff. Histopathology 43:444–452. https://doi.org/10.1046/j.1365-2559.2003.01674.x
Liu H, Shi J, Wilkerson ML, Lin F (2012) Immunohistochemical evaluation of GATA3 expression in tumors and normal tissues: a useful immunomarker for breast and urothelial carcinomas. Am J Clin Pathol 138:57–64. https://doi.org/10.1309/AJCP5UAFMSA9ZQBZ
Miettinen M, McCue PA, Sarlomo-Rikala M, Rys J, Czapiewski P, Wazny K, Langfort R, Waloszczyk P, Biernat W, Lasota J, Wang Z (2014) GATA3: A multispecific but potentially useful marker in surgical pathology: a systematic analysis of 2500 epithelial and nonepithelial tumors. Am J Surg Pathol 38:13–22. https://doi.org/10.1097/PAS.0b013e3182a0218f
Berg KB, Churg A (2017) GATA3 immunohistochemistry for distinguishing sarcomatoid and desmoplastic mesothelioma from sarcomatoid carcinoma of the lung. Am J Surg Pathol 41:1221–1225. https://doi.org/10.1097/PAS.0000000000000825
Prabhakaran S, Hocking A, Kim C, Hussey M, Klebe S, Frcpa (2020) The potential utility of GATA binding protein 3 for diagnosis of malignant pleural mesotheliomas. Hum Pathol 105:1–8. https://doi.org/10.1016/j.humpath.2020.08.005
Klebe S, Brownlee NA, Mahar A, Burchette JL, Sporn TA, Vollmer RT, Roggli VL (2010) Sarcomatoid mesothelioma: a clinical-pathologic correlation of 326 cases. Mod Pathol 23:470–479. https://doi.org/10.1038/modpathol.2009.180
Rdzanek M, Fresco R, Pass HI, Carbone M (2006) Spindle cell tumors of the pleura: differential diagnosis. Semin Diagn Pathol 23:44–55. https://doi.org/10.1053/j.semdp.2006.06.002
Chu AY, Litzky LA, Pasha TL, Acs G, Zhang P (2005) Utility of D2-40, a novel mesothelial marker, in the diagnosis of malignant mesothelioma. Mod Pathol 18:105–110. https://doi.org/10.1038/modpathol.3800259
Kushitani K, Takeshima Y, Amatya VJ, Furonaka O, Sakatani A, Inai K (2008) Differential diagnosis of sarcomatoid mesothelioma from true sarcoma and sarcomatoid carcinoma using immunohistochemistry. Pathol Int 58:75–83. https://doi.org/10.1111/j.1440-1827.2007.02193.x
Hinterberger M, Reineke T, Storz M, Weder W, Vogt P, Moch H (2007) D2-40 and calretinin- a tissue microarray analysis of 341 malignant mesotheliomas with emphasis on sarcomatoid differentiation. Mod Pathol 20:248–255. https://doi.org/10.1038/modpathol.3800736
Husain AN, Colby TV, Ordonez NG, Allen TC, Attanoos RL, Beasley MB, Butnor KJ, Chirieac LR, Churg AM, Dacic S, Galateau-Sallé F, Gibbs A, Gown AM, Krausz T, Litzky LA, Marchevsky A, Nicholson AG, Roggli VL, Sharma AK, Travis WD, Walts AE, Wick MR (2018) Guidelines for pathologic diagnosis of malignant mesothelioma 2017 update of the consensus statement from the international mesothelioma interest group. Arch Pathol Lab Med 142:89–108. https://doi.org/10.5858/arpa.2017-0124-RA
Chang A, Brimo F, Montgomery EA, Epstein JI (2013) Use of PAX8 and GATA3 in diagnosing sarcomatoid renal cell carcinoma and sarcomatoid urothelial carcinoma. Hum Pathol 44:1563–1568. https://doi.org/10.1016/j.humpath.2012.12.012
Haraguchi T, Miyoshi H, Hiraoka K, Yokoyama S, Ishibashi Y, Hashiguchi T, Matsuda K, Hamada T, Okawa T, Shiba N, Ohshima K (2016) GATA3 expression is a poor prognostic factor in soft tissue sarcomas. PLoS One 11:e0156524. https://doi.org/10.1371/journal.pone.0156524
Cigognetti M, Lonardi S, Fisogni S, Balzarini P, Pellegrini V, Tironi A, Bercich L, Bugatti M, Rossi G, Murer B, Barbareschi M, Giuliani S, Cavazza A, Marchetti G, Vermi W, Facchetti F (2020) BAP1 (BRCA1-associated protein 1) is a highly specific marker for differentiating mesothelioma from reactive mesothelial proliferations. Mod Pathol 28:1043–1057
Author information
Authors and Affiliations
Contributions
ZHP and XCZ conceived and designed the study; JYC performed the experiment; ZHP analyzed data, wrote, edited, and reviewed the manuscript. All authors gave final approval for publication. ZHP takes full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript.
Corresponding author
Ethics declarations
The data and experiments reported here were performed in accordance with the Declaration of Helsinki principles and the Ethics Committee of the Ningbo Diagnostic Pathology Center.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The formula Sensitivity = TP/(TP + FP) x 100% under the section “Statistical analysis” has been corrected as follows:
Sensitivity = TP/(TP + FN) x 100%
Specificity = TN/(TN + FP) x 100%
This article is part of the Topical Collection on Quality in Pathology
Highlights
1. We found GATA3 as an excellent specific marker for distinguishing sarcomatoid malignant mesothelioma from lung sarcomatoid carcinoma and organizing pleuritis. This is a new finding, and there is only one related study at present.
2. Expression for mesothelial markers such as WT-1 and D2-40 in organizing pleuritis cases, it is a pitfall for differential diagnosis from desmoplastic mesotheliomas.
Supplementary Information
Supplementary Material 1
(DOCX 56 kb).
Rights and permissions
About this article
Cite this article
Piao, Z.H., Zhou, X.C. & Chen, J.Y. GATA3 is a useful immunohistochemical marker for distinguishing sarcomatoid malignant mesothelioma from lung sarcomatoid carcinoma and organizing pleuritis. Virchows Arch 479, 257–263 (2021). https://doi.org/10.1007/s00428-021-03048-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-021-03048-y