Abstract
Analysis of breast cancer prognostic and predictive factors is still nowadays poorly accurate and standardized. The advent of multi-gene expression profiles (MGEPs) has improved the prediction of breast cancer outcome, particularly regarding early luminal breast cancers (LBCs). The availability in our Institute of EndoPredict® (EP), a last-generation prognostic gene signature assay, has prompted us to study a series of LBCs, firstly verifying its reproducibility on six routine representative cases, either presenting non-optimal preanalytical conditions or different tumor samples from the same patient; secondly, correlating EP results on 8 retrospectively recruited samples with patients’ follow-up; thirdly, applying prospectively EP on 100 routinely diagnosed cases, assessing the oncologists’ and pathologists’ attitude toward it. The complete reproducibility of EP on all the samples investigated in the first phase allowed to state that EP overcomes the detrimental effects of an inaccurate pre-analytic phase, determining the most appropriate prognostic and predictive parameters of breast cancer. The second phase confirmed EP as a fundamental tool in guiding therapeutic decision, improving the classical bio-pathological characterization and recovering 38% patients’ inadequately managed. Finally, the study disclosed how oncologists sometimes inadequately requested EP, but also how it allows a better stratification of breast cancer otherwise considered poorly aggressive and not requiring an EP test, such as G1 neoplasms or tubular histotype. In conclusion, the introduction of EP test in an Anatomic Pathology Department emerges as a useful tool in routine breast cancer diagnosis, both for the characterization of individual cases and, as a result, for more appropriate therapeutic choices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is a wide perception that the methodologies which analyze breast cancer for prognostic and predictive purposes are neither accurate nor standardized [1, 2].
One of the main reason is the intrinsic limit of immunohistochemical reactions, particularly due to both the conspicuous effects of the pre-analytical phase [3] and the subjectivity of interpretative criteria [4], which are often based on unclear guidelines [5, 6].
In addition, another source of poor reproducibility is the extreme intra- and inter-tumoral heterogeneity [7], which is poorly framed by the current classification schemes.
The introduction of both molecular breast cancer classification [8] and its immunohistochemical surrogate [9] has attenuated some discrepancies, ameliorating the therapeutic choices, particularly as regards the category of luminal breast cancers (LBCs). This clinical-pathological category encompasses the luminal A-like carcinomas—characterized by estrogen receptor (ER) and progesterone receptor (PgR) positivity, HER2 negativity, and a low proliferation index (Ki-67)—which (either in adjuvant or neoadjuvant setting) need only endocrine therapy and luminal B-like carcinomas—ER and PgR positive or negative, either HER2 positive or negative and Ki-67 high neoplasms—for which (adjuvant or neoadjuvant) chemotherapy could be added to endocrine therapy [9]. However, the category of LBCs, which includes about 65% of all breast cancer cases, is characterized by a remarkable variability of biological behaviors and, consequently, diverse response to therapies. This scenario strongly disagrees with the needs of a true precision medicine which presupposes a precision pathology [10,11,12].
Multi-gene expression profiles (MGEPs) have significantly improved our skills to predict the outcome of LBCs; moreover, they, in addition to conventional prognostic and predictive markers, significantly help treatment decisions (chemotherapy use and/or extended endocrine therapy) [3], as currently recommended by several guidelines [13]. Among MGEPs, EndoPredict® (EP Myriad Genetics®, Salt Lake City, USA) is a last-generation prognostic gene signature assay that uses gene expression to calculate the risk of metastases within 10 years from the initial diagnosis of breast cancer [14]. This test has been specifically developed for LBCs. In contrast with almost all the other commercially available multigene tests, it is suitable for decentralized use instead of a single reference laboratory and allows to correlate molecular data and clinical-pathological parameters in a single integrated diagnostic algorithm useful for clinical decisions [15,16,17].
