Abstract
This prospective cohort study evaluates associations between structural and ultrastructural parameters in baseline biopsies from human kidney transplants and long-term graft survival after more than 14 years’ follow-up. Baseline kidney graft biopsies were obtained prospectively from 54 consecutive patients receiving a kidney transplant at a single institution. Quantitative measurements were performed on the baseline biopsies by computer-assisted light microscopy and electron microscopy. Stereology-based techniques estimated the fraction of interstitial tissue, the volume of glomeruli, mesangial fraction, and basement membrane thickness of glomerular capillaries. The fraction of occluded glomeruli and scores according to the Banff classification were achieved. Kidney graft survival was analyzed by Kaplan–Meier estimates and Cox regression. Association to long-term kidney function was also analyzed. The long-term surviving kidney transplants were characterized at implantation by less arteriolar hyaline thickening (P < 0.001) and less interstitial fibrosis (P = 0.001), as well as a lower fraction of occluded glomeruli (P = 0.004) and lower glomerular volume (P = 0.03). At the latest follow-up, eGFR was decreased by 12 ml/min/1.73 m2 per unit increase in the score for arteriolar hyalinosis at implantation (P = 0.02), and eGFR was decreased by 19 ml/min/1.73 m2 per 106 μm3 increase in glomerular volume at baseline (P = 0.03). The unbiased Cavalieri estimate of glomerular volume and the ultrastructural parameters are the first to be evaluated in a cohort study with prospective follow-up for more than 14 years. The study shows that baseline biopsies from human kidney grafts contain extraordinary long-term prognostic information, and it highlights the importance of these intrinsic graft factors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Deterioration of long-term kidney function after transplantation remains a problem [1].
Several studies have shown that the graft may show histological abnormalities at the time of transplantation [2,3,4]. Cohort studies of baseline biopsies with very long-term follow-up are sparse as is the application of stereology-based, design-unbiased methods for morphometric assessment of kidney grafts [5, 6].
The hypothesis of this study is that structural deviations in implantation kidney biopsies have prognostic impact on long-term graft function. As these intrinsic graft factors seem difficult to categorize as immunological or non-immunological factors, we use the term “structural parameters.”
Formerly, the terms “chronic rejection” and “chronic allograft nephropathy” (CAN) have been used as both a clinical and histological diagnosis [7, 8]. The term CAN was a part of the early Banff classification systems [9, 10], but was later discarded and replaced by the term IF/TA (interstitial fibrosis/tubular atrophy) [11]. Regardless of the terminology used, the histological changes have been reported in a large proportion of transplanted kidneys [12,13,14,15]. Structural factors have been shown to be associated with the kidney function in native kidney diseases [16, 17] and in transplanted kidneys [18,19,20,21,22,23,24,25,26], and are still a focus point [27,28,29].
In the present study, baseline biopsies were scored according to the Banff 97 classification [10]. Furthermore, by light microscopy and an unbiased technique, cortical interstitium was estimated by test points. The frequency of sclerosed glomerular profiles was counted, and glomerular volume was obtained by the Cavalieri estimator; stereological methods and electron microscopy were used for estimation of glomerular capillary basement membrane thickness and mesangial volume [30]. Finally, the associations between baseline structural parameters and graft failure were evaluated by Cox regression and illustrated by Kaplan–Meier plots. We focus on graft failure as an end point because of its significance and also evaluate the association between baseline factors and kidney function.
Materials and methods
The study is a prospective long-term continuation of a short-term study [31].
Clinical data
Baseline kidney biopsies were obtained prospectively from 54 consecutive patients receiving a kidney transplant from September 1997 to November 1998 in the Kidney Transplantation Center in Aarhus, Denmark. Baseline donor and recipient data were registered at the time of transplantation.
Recipient follow-up
The recipients were prospectively followed up with clinical control every 3 months during the first year, then once a year or on clinical indication. Standard clinical assessments were performed. During the first 3 years, clinical data were noted in a special chart in the patient file. Then, annual clinical data were obtained from the files and from the Scandinavian transplantation cooperative Scandiatransplant. The date of the cessation of renal function was registered together with the clinical cause. Latest follow-up was December 31, 2012 according to the Health Research Ethics permission. Four clinical endpoint groups were defined: recipient alive with kidney function, recipient alive without kidney function due to re-transplantation or return to dialysis, recipient died with kidney function, and recipient died without kidney function [32].
