Abstract
Whether pT3 urothelial carcinoma of the renal pelvis (UCRP) and urothelial carcinoma of the ureter (UCU) have the same prognosis is controversial, this study compared the prognosis of pT3 UCRP with that of pT3 UCU. We retrospectively evaluated 954 patients who underwent nephroureterectomy at our institutions between January 1983 and December 2017. All surgical specimens were reviewed by a single genitourinary pathologist. Cases of pT3 UCRP were subclassified as pT3a (urothelial carcinomas extending only to the renal medulla) and pT3b (urothelial carcinomas extending into the renal cortex and/or peripelvic adipose tissue). Fine and Gray’s model was used to predict recurrence-free survival (RFS) and cancer-specific survival (CSS). A total of 493 (51.7%) had UCRP and 461 (48.3%) had UCU. Within this group, 202 patients had pT3 UCRP and 146 had pT3 UCU. The pT3 subclassification of UCRP resulted in 79 cases of pT3a and 120 of pT3b. The difference in 5-year CSS among the pT3a UCRP, pT2 UCRP, and pT2 UCU subgroups was not statistically significant (pT3a UCRP vs pT2 UCRP, HR = 0.69, p = 0.56; pT3a UCRP vs pT2 UCU, HR = 0.66, p = 0.31) However, RFS and CSS were significantly higher in the pT3a UCRP group than in the pT3b group (pT3a vs pT3b, HR = 2.59, p = 0.0038 and pT3a vs pT3b, HR = 3.10, p = 0.001). The results suggest that our proposed pT3 subclassification better predicts the prognosis of UCRP patients than does the pT3 of the current AJCC/UICC classification.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Upper tract urothelial carcinoma (UTUC) is a relatively uncommon disease that accounts for ~ 10% of all renal tumors and ~ 5% of all urothelial carcinomas [23, 26]. Although ~ 60% of UTUCs are invasive tumors at diagnosis [12], few studies have addressed the pathological prognostic factors in detail. Pathological tumor stage (pT), the presence of lymphovascular invasion (LVI), tumor location, histological grade, and lymph node metastasis are well-known and important pathological prognostic factors [11, 14,15,16, 20, 29]. Interestingly, in some studies, patients with urothelial carcinoma of the renal pelvis (UCRP) had a significantly better prognosis than those with urothelial carcinoma of the ureter (UCU) [1, 17], whereas in others the prognosis was the same [7, 10, 21].
In the 2017 American Joint Committee on Cancer (AJCC) and Union for International Cancer Control (UICC) classification, UCRPs infiltrating the renal parenchyma are defined as pT3 tumors [2, 4]. However, the depth of renal parenchymal infiltration is not considered. In some reports, the degree of infiltration into the renal parenchyma was shown to correlate with the prognosis of patients with pT3 UCRP and several subclassification systems were developed accordingly [22, 24, 25]. However, their usefulness is unclear, as is the mechanism underlying this finding.
In this study, we examined the prognoses of UCRP and UCU patients according to our proposed subclassified UTUC pT classification, which is based on precise anatomical criteria. Our aim was to reveal the exact mechanism underlying the difference in prognosis between pT3 UCRP and pT3 UCU patients.
Materials and methods
In total, 1289 patients with UTUC underwent radical nephroureterectomy (RNU) with bladder cuff excision between January 1983 and December 2017. The records were retrieved from the treating physicians and five participating institutions. Patients with the following characteristics were excluded: multifocal tumors, unknown clinical details, concomitant ipsilateral ureteral cancer, distant metastasis, concomitant invasive bladder cancer, and neoadjuvant chemotherapy. Of the remaining patients, 954 were eligible for this study (Fig. 1). The clinicopathological data included sex, age at diagnosis, laterality, operative method, pathological tumor characteristics, pT, tumor location, WHO/ISUP grade, histological variant, LVI, and pathological lymph node stage (pN). Adjuvant chemotherapy was performed with regimens of methotrexate, vinblastine, doxorubicin, and cisplatin, or gemcitabine and cisplatin.
The primary endpoint of this study was overall survival (OS), which was defined as the time from RNU to death from any cause. The secondary endpoint was recurrence-free survival (RFS) and cancer-specific survival (CSS). RFS was defined as the time from RNU to extravesical recurrence. CSS was defined as the time from RNU to death attributable to a cancer-related complication. Extravesical recurrence (lymph node metastasis, distant metastasis, and local recurrence) was an endpoint in this study, but intravesical recurrence was not.