Using a quantitative RT-qPCR on formalin-fixed, paraffin-embedded (FFPE) samples, EP is based on mRNA expression of 12 genes—UBE2C, BIRC5, and DHCR7 (proliferation-associated genes); RBBP8, IL6S7, AZGP1, MGP, and STC2 (estrogen receptor-associated genes); CALM2, OAZ1, and RPL37A (reference genes); and a control gene for the presence of DNA contamination [14, 15, 18]—to calculate the EndoPredict score (EP score), which, using a validated cutoff value of 5, can classify patients with LBCs into either low- or high-risk class for developing metastases after endocrine therapy [14, 19]. The EP score can be combined with the nodal status and tumor size to obtain the integrated molecular and clinical risk score (EP clin), which allows a better risk stratification in terms of recurrences at 10 years; according to the methods described by Filipits et al. [14], a cutoff point of 3.3 corresponds to a 10-year risk (EP clin risk) of 10%.
The availability of EndoPredict® (EP) has given us the opportunity to have an experience in this field, which is reported in the current study, highlighting, in particular, how such a technique can greatly improve the service that anatomic pathology provides for the benefit of patients suffering from breast cancer.
Materials and methods
EndoPredict ®
The EP test was performed on total RNA, which, according to the instructions [19], was manually isolated from FFPE blocks of the primary resected tumor. For isolation, a 10-μm section of an FFPE block (surgical specimen or core biopsy) was used. The portion of the tumor must have been at least 30% (ratio of invasive tumor tissues to total tissue area). Adipose tissue is not to be considered as tissue, as it hardly contains RNA. The evaluation of the portion of the tumor was determined using the adjacent Hematoxylin and Eosin section. If the tumor section content was less 30%, the proportion of the tumor was enriched by macro-dissection. The isolation procedure started with the removal of the paraffin and the lysis of the tissue, and subsequently, the nucleic acid was extracted using silica-coated magnetic particles. After extraction and elution of the nucleic acids, a DNase digest was carried out. As a sufficient quantity of RNA and efficient DNA digestion is required for a valid EndoPredict test, all the samples were controlled with the EndoPredict QC Strip to verify the adequate level of RNA before subsequent analysis, according to the manufacturer’s instructions. [19].
After a specific training, we started the implementation of EP in our Department, strictly following the manufacturer’s instructions [19].
Study design and patients selection
The study was divided into three phases. Phase 1 aimed to verify the reproducibility of results of EP in difficult technical conditions, both regarding the pre-analytical phase of tumoral tissues collection—presence or absence of inadequate fixation artifacts such as tissue shrinkage, loss of cellular cohesiveness, cytoplasmic clumping, indistinct nucleoli, and loss of other cellular details; thermal stress; scarcity of useful tissue derived from core biopsies; differences between FFPE specimens which underwent a previous intraoperative examination by cryostat and thereafter routinely formalin-fixed and paraffin-embedded—and comparing the primary invasive tumors with their own axillary node metastases or their own in situ component, which were prepared for the molecular test by macrodissection, as above-mentioned for tumor section content less 30%. For this purpose, 12 samples of 6 representative cases were retrospectively recruited.
In the phase 2, the EP was applied on representative FFPE blocks of eight patients selected from our archive, having both an adequate follow-up (period of follow-up 2005–2016) and a complete therapeutic history, in order to firstly analyze retrospectively the concordance between the results of EP test and the prognostic grouping based on conventional bio-pathological parameters, the therapeutic choices made at the time of the diagnosis, the patients’ outcomes, and the variations of therapeutic choice that EP test would determine if adopted at the time of the original diagnosis.
In phase 3, the EP test was applied in daily clinical practice (period of diagnoses from 2015 to 2018) based either on pathologists’ autonomous decision—whenever a breast carcinoma belonged to the luminal B category of breast cancer or presented clinical-pathological discrepancies, such as inconsistency between tumor grading/histotype and nodal status or ambiguous bio-pathological characterization—or on oncologists’ requests—collected both as oncologists’ opinions in the frame of multidisciplinary teams and as oncologists’ independent requests—in order to assess their attitude toward it. These 100 cases, randomly collected based on the chronological order of the request, were therefore stratified in 3 risk classes (low, high, and equivocal risk), according to both clinical-pathological characteristics and the main international guidelines in force at the time of the diagnosis [20,21,22,23,24].
Descriptive statistical methods were used for both analyzing and representing all the obtained data, displaying them as numbers, counts, and percentages.