Kidney biopsies
Baseline biopsies
Before wound closure, two baseline biopsies were obtained with an 18-G needle. The biopsy core length was 10 mm. The biopsies were performed at an angle of approximately 30° to the kidney surface to obtain primarily cortical tissue. One of the biopsies was fixed in 4% buffered formaldehyde, paraffin-embedded, serially sectioned at 4 μm, and stained by standard procedures [10]. The other biopsy provided tissue for plastic embedding; it was fixed in 2% glutaraldehyde, divided in blocks of approximately 1 mm3 and Epon-embedded, and a specialized sectioning procedure was applied, see Online Resource page 2. This provided material for measurement of glomerular volume and for electron microscopical evaluation.
Banff scores
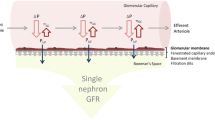
The paraffin-embedded biopsy was scored by an experienced renal pathologist (NM) according to the Banff 97 classification [10]. The scoring was anonymized. The scores for arteriolar hyaline thickening “ah,” interstitial fibrosis “ci,” tubular atrophy “ct,” allograft glomerulopathy “cg,” and arterial fibrous intimal thickening “cv” were the study parameters. Figure 1 presents an overview of applied methods
Morphometry
Quantification was performed at baseline blinded to clinical information, and by a stereology-based method when applicable. The techniques used have been presented [31] and are partly accessible in Online Resources. Biopsies were anonymized by numbering. The objective quantification was performed to lower bias based on subjectivity, and the counting was performed by one person to minimize observer variability. The study parameters were defined to fulfill method-dependent inclusion criteria.
Measurements by light microscopy
The measurements of the sections were performed with computer-assisted light microscopy (Grid®, Zeiss A/S, Denmark). Live video images of the field of vision in the microscope were transmitted to a computer screen. For details, see Online Resource page 2.
Interstitial volume fraction
A stereology-based point counting technique [33] was applied to estimate the volume fraction of the interstitium per glomerular cortex, VvC(interstitium/cortex). All biopsies with cortical tissue were included. The section with the largest biopsy area stained with periodic acid-Schiff (PAS) was used for the quantification of interstitial tissue. In five patients, the measurements were repeated on two consecutive days.
Fraction of occluded glomeruli
The frequency of glomerular occlusion, also called glomerulosclerosis, was expressed as the proportion of corpuscle profiles that were totally occluded. The inclusion criterion was a minimum of 14 glomerular profiles present.
Glomerular volume
The volume of individual glomeruli was obtained by the Cavalieri estimator [30, 34, 35] on systematic sampled 1-μm plastic sections obtained by exhaustive sectioning; Online Resource page 3. Biopsies containing at least seven glomeruli were included.
Measurements by electron microscopy
Digital images were obtained with a Philips CM10 electron microscope equipped with the computer software SIS (AnalySIS 3.0, Soft Imaging System). For details, see Online Resource page 4.
Mesangium
The volume fraction of mesangium per glomerular tuft, VvG(mesangium/glomerular tuft), was estimated by a stereology-based point counting technique. Images were obtained at a low magnification of × 1450 with three glomeruli assessed at two levels.
Basement membrane thickness
The glomerular capillary basement membrane thickness (BMT) was estimated using the orthogonal intercept method [35] by images obtained at a magnification of × 5800 with three glomeruli assessed at two levels each. For calculation of the stereology-based estimates, a computer program was used (Dimac, Digital Image Company, CHAMP).
Follow-up biopsies
Follow-up biopsies were performed on clinical indication. The histological diagnosis was established by an experienced renal pathologist (NM) according to the Banff 97 classification; chronic changes should be with no evidence of specific etiology, in accordance with the IF/TA definition [11].
Kidney function
The renal allograft function was evaluated by creatinine values, and glomerular filtration rate was estimated by the abbreviated MDRD equation (eGFR). When patients were alive and on dialysis or re-transplanted, eGFR was assigned the value zero. eGFR groups were established according to chronic kidney disease stages, CKD stages (www.renal.org).