Pathological evaluation
All HE slides in this study were retrospectively reviewed by one genitourinary pathologist (T.T.). Pathological evaluations were concerned with the pT stage, pN, WHO/ISUP grade, LVI, and histological variant [24].
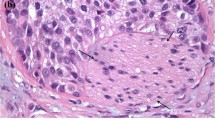
We subclassified pT3 into pT3a and pT3b according to the depth of tumor invasion, as reported previously [24]. Briefly, pT3a was defined as tumors extending only into the renal medulla, without normal glomeruli surrounded by carcinoma cells. pT3b was defined as tumors extending into the renal cortex (with normal glomeruli surrounded by carcinoma cells) or into the peripelvic adipose tissue (Fig. 2 and Supplementary Figure 1).
Anatomical features of the renal pelvis and ureter, and definition of subclassification of pT3 urothelial carcinoma. a Proposed pT3 subclassification—pT3a, carcinoma only infiltrates the renal medulla without peripelvic adipose tissue invasion. pT3b, carcinoma infiltrates the renal cortex or the peripelvic adipose tissue. Anatomical features of the renal pelvis and ureter. b Note the lack of the muscularis propria in the pyramid (right side). By contrast, the muscularis propria is present adjacent to the adipose tissue on the left side. The pyramid is located at the corticomedullary junction, which lacks a muscularis propria. c The ureteral wall is histologically composed of three layers: the mucosa, smooth muscle layer, and adventitia
Statistical analyses
The clinicopathological features of the two groups were compared using Fisher’s exact test for categorical variables and the Wilcoxon signed-rank test for continuous variables. Cumulative incidence curves were used in a competing-risks setting to calculate the probabilities of CSS and RSS. The cumulative incidence curves for patients stratified by tumor stage and location were compared using Gray’s test. The influence of prognostic factors on CSS and RFS was estimated using Fine and Gray’s model [8]. OS was estimated using the Kaplan–Meier method and compared among groups using the log-rank test. Multivariate analyses using the Cox proportional hazards model were performed to evaluate the influence of prognostic factors for OS. p values < 0.05 were considered significant. All statistical analyses were performed using SAS software (version 9.3; SAS Institute, Cary, NC, USA).
Results
The clinicopathological characteristics of the patients are listed in Table 1. In all, 493 patients (51.7%) with UCRP and 461 patients (48.3%) with UCU were included in the analyses. Average and median patient age was 67.5 and 69 years (range 33–88 years). The median follow-up period was 57 months (range 2–340 months). A total of 896 patients (94%) were diagnosed as pure urothelial carcinomas (renal pelvis 458, ureter 438) and 58 patients (6%) contained variant histology (renal pelvis 35, ureter 23). The detail of histology in each organ was shown in Table 1. A total of 202 cases were pT3 UCRP and 146 were pT3 UCU. The pT3 subclassification of UCRP resulted in 79 pT3a and 120 pT3b cases. There were more non-papillary (58% vs 28%), pN-positive (32% vs 8%), and LVI cases (66% vs 19%) among patients with pT3b UCRPs. pT3b tumors tended to have worse prognostic factors.
Recurrence-free survival and cancer-specific survival
Overall, 100 patients (20%) with UCRP and 103 (22%) with UCU experienced extravesical recurrence; 97 (19.6%) and 85 patients (18.4%) died of their disease during follow-up.
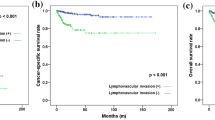
The RFS and CSS rates in patients with UCRP and UCU decreased according to the pT stage (Fig. 3). Table 2 shows the 5-year RFS and CSS stratified by pT and location. We examined the hazard ratios (HRs) of RFS and CSS in each pathological stage (Table 3). No significant differences were found in the 5-year RFS or 5-year CSS among pT3a UCRP, pT2 UCRP, and pT2 UCU (5-year RFS, pT3a UCRP 81.7% vs pT2 UCRP 80% (p = 0.74) and pT3a UCRP vs pT2 UCU 81.8% (p = 0.72); 5-year CSS, pT3a UCRP 80.6% vs pT2 UCRP 87.4% (p = 0.57) and pT3a UCRP 81.7% vs pT2 UCU 88.2% (p = 0.31)). Both the RFS and CSS rates were significantly higher in pT3a UCRP than in pT3b UCRP patients (5-year RFS, pT3a UCRP 81.7% vs pT3b UCRP 42.9%, HR = 2.59, p = 0.0038; 5-year CSS, pT3a UCRP 80.6% vs pT3b UCRP 42%, HR = 3.10, p = 0.001) and pT3 UCU patients (5-year RFS, pT3a UCRP 81.7% vs pT3 UCU 40.6%, HR = 2.34, p = 0.0084; 5-year CSS, pT3a UCRP 80.6% vs pT3 UCU 50.8%, HR = 2.29, p = 0.0179) (Table S1).