Results
Phase 1—reproducibility of EP test
The first phase of the study (Table 1) included 12 specimens obtained from 6 patients, with median age of 62.5 years and with no special type (NST) breast carcinomas G2 and G3.
Among these, two (33%) cases were studied for highlighting the eventual differences in EP test results between specimens from the same patient processed one as frozen biopsy (and afterward underwent the routinely FFPE processing) and the other routinely FFPE. There were not any differences among the results in these cases. Comparing the EP test performed both on the primary cancer and on one out of its two axillary node metastasis (20 axillary nodes totally examined), there were no discrepancies among the results obtained. Moreover, no differences in the results of EP test in case of core biopsy material versus (vs) surgical specimen, adequate vs inadequate fixation of the tumor tissue, and invasive vs in situ component of breast carcinoma were found.
Phase 2—concordance analysis of patients’ risk stratification
The second phase of the study (Table 2) included eight cases having a mean follow-up of 95.5 months (median follow-up of 96 months, range 51–132 months).
The EP test results showed four (50%) breast cancers belonging to the low EPclin risk class and four (50%) cases included into the high EPclin risk class.
Comparing the EP test results with the patients’ outcomes, we highlighted that three (38%) cases could have received a more appropriate therapy. In fact, one (33%) patient, still alive and well (AW), belonging to the low EPclin risk class underwent adjuvant chemotherapy, while only adjuvant endocrine therapy (ET) should have been enough for her. Moreover, two (67%) deceased patients could have undergone adjuvant chemotherapy (CHT) if they had known, at the time of diagnosis, that their breast cancer belonged to the high EPclin risk class.
On the other hand, two (25%) patients still AW underwent only adjuvant ET, resulting in the correct therapeutic choice, as their low EPclin risk class confirmed. Furthermore, one (13%) patient with EP test result of high-risk disease underwent neoadjuvant CHT and showed complete response.
One (13%) case underwent the appropriate treatment at the time of diagnosis (adjuvant ET+ CHT), but this male patient died, consistently with the high-risk class demonstrated by EP test.
Finally, one (13%) case with low EPclin risk had a partial response after neoadjuvant CHT.
Phase 3—application of EP test in routinely clinical practice
As regards the third phase of the study (Table 3), EP test was performed for 100 patients, according either to pathologists’ or oncologists’ requests.
Although the EP has been specifically developed for early LBC risk stratification, the motivation for EP test request in our study also encompassed a range of features involved in therapeutic decisions. In particular, while involved pathologist chooses EP mainly (41% of cases) for borderline bio-pathological aspects (e.g., Ki-67 close to the cut-off [20]—30%—or low hormonal receptor expression—11%), oncologists’ recourse to EP test was often (43%) connected with either tumoral staging interpretation (e.g., tumor diameter less than 10 mm—8%—minimal nodes involvement—13%) or, even, with 1–3 positive node cases (23%).
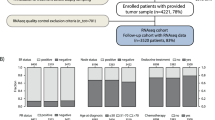
Applying the EP test in this way, both unexpected and clinically useful results have been reached, such as the finding of two (2%) G1 breast carcinomas or of a usually harmless tubular histotype with high likelihood of aggressive biological behavior (Fig. 1).
An example of a LBCs belonging to the high EP clin class, the histotype (tubular) of which usually does not show an aggressive clinical behavior. ER = estrogen receptor; PR and PgR = progesterone receptor; Ki67 = proliferative index; *surrogate = immunohistochemical surrogate classification according to St Gallen guidelines [20]; CHT = adjuvant chemotherapy; ET = endocrine therapy. HE = Hematoxylin and Eosin. The cross pointer in the chart indicates the patient’s risk stratification class in terms of recurrences at 10 years
Clinical-pathological risk prediction
As regards the clinical-pathological point of view, the EP test revealed its fundamental importance for the therapeutic choices of the 100 cases analyzed, as highlighted in Fig. 2.