Statistical methods
We used Kaplan–Meier plots and Cox proportional hazards regression to analyze renal allograft survival. Recipients who died with functioning graft were censored at the time of death [32]. Recipients with immediate complications were excluded. The association of kidney function to baseline structural values was analyzed with linear regression. P values less than 0.05 were considered significant. Analyzes were performed with Stata 13 (StataCorp LCC, Texas, USA).
Results
Clinical data
Baseline donor (n = 54) and recipient (n = 54) data are presented in Table 1. One year after transplantation, eight patients were alive without kidney function; among these, two patients had lost the kidney transplant very early: one due to arterial torsion with infarction, one due to an immediate renal vein thrombosis. One graft was histologically diagnosed with continuous acute rejection grade 3, and later vein thrombosis. Two patients were on dialysis with their grafts in situ; they were later explanted and diagnosed with IF/TA. Four grafts were explanted due to clinically and histologically verified acute rejection in a combination with IF/TA; one of these patients had died. At the latest follow-up December 31, 2012, 14 patients were alive without kidney function: Among these was the patient with early complication caused by immediate renal vein thrombosis, and the patient with early continued acute rejection. Two transplants were explanted due to acute rejection and IF/TA; six had been explanted solely due to IF/TA. One patient had been re-transplanted based on biopsy-proven IF/TA. One was on dialysis without a diagnosis. Two were on dialysis/explanted with histological diagnoses as a mixture of chronic changes and diabetic complications. Six patients had died without kidney function. Among these was the one with early complication due to arterial torsion. One patient was on dialysis and had a biopsy verified IF/TA. One was on dialysis with the graft in situ, clinically diagnosed as chronic rejection, but not confirmed by histology. Furthermore, one patient was on dialysis without biopsy. Two grafts had been explanted due to acute rejection and IF/TA. Twelve patients died with graft function; two patients died during the first year, one with cerebral infarction, one due to acute pancreatitis after a cholecystectomy. Until 5 year’s follow-up, another three patients died with transplant function; one due to an acute myocardial infarction, two with unspecified cause. After 5 years until the last date of follow-up, further seven recipients died with transplant function: three with cancer diagnosed within 5 to 7 years after transplantation (pulmonal, thyroid and esophageal), and one caused by infection (Pneumocystis carinii). Three with unspecified cause. The follow-up period was up to 15.3 years. Clinical status is illustrated in Table 2. The mean overall kidney survival time was 8.6 years (median = 8.6, SD = 6.05, range 0.03–15.3, n = 54). Figure 2 illustrates survival as a combination of recipient and graft survival. The mean number of histologically verified acute rejections during the first year was 0.46 (range 0–3), borderline rejections was 0.66 (0–4).
Recipient and graft survival, illustrated by Kaplan–Meier curves. Text in the graph area according to the current outcome. At the end of the study, 66.7% recipients were alive, 40.7% were alive with kidney transplant function, and 33.3% died during follow-up; of these, two-thirds (22.2%) had a functioning kidney transplant. Numbers at risk are shown in Table 2
Baseline biopsies
Results are presented in Table 3, and includes Banff scores, Measurements by light microscopy, and Measurements by electron microscopy.
Kidney function
Plasma creatinine values for kidneys functioning after 1 year were on average 163 μmol/l (median = 149, SD = 55, range 86–306, n = 43). Mean eGFR value for these patients was 42 ml/min/1.73 m2 (median = 41, SD = 15, range 16–93, n = 43). Median CKD group value was 3 (range 1–5, n = 51). Mean plasma creatinine values for kidneys functioning at the end of 2012 was 144 μmol/l (median = 129, SD = 50, range 62–258, n = 22). Mean eGFR value for kidneys functioning was 48 ml/min/1.73 m2 (median = 47, SD = 23, range 23–127, n = 22). Median CKD group value was 4 (range 1–5, n = 36).
Baseline allograft factors as predictors of allograft failure
Table 4 shows the results of Cox regression analysis of baseline allograft factors as predictors of allograft failure during the follow-up period. Results are expressed by hazard ratios, and includes analysis at 5 years and at the end of the study.