According to Fine and Gray’s model of RFS, pT stage (p < 0.001), pN (HR = 1.72, p = 0.011), WHO/ISUP grade (HR = 3.93, p = 0.008), and LVI (HR = 1.58, p = 0.015) were associated with tumor recurrence (Table 3). Squamous cell differentiation (HR = 1.62, p = 0.007) and sarcomatoid change (HR = 3.54, p < 0.001) were also independent risk factors for predicting RFS. The significance of other histological variants was not statistically significant (HR = 1.79, p < 0.240). The evaluation for individual of them was impossible because of their small number.
In an analysis of CSS using the same model, pT stage (p < 0.001), pN (HR = 2.33, p < 0.001), operative method (HR = 0.64, p = 0.004), histological variant (HR = 2.27 p < 0.001), LVI (HR = 1.46, p = 0.049), gross type (HR = 1.58, p = 0.0099), and adjuvant chemotherapy (HR = 0.65, p = 0.037) were associated with cancer death (Table 3). Squamous cell differentiation (HR = 2.03, p < 0.001) and sarcomatoid change (HR = 5.57, p < 0.001) were also independent risk factors for predicting CCS. The significance of other histological variants was not statistically significant (HR = 1.74, p < 0.250).
Overall survival
In this cohort, death occurred in 148 patients (30%) with UCRP, and in 144 patients (31.2%) with UCU. Figure 3c shows the OS curves after stratification by tumor location, and Table 2 shows the 5-year OS after stratification by pT and location.
In the Cox regression multivariate analyses, pT stage (p < 0.001), age (p < 0.001), pN (HR = 1.78 p = 0.0026), operative method (HR = 0.73 p = 0.0098), histological variant (HR = 1.89, p = 0.0017), LVI (HR = 1.45 p = 0.028), and gross type (HR = 1.45, p = 0.005) were independent risk factors for predicting OS (Table 3). Squamous cell differentiation (HR = 1.86, p = 0.009) and sarcomatoid change (HR = 3.65, p < 0.001) were also independent risk factors for predicting OS. The significance of other histological variants was not statistically significant (HR = 1.89, p < 0.140).
Discussion
Few studies have reported the significance of tumor location for oncological outcomes of UTUC, although the anatomical structure of the renal pelvis differs from that of the ureter [7, 10, 21]. Ouzzane et al. reported that the prognosis of UCRP is better than that of UCU (5-year CSS, UCRP 86.8% vs UCU 68.9%; HR = 1.7, p = 0.02) [17].
Park et al. reported that the CSS rate of pT3 UCU patients was significantly lower than that of pT3 UCRP patients, while there was no difference between UCRP and UCU patients with pathological stage pT2 or less [18]. The 5-year CSS was 76.1% in pT3 UCRP patients and 43.1% in pT3 UCU patients (p = 0.009). Tai et al. reported a longer RFS in patients with pT3 UCRP than in those with pT3 UCU (5-year RFS, pT3 UCRP 71% vs pT3 UCU 50%, p = 0.047), although the difference in CSS was not significant [27].
In contrast to the urothelium, renal calyces are composed of protruding papillae with a thin mucosal layer and the absence of a lamina propria and muscle layers (Fig. 2) [6]. These anatomical structures are different from those of the ureter and renal pelvis, located outside the kidney. Considering these anatomical differences, we determined two patterns of tumor invasion in pT3 UCRP [24]: (1) pT3a, carcinoma cells extend into the collecting duct, with limited invasion of the renal medulla; (2) pT3b, carcinoma cells invade the renal cortex or peripelvic adipose tissue. In general, the former pattern showed minimal parenchymal invasion, while the latter was characterized by extensive invasion. Therefore, we hypothesized that the renal medulla plays the same role as the muscularis propria of the ureter and renal pelvis outside the kidney.