Among the 28 cases judged as high risk based upon clinical-pathological findings, the EP test allowed to identify four (14%) patients belonging to the low-risk class of EPclin. On the other hand, the EP test found 1 (5%) out of 21 cases assessed as low risk after clinical-pathological evaluation which presented, actually, a high EPclin risk, requiring CHT treatment. Finally, EP test helped to take a proper therapeutic decision for the 51 cases with equivocal risk.
Discussion
The decision of adding chemotherapy (CHT) to endocrine therapy (ET) in early ER-positive, HER2-negative breast cancer is often controversial and it is known that most patients can safely avoid it [25, 26]. In fact, conventional histopathological features (size, histotype, grade, vascular invasion, and node status) and biomarkers (ER, PgR, HER2, and Ki-67), together with guidelines and additional on-line predictive tools, are often clear enough for oncologists to either recommend or omit CHT. However, given the heterogeneity of luminal breast cancers (LBCs, about 65% of the total), accurate estimation in terms of risk of recurrence after ET alone is not possible. In fact, these parameters are unable to estimate the risk of late distant recurrence (5–10 years), particularly in women with node-negative disease or with limited nodal involvement, and to identify the subset of patients who remain at low risk of distant recurrence even after 10 years [27]. On the other hand, these bio-pathological characteristics are not always predictive for possible CHT benefit and a substantial overtreatment in these patients is a logical consequence.
On this background, the main purpose of our study was both to describe and to analyze the impact deriving from the implementation of a gene expression assay for LBCs in the routine diagnostic activity of a department of anatomic pathology, focusing on difficulties of implementing this test in routine practice and on improvements, in terms of prognostic and predictive stratification, compared with conventional bio-pathological parameters and, consequently, on oncologists’ behavior towards this useful aid for therapeutic choices.
Based on our experience, EP test can be easily implemented in an Anatomic Pathology Department which has sufficient experience in the bio-pathological characterization of breast carcinomas [15].
The first part of our study had tested the reproducibility of EP. According to the concordance of results obtained on material that undergone different pre-analytic phases, such as either frozen versus formalin fixation or adequate versus inadequate formalin fixation, we showed that EP is a resilient tool which can overcome the detrimental effects of an inaccurate pre-analytic phase [3, 12], allowing a good assessment of prognostic and predictive parameters of breast carcinoma, even in tumoral tissues suboptimally preserved. It is well known that all these conditions make immunohistochemical (IHC) interpretation of biomarkers’ expression difficult or impossible; therefore, EP test, compensating these intrinsic limitations of IHC—particularly issues regarding both accuracy and reproducibility of cell proliferation determination using the monoclonal antibody Ki-67 [4]—could improve the pathologists’ role for a more precise diagnosis and treatment of breast cancer.
An additional result, worthy of further studies, is the similar gene expression profiles obtained comparing either carcinomas in situ to their invasive counterpart, primary tumors to their nodal metastases or core biopsies to respective surgical specimens [18]. All these observations seem to support a possible ability of the EP test to overcome conditions of strong intra- and inter-tumor heterogeneity or limited representativeness of the sample.
As regards the evidences derived from the second phase of our study, we confirm the literature data [14, 17], according to which the EP test is a more useful tool for a better risk stratification of patient with early LBCs than conventional clinical-pathological parameters, allowing the choice of more personalized treatment. In fact, according to the retrospectively obtained results of the EP test, among the group of still AW patients, one received probably overtreatment with adjuvant CHT; on the other hand, two out of deceased patients did not receive adjuvant CHT treatment, either based upon oncologist’s decision or upon patient’s refusal. Obviously, if at that time, the oncologist had had the EP test available, the therapeutic choices would have been significantly different. It is also important to note that one patient died despite appropriate therapy due to an intrinsic greater aggressiveness of their tumors, as their high class of EPclin risk demonstrated.
Regarding the third phase of our study—the routine use of the EP test on current cases—one of the main aspects that we noticed was the attitude of the oncologists towards this new test. In fact, although we had proposed the introduction of the EP test as a tool for decision aids, their rate of requests was extremely low and not very enthusiastic. Thereafter, since scientific societies and international conferences have allowed the gradual emergence of specific recommendations in the guidelines issued [13, 20, 22], oncologists’ interest in the EP test grew progressively, extending their requests not only to LBCs, but also to carcinomas with other types of therapeutic uncertainty, regardless of tumor diameter, histotype, grading, and node status (Table 3). This evolution shows that EP test can alleviate the heaviness of these colleagues’ therapeutic choices [28].