The Banff “ah” score at implantation was associated with loss of function of the transplanted kidney. For each unit increase in “ah” score at baseline, the incidence of allograft failure increased by a factor of 3.28, P < 0.001. An “ah” score above 1 had a worse outcome, Fig. 3. The “cv” score was marginally significant after 5 years. For each unit increase in “ci” score at baseline, the incidence of allograft failure increased by a factor of 5.98, P = 0.001. The proportion of interstitium in the glomerular cortex at baseline was also associated with loss of function of the transplanted kidney. For each percent increase in interstitial tissue, the incidence of lost kidney transplant function increased by 14%, P = 0.01. Per 10% increase in VvC(interstitium/glomerular cortex), the incidence of lost kidney transplant function increased by a factor of 3.58 (95% CI 1.38, 9.26; P = 0.01). Per 10 years increase in donor age, the hazard ratio was 1.60 (95% CI 1.06, 2.41; P = 0.03).
Illustration of survival by Kaplan-Meier plots for two groups. Cutpoint value was guided by the mean value of Banff “ah” score in the baseline biopsies. Death censored graft survival as a function of arteriolar hyaline thickening score in the baseline biopsy. Cox regression for Banff “ah” score 2-3 compared to Banff “ah” score 0–1. P < 0.001; Hazard Ratio = 8.18 (CI 2.83, 23.6) at the end of the study
Figures 4 and 5 and Online Resource Figures 1–4 illustrate grouped graft survival for the baseline parameters. Hazard ratio for the number of acute rejections in the first year was 2.09, P = 0.01 (95% CI 1.18, 3.66). The number of borderline rejections in the first year, AB and DR–mismatches and the cold ischemia time did not show significance in the Cox regressions. When applying a multifactorial model, the effect of “ah” score was robust. The Banff “ah” score was also the single most important factor when donor age was included in the assessment.
Illustration of survival by Kaplan-Meier plots for two groups. Death censored graft survival as a function of glomerular volume in the baseline biopsy. Cox regression for glomerular volume above 3 × 106 μm3 compared to glomerular volume below 3 × 106 μm3. P = 0.06; hazard ratio = 7.44 (CI 0.03, 58.95) at the end of study
Illustration of survival by Kaplan–Meier plots for two groups. Death censored graft survival as a function of Banff score of cortical interstitial tissue in the baseline biopsy. Cox regression for Banff “ci” score 1–3 compared to Banff “ci” score 0. P = 0.001; hazard ratio = 5.98 (CI 2.06, 17.34) at the end of study
Baseline allograft factors correlated to kidney function during follow-up
The eGFR after 1 year of follow-up was statistically significantly correlated with arteriolar hyalinosis at baseline, as well as with the number of sclerosed glomerular profiles and glomerular volume at baseline, Table 5. Further analysis of kidney function at year 1 is presented in Online Resource page 5. Banff arteriolar hyalinosis score and glomerular volume still correlated with eGFR at 5- and 10-year follow-up, and at the latest follow-up (results shown for the latter). At the end of the study, eGFR was decreased by 12 ml/min/1.73 m2 per unit increase in the score for arteriolar hyalinosis at implantation (P = 0.02); per 106 μm3 increase in glomerular volume at baseline, the eGFR at the latest follow-up was decreased by 19 ml/min/1.73 m2 (P = 0.03).
Discussion
We hypothesized that long-term kidney graft survival is related to structural parameters in baseline biopsies, and also studied the association between baseline structural parameters and long-term graft function. Baseline factors have been reviewed in detail [36, 37]; few studies have been very long-term prospective cohort studies [6]. We analyzed factors in three structural compartments (vessels, interstitial tissue, glomeruli) and evaluated together with donor age. The main finding of our study is that the Banff score for arteriolar hyaline thickening (“ah” score) is the single factor with the greatest impact on long-term graft survival.
The “ah” score also associated with long-term renal function in the surviving grafts, as also shown with a shorter period of follow-up [26]. This was also the case in a long-term study on retrospectively reviewed early indication biopsies [38], but are in contrast to another study by the same group [39]; the discrepancy might rely on the type of biopsy and different inclusion criteria with a group of biopsies “on request” from surgeon and non-consecutively enrolment for the latter. We did not perform quantitative estimates for arteries.
The quantitative estimate of baseline cortical interstitial tissue, VvC(interstitium/cortex), and the baseline