Although our study showed that the depth of tumor invasion into the renal parenchyma is a critical prognostic determinant, it is not considered by the current AJCC TNM classification. As a result, UCRPs that infiltrate only into the renal medulla are classified as pT3, not pT1 or pT2. Cho et al. proposed that tumors with invasion limited to the renal medulla be classified as pT2, while those with invasion extending into the renal cortex should be considered as pT3 [5]. However, we reclassified renal medullary invasion as pT3a, and renal cortical invasion as pT3b, because pT2 in the current TNM staging system corresponds to tumor invasion into the muscle layer, not the renal parenchyma. Restricting the degree of modification of the pT classification will avoid unnecessary confusion. Patients with pT3b UCRP had a significantly worse prognosis than those with pT3a tumors. The 5-year CSS rates for pT3a and pT3b patients were 84.6% and 37.3% (p = 0.008), respectively.
Several authors have proposed cutoffs for the depth of parenchymal invasion (Table S2). Yoshimura et al. demonstrated that the degree of parenchymal invasion (not deeper than 5 mm from the basement membrane or deeper than 5 mm) influenced the prognosis of UCRP patients [30] (3-year cause-specific survival; 76.4% [≤ 5 mm] vs 25.2% [> 5 mm], p < 0.0001). Wu et al. also found that superficial parenchymal invasion (not deeper than 5 mm) was associated with a better oncological outcome than extensive parenchymal invasion (deeper than 5 mm) or peripelvic and periureteral adipose tissue invasion, in a study of 72 patients with pT3 UTUC located in either the renal pelvis or the ureter [29]. However, Park did not find evidence of a survival difference according to parenchymal invasion depth (5-year RFS, microscopic 84.6% vs extensive 60.5%, p = 0.218; 5-year CSS, microscopic 92.3% vs extensive 81.7%, p = 0.864). Rather, the prognosis of patents with tumors invading the peripelvic adipose tissue was worse than that of patients whose tumors invaded the renal parenchyma [19].
Shariat et al. classified pT3 UCRPs into pT3a (microscopic parenchymal invasion) and T3b (macroscopic parenchymal invasion or invasion into peripelvic adipose tissue). In their study of 266 patients with pT3 UCRP, those with pT3b had significantly lower 10-year RFS and CSS rates than patients with pT3a (RFS, 58% vs 38%; CSS, 60% vs 39%; p < 0.001 and p = 0.002, respectively) [25]. Roscigno et al. assessed the prognostic value of the pT3 subclassification in 284 international patients with pT3 UCRPs. Those with pT3b tumors were shown to be at increased risk of disease recurrence and cancer-specific mortality. However, in multivariate analyses, the subclassification of pT3 tumors was not associated with tumor recurrence or CSS [22].
In the categories proposed by Yoshimura or Shariat, microscopic invasion includes minimal or limited renal medulla invasion, in contrast to extensive parenchymal invasion. However, their categories are subjective and have no quantitative basis. Junior residents with little experience often evaluate the macroscopic findings, but the findings of senior pathologists may differ. Therefore, the subclassification may not be reliable [28]. Our proposed boundary at the corticomedullary junction was clear and reproducible. In this study, we validated this subclassification of pT3 UCRPs in a larger number of patients. We reconfirmed that patients with pT3b UCRPs have a significantly worse prognosis than pT3a UCRPs (5-year RFS, pT3a 81.7% vs pT3b 42.9%, p = 0.0038; 5-year CSS, pT3a 80.6% and pT3b 42%, p = 0.001).
Furthermore, we compared the oncological results of both UCRP and UCU in a relatively large number of patients. The prognosis of patients with our proposed pT3a UCRP was similar to that of patients with pT2 UCRP or UCU. Moreover, the prognosis of pT3b UCRP was similar to that of pT3 UCU. These results show that the renal parenchyma had a protective effect against tumor invasion in the renal pelvis where it lacks a muscular layer. Our proposed subclassification, which distinguishes pT3a from pT3b, clearly showed a correlation between TNM stage and the oncological results.
Multivariate analyses revealed that patients who received adjuvant chemotherapy had a better CSS than patients who did not. Current clinical guidelines do not strongly recommend adjuvant chemotherapy and many reports suggest that adjuvant therapy for UTUC has no impact on prognosis. However, some retrospective studies of UTUC patients (pT3N0M0) have shown that adjuvant chemotherapy improves CSS [9, 13].