Although remarkably interesting, the results obtained need additional validation on a larger number of samples, particularly regarding the usefulness of EP test to overcome conditions related to either inadequate preanalytical phase or tumor heterogeneity, observed in the first phase of the current study. Moreover, it could be interesting to confirm the accuracy of therapeutic choices taken according to EP test results once an adequate follow-up period of at least 10 years will be achieved by the patients enrolled in the third phase of the study and, consequently, corroborate the evidence of EP as a helpful tool in routine breast cancer diagnosis and management.
Conclusions
EP test, if appropriately performed in selected cases, is able to identify subtypes of LBCs with prognostic and predictive factors otherwise not accurately highlighted by conventional clinical-pathological characterization. EP test is a useful supplement to pathological routinely used methods, compensating the intrinsic limitations of both laboratory techniques and the marked intra- and inter-tumor heterogeneity, which characterize this disease. EP test, if applied widely, enhances the informative value of pathologists’ report, allowing both oncologists and patients to solve the prognostic assessment and therapeutic uncertainties, particularly avoiding chemotherapy overtreatment in breast cancer. In the era of diagnosis and stratification of distinct disease subtypes at cellular level, the multi-gene expression profiles represent an attempt to best identify the prognosis and the optimal therapeutic choices for individual patients. The pathologists’ perception is that EP test is able to introduce remarkable and useful improvements in the characterization of individual cases and, consequently, in more appropriate therapeutic choices.
Data availability
The request for accessing the properly anonymized datasets analyzed in this article can be directed to Professor Angelo Sidoni: angelo.sidoni@unipg.it.
References
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC, American Society of Clinical Oncology, College of American Pathologists (2010) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med 134(7):e48–e72. https://doi.org/10.1043/1543-2165-134.7.e48
Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF (2007) American Society of Clinical O, and College of American P American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25(1):118–145. https://doi.org/10.1200/JCO.2006.09.2775
Khoury T (2018) Delay to formalin fixation (cold ischemia time) effect on breast cancer molecules. Am J Clin Pathol 149(4):275–292. https://doi.org/10.1093/ajcp/aqx164
Denkert C, Budczies J, von Minckwitz G, Wienert S, Loibl S, Klauschen F (2015) Strategies for developing Ki67 as a useful biomarker in breast cancer. Breast 24(Suppl 2):S67–S72. https://doi.org/10.1016/j.breast.2015.07.017
Rakha EA, Starczynski J, Lee AH, Ellis IO (2014) The updated ASCO/CAP guideline recommendations for HER2 testing in the management of invasive breast cancer: a critical review of their implications for routine practice. Histopathology 64(5):609–615. https://doi.org/10.1111/his.12357
Singh K, Tantravahi U, Lomme MM, Pasquariello T, Steinhoff M, Sung CJ (2016) Updated 2013 College of American Pathologists/American Society of Clinical Oncology (CAP/ASCO) guideline recommendations for human epidermal growth factor receptor 2 (HER2) fluorescent in situ hybridization (FISH) testing increase HER2 positive and HER2 equivocal breast cancer cases; retrospective study of HER2 FISH results of 836 invasive breast cancers. Breast Cancer Res Treat 157(3):405–411. https://doi.org/10.1007/s10549-016-3824-x
Koren S, Bentires-Alj M (2015) Breast tumor heterogeneity: source of fitness, hurdle for therapy. Mol Cell 60(4):537–546. https://doi.org/10.1016/j.molcel.2015.10.031