The recent randomized and prospective POUT study showed that combination platinum-based chemotherapy containing cisplatin or carboplatin (GC or GCarbo therapy), administered within 90 days to patients undergoing RNU, improved the 3-year DFS and 3-year PFS rates [3]. Most of our patients are treated with platinum-based regimens such as MVAC, GC, or GCarbo as adjuvant therapy, which may have helped to improve the CSS of our cohort.
Our distinct and reproducible criteria for classification of the degree of parenchymal invasion at the corticomedullary boundary allowed one genitourinary pathologist to accurately evaluate a large number of specimens. We therefore believe that this subclassification will be useful for precisely predicting the prognosis of pT3 UCRP patients.
Our study had several limitations. The data were collected retrospectively, and the research was multi-institutional. Surgical procedures including lymphadenectomy and the chemotherapy regimen have changed over the past 30 years and are determined by physicians’ choice. Nevertheless, the large number of enrolled patients provided reliable results.
In conclusion, we examined the validity of the pT3 classification for UCRP from an anatomical point of view. Our results suggest that our proposed pT3 subclassification predicts the prognosis of UCRP more accurately than pT3 of the current AJCC/UICC classification, that is, pT3a UCRP had almost the same prognosis as pT2 UCRP and UCU, whereas pT3b UCRP had almost the same prognosis as pT3 UCU. An accurate diagnosis of pT3a makes it possible to avoid unnecessary postoperative chemotherapy. A prospective trial is needed to examine the usefulness of postoperative chemotherapy.
Our findings on the proposed pT3 subclassification may enhance the predictive value of UCRP prognosis than the current AJCC/UICC classification.
References
Akdogan B, Dogan HS, Eskicorapci SY, Sahin A, Erkan I, Ozen H (2006) Prognostic significance of bladder tumor history and tumor location in upper tract transitional cell carcinoma. J Urol 176:48–52
Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK (2017) AJCC cancer staging manual. Springer, New York
Birtle A, Johnson M, Chester J, Jones R, Dolling D, Bryan RT, Harris C, Winterbottom A, Blacker A, Catto JWF, Chakraborti P, Donovan JL, Elliott PA, French A, Jagdev S, Jenkins B, Keeley FX Jr, Kockelbergh R, Powles T, Wagstaff J, Wilson C, Todd R, Lewis R, Hall E (2020) Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): a phase 3, open-label, randomised controlled trial. Lancet 395:1268–1277
Brierley JD, Gospodarowicz MK, Wittekind C (2017) TNM classification of malignant tumours. Wiley-Blackwell, London
Cho KS, Cho DS, Choi YD, Hong SJ, Kim SJ, Cho NH (2007) A proposal for a novel staging system in renal pelvicaliceal urothelial carcinomas. Hum Pathol 38:1639–1648
Chung SD, Wang SM, Lai MK, Huang CY, Liao CH, Huang KH, Pu YS, Chueh SC, Yu HJ (2009) Lymphovascular invasion predicts poor outcome of urothelial carcinoma of renal pelvis after nephroureterectomy. BJU Int 103:1047–1051
Favaretto RL, Shariat SF, Chade DC, Godoy G, Adamy A, Kaag M, Bochner BH, Coleman J, Dalbagni G (2010) The effect of tumor location on prognosis in patients treated with radical nephroureterectomy at Memorial Sloan-Kettering Cancer Center. Eur Urol 58:574–580
Fine J, Gray R (1999) A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94:496–509
Huang YC, Chen MF, Shi CS, Shindel AW, Huang CE, Pang ST, Chuang CK, Chen CS, Chang YH, Lin WY, Ho DR, Chin CC, Kuo YH, Wu CF (2015) The efficacy of postoperative adjuvant chemotherapy for patients with pT3N0M0 upper tract urothelial carcinoma. J Urol 194:323–329
Isbarn H, Jeldres C, Shariat SF, Liberman D, Sun M, Lughezzani G, Widmer H, Arjane P, Pharand D, Fisch M, Graefen M, Montorsi F, Perrotte P, Karakiewicz PI (2009) Location of the primary tumor is not an independent predictor of cancer specific mortality in patients with upper urinary tract urothelial carcinoma. J Urol 182:2177–2181