Perou CM, Sorlie T, Eisen MB, Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D et al (2000) Molecular portraits of human breast tumours. Nature 406(6797):747–752. https://doi.org/10.1038/35021093
Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ, Panel M (2011) Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol;22(8):1736–47. https://doi.org/10.1093/annonc/mdr304
Donovan MJ, Cordon-Cardo C (2017) Implementation of a precision pathology program focused on oncology-based prognostic and predictive outcomes. Mol Diagn Ther 21(2):115–123. https://doi.org/10.1007/s40291-016-0249-5
Kaul KL (2017) Preparing pathology for precision medicine: challenges and opportunities. Virchows Arch 471(2):141–146. https://doi.org/10.1007/s00428-017-2141-z
Compton CC, Robb JA, Anderson MW, Berry AB, Birdsong GG, Bloom KJ, Branton PA, Crothers JW, Cushman-Vokoun AM, Hicks DG, Khoury JD, Laser J, Marshall CB, Misialek MJ, Natale KE, Nowak JA, Olson D, Pfeifer JD, Schade A, Vance GH, Walk EE, Yohe SL (2019) Preanalytics and precision pathology: pathology practices to ensure molecular integrity of cancer patient biospecimens for precision medicine. Arch Pathol Lab Med 143(11):1346–1363. https://doi.org/10.5858/arpa.2019-0009-SA
Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio IT, Zackrisson S, Senkus E (2019) Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 30(8):1194–1220. https://doi.org/10.1093/annonc/mdz189
Filipits M, Rudas M, Jakesz R, Dubsky P, Fitzal F, Singer CF, Dietze O, Greil R, Jelen A, Sevelda P, Freibauer C, Muller V, Janicke F, Schmidt M, Kolbl H, Rody A, Kaufmann M, Schroth W, Brauch H, Schwab M, Fritz P, Weber KE, Feder IS, Hennig G, Kronenwett R, Gehrmann M, Gnant M, Investigators EP (2011) A new molecular predictor of distant recurrence in ER-positive, HER2-negative breast cancer adds independent information to conventional clinical risk factors. Clin Cancer Res 17(18):6012–6020. https://doi.org/10.1158/1078-0432.CCR-11-0926
Denkert C, Kronenwett R, Schlake W, Bohmann K, Penzel R, Weber KE, Hofler H, Lehmann U, Schirmacher P, Specht K, Rudas M, Kreipe HH, Schraml P, Schlake G, Bago-Horvath Z, Tiecke F, Varga Z, Moch H, Schmidt M, Prinzler J, Kerjaschki D, Sinn BV, Muller BM, Filipits M, Petry C, Dietel M (2012) Decentral gene expression analysis for ER+/Her2- breast cancer: results of a proficiency testing program for the EndoPredict assay. Virchows Arch 460(3):251–259. https://doi.org/10.1007/s00428-012-1204-4
Kronenwett R, Bohmann K, Prinzler J, Sinn BV, Haufe F, Roth C, Averdick M, Ropers T, Windbergs C, Brase JC, Weber KE, Fisch K, Muller BM, Schmidt M, Filipits M, Dubsky P, Petry C, Dietel M, Denkert C (2012) Decentral gene expression analysis: analytical validation of the Endopredict genomic multianalyte breast cancer prognosis test. BMC Cancer 12:456. https://doi.org/10.1186/1471-2407-12-456
Muller BM, Keil E, Lehmann A, Winzer KJ, Richter-Ehrenstein C, Prinzler J, Bangemann N, Reles A, Stadie S, Schoenegg W, Eucker J, Schmidt M, Lippek F, Johrens K, Pahl S, Sinn BV, Budczies J, Dietel M, Denkert C (2013) The EndoPredict gene-expression assay in clinical practice - performance and impact on clinical decisions. PloS One 8(6):e68252. https://doi.org/10.1371/journal.pone.0068252
Muller BM, Brase JC, Haufe F, Weber KE, Budzies J, Petry C, Prinzler J, Kronenwett R, Dietel M, Denkert C (2012) Comparison of the RNA-based EndoPredict multigene test between core biopsies and corresponding surgical breast cancer sections. J Clin Pathol 65(7):660–662. https://doi.org/10.1136/jclinpath-2012-200716
EndoPredict®Instruction Manual. Sividon Diagnostics GmbHAs of: 26.02.2014
Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thurlimann B, Senn HJ, Panel M (2013) Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 24(9):2206–2223. https://doi.org/10.1093/annonc/mdt303
Coates AS, Winer EP, Goldhirsch A, Gelber RD, Gnant M, Piccart-Gebhart M, Thurlimann B, Senn HJ, Panel M (2015) Tailoring therapies--improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 26(8):1533–1546. https://doi.org/10.1093/annonc/mdv221
Curigliano G, Burstein HJ, E PW, Gnant M, Dubsky P, Loibl S, Colleoni M, Regan MM, Piccart-Gebhart M, Senn HJ, Thurlimann B, Panel Members of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast C (2019) De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol;30(7):1181. https://doi.org/10.1093/annonc/mdy537
Senkus E, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rutgers E, Zackrisson S, Cardoso F, Committee EG (2015) Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 26(Suppl 5):v8–v30. https://doi.org/10.1093/annonc/mdv298
Paluch-Shimon S, Pagani O, Partridge AH, Abulkhair O, Cardoso MJ, Dent RA, Gelmon K, Gentilini O, Harbeck N, Margulies A, Meirow D, Pruneri G, Senkus E, Spanic T, Sutliff M, Travado L, Peccatori F, Cardoso F (2017) ESO-ESMO 3rd international consensus guidelines for breast cancer in young women (BCY3). Breast 35:203–217. https://doi.org/10.1016/j.breast.2017.07.017
Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, Geyer CE Jr, Dees EC, Goetz MP, Olson JA Jr, Lively T, Badve SS, Saphner TJ, Wagner LI, Whelan TJ, Ellis MJ, Paik S, Wood WC, Ravdin PM, Keane MM, Gomez Moreno HL, Reddy PS, Goggins TF, Mayer IA, Brufsky AM, Toppmeyer DL, Kaklamani VG, Berenberg JL, Abrams J, Sledge GW Jr (2018) Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med 379(2):111–121. https://doi.org/10.1056/NEJMoa1804710
Nitz U, Gluz O, Christgen M, Kates RE, Clemens M, Malter W, Nuding B, Aktas B, Kuemmel S, Reimer T, Stefek A, Lorenz-Salehi F, Krabisch P, Just M, Augustin D, Liedtke C, Chao C, Shak S, Wuerstlein R, Kreipe HH, Harbeck N (2017) Reducing chemotherapy use in clinically high-risk, genomically low-risk pN0 and pN1 early breast cancer patients: five-year data from the prospective, randomised phase 3 West German Study Group (WSG) PlanB trial. Breast Cancer Res Treat 165(3):573–583. https://doi.org/10.1007/s10549-017-4358-6
Dubsky P, Brase JC, Jakesz R, Rudas M, Singer CF, Greil R, Dietze O, Luisser I, Klug E, Sedivy R, Bachner M, Mayr D, Schmidt M, Gehrmann MC, Petry C, Weber KE, Fisch K, Kronenwett R, Gnant M, Filipits M, Austrian Breast and Colorectal Cancer Study Group (2013) The EndoPredict score provides prognostic information on late distant metastases in ER+/HER2- breast cancer patients. Br J Cancer 109(12):2959–2964. https://doi.org/10.1038/bjc.2013.671
Fallowfield L, Matthews L, May S, Jenkins V, Bloomfield D (2018) Enhancing decision-making about adjuvant chemotherapy in early breast cancer following EndoPredict testing. Psycho-oncology 27(4):1264–1269. https://doi.org/10.1002/pon.4664
Author information
Authors and Affiliations
Contributions
All the authors have contributed to both conception, design of the study, acquisition, analysis, and interpretation of data, either drafting the article or revising it critically. All the authors have read and approved the submitted version of the manuscript.
Corresponding author
Ethics declarations
All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
Dr. Sidoni received speaker’s fees from Myriad Genetics. The other authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Quality in Pathology
Rights and permissions
About this article
Cite this article
Pelliccia, C., Caselli, E., Mandarano, M. et al. The implementation of a commercially available multi-gene profile test for breast cancer characterization in a department of pathology: what have we learned from the first 100 cases?. Virchows Arch 478, 1079–1087 (2021). https://doi.org/10.1007/s00428-020-02994-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-020-02994-3