Kikuchi E, Horiguchi Y, Nakashima J, Hatakeyama N, Matsumoto M, Nishiyama T, Murai M (2005) Lymphovascular invasion independently predicts increased disease specific survival in patients with transitional cell carcinoma of the upper urinary tract. J Urol 174:2120–2123
Margulis V, Shariat SF, Matin SF, Kamat AM, Zigeuner R, Kikuchi E, Lotan Y, Weizer A, Raman JD, Wood CG (2009) Outcomes of radical nephroureterectomy: a series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer 115:1224–1233
Nakagawa T, Komemushi Y, Kawai T, Otsuka M, Miyakawa J, Uemura Y, Kanatani A, Taguchi S, Naito A, Suzuki M, Nishimatsu H, Hirano Y, Tanaka Y, Matsumoto A, Miyazaki H, Fujimura T, Fukuhara H, Kume H, Igawa Y, Homma Y (2017) Efficacy of post-nephroureterectomy cisplatin-based adjuvant chemotherapy for locally advanced upper tract urothelial carcinoma: a multi-institutional retrospective study. World J Urol 35:1569–1575
Novara G, De Marco V, Gottardo F, Dalpiaz O, Bouygues V, Galfano A, Martignoni G, Patard JJ, Artibani W, Ficarra V (2007) Independent predictors of cancer-specific survival in transitional cell carcinoma of the upper urinary tract: multi-institutional dataset from 3 European centers. Cancer 110:1715–1722
Novara G, Matsumoto K, Kassouf W, Walton TJ, Fritsche HM, Bastian PJ, Martinez-Salamanca JI, Seitz C, Lemberger RJ, Burger M, El-Hakim A, Baba S, Martignoni G, Gupta A, Karakiewicz PI, Ficarra V, Shariat SF (2010) Prognostic role of lymphovascular invasion in patients with urothelial carcinoma of the upper urinary tract: an international validation study. Eur Urol 57:1064–1071
Olgac S, Mazumdar M, Dalbagni G, Reuter VE (2004) Urothelial carcinoma of the renal pelvis: a clinicopathologic study of 130 cases. Am J Surg Pathol 28:1545–1552
Ouzzane A, Colin P, Xylinas E, Pignot G, Ariane MM, Saint F, Hoarau N, Adam E, Azemar MD, Bensadoun H, Cormier L, Cussenot O, Houlgatte A, Karsenty G, Bruyere F, Maurin C, Nouhaud FX, Phe V, Polguer T, Roumiguie M, Ruffion A, Roupret M (2011) Ureteral and multifocal tumours have worse prognosis than renal pelvic tumours in urothelial carcinoma of the upper urinary tract treated by nephroureterectomy. Eur Urol 60:1258–1265
Park J, Ha SH, Min GE, Song C, Hong B, Hong JH, Kim CS, Ahn H (2009) The protective role of renal parenchyma as a barrier to local tumor spread of upper tract transitional cell carcinoma and its impact on patient survival. J Urol 182:894–899
Park J, Park S, Song C, Hong JH, Kim CS, Ahn H (2014) Peripelvic/periureteral fat invasion is independently associated with worse prognosis in pT3 upper tract urothelial carcinoma. World J Urol 32:157–163
Park S, Hong B, Kim CS, Ahn H (2004) The impact of tumor location on prognosis of transitional cell carcinoma of the upper urinary tract. J Urol 171:621–625
Raman JD, Ng CK, Scherr DS, Margulis V, Lotan Y, Bensalah K, Patard JJ, Kikuchi E, Montorsi F, Zigeuner R, Weizer A, Bolenz C, Koppie TM, Isbarn H, Jeldres C, Kabbani W, Remzi M, Waldert M, Wood CG, Roscigno M, Oya M, Langner C, Wolf JS, Strobel P, Fernandez M, Karakiewcz P, Shariat SF (2010) Impact of tumor location on prognosis for patients with upper tract urothelial carcinoma managed by radical nephroureterectomy. Eur Urol 57:1072–1079
Roscigno M, Cha EK, Rink M, Seitz C, Novara G, Chromecki TF, Fritsche HM, Matsumoto K, Walton TJ, Carballido J, Filippo Da Pozzo L, Bertini R, Ficarra V, Otto W, Karakiewicz PI, Pycha A, Fajkovic H, Naspro R, Scherr DS, Montorsi F, Shariat SF (2012) International validation of the prognostic value of subclassification for AJCC stage pT3 upper tract urothelial carcinoma of the renal pelvis. BJU Int 110:674–681
Roupret M, Babjuk M, Comperat E, Zigeuner R, Sylvester RJ, Burger M, Cowan NC, Gontero P, Van Rhijn BWG, Mostafid AH, Palou J, Shariat SF (2018) European Association of Urology Guidelines on upper urinary tract urothelial carcinoma: 2017 Update. Eur Urol 73:111–122
Sassa N, Tsuzuki T, Fukatsu A, Majima T, Kimura T, Nishikimi T, Yoshino Y, Hattori R, Gotoh M (2012) Is pT3 urothelial carcinoma of the renal pelvis a homogeneous disease entity? Proposal for a new subcategory of the pT3 classification. Histopathology 61:620–628
Shariat SF, Zigeuner R, Rink M, Margulis V, Hansen J, Kikuchi E, Kassouf W, Raman JD, Remzi M, Koppie TM, Bensalah K, Guo CC, Mikami S, Sircar K, Ng CK, Haitel A, Kabbani W, Chun FK, Wood CG, Scherr DS, Karakiewicz PI, Langner C (2012) Subclassification of pT3 urothelial carcinoma of the renal pelvicalyceal system is associated with recurrence-free and cancer-specific survival: proposal for a revision of the current TNM classification. Eur Urol 62:224–231
Siegel RL, Miller KD, Jemal A (2020) Cancer statistics, 2020. CA Cancer J Clin 70:7–30
Tai YS, Chen CH, Huang CY, Tai HC, Wang SM, Pu YS (2016) The effect of tumor location on oncologic outcomes in patients with upper urinary tract urothelial carcinoma stratified by pathologic stage. Urol Oncol 34:4 e19–4 e25
Tsuzuki T, Sassa N, Gotoh M (2012) Re: Shahrokh F. Shariat, Richard Zigeuner, Michael Rink, et al. Subclassification of pT3 urothelial carcinoma of the renal pelvicalyceal system is associated with recurrence-free and cancer-specific survival: proposal for a revision of the current TNM classification. Eur Urol 61:e61
Wu CF, Pang ST, Chen CS, Chuang CK, Chen Y, Lin PY (2007) The impact factors on prognosis of patients with pT3 upper urinary tract transitional cell carcinoma. J Urol 178:446–450
Yoshimura K, Arai Y, Fujimoto H, Nishiyama H, Ogura K, Okino T, Ogawa O (2002) Prognostic impact of extensive parenchymal invasion pattern in pT3 renal pelvic transitional cell carcinoma. Cancer 94:3150–3156
Author information
Authors and Affiliations
Contributions
Tomoyasu Sano: data curation, formal analysis, project administration, investigation, and writing original draft
Masashi Kato: data curation and project administration
Naoto Sassa: data curation, formal analysis, and project administration
Ryo Sadachi: formal analysis and statistics
Akihiro Hirakawa: formal analysis and statistics
Osamu Kamihira: data curation
Tsuyoki Hirabayashi: data curation
Toshinori Nishikimi: data curation
Satoshi Katsuno: data curation
Toru Kimura: data curation
Ryohei Hattori: conceptualization, data curation, supervision, writing original draft, and writing-review
Momokazu Gotoh: funding acquisition, supervision, and writing-review
Toyonori Tsuzuki: conceptualization, formal analysis, project administration, investigation, funding acquisition, supervision, writing original draft, writing-review, and editing
Corresponding author
Ethics declarations
This study is approved by institutional review boards of Nagoya University Hospital and all affiliated institutions.
Conflicts of interest
Toyonori Tsuzuki: honoraria, AstraZeneca, Chugai.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Quality in Pathology
Supplementary information
Supplementary Fig. 1
Representative figure of pT3a and pT3b urothelial carcinoma of the renal pelvis. a: pT3a: Cancer cells infiltrate only into the renal medulla. b: pT3b: Cancer cells infiltrate into the renal cortex. c: pT3b: Cancer cells infiltrate into the perirenal adipose tissue (PNG 10704 kb)
Table S1
Recurrence-free survival / Cancer-specific survival and variables with reference to pT3a (DOCX 21 kb)
Table S2
The prognostic value of different subclassifications for pT3 pelvicalyceal upper urinary tract carcinomas (DOCX 17 kb)
Rights and permissions
About this article
Cite this article
Sano, T., Kato, M., Sassa, N. et al. pT3 subclassification of renal pelvic cancer considering the tumor location improves the patients’ prognostic accuracy. Virchows Arch 478, 1089–1097 (2021). https://doi.org/10.1007/s00428-020-02973-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-020-02973-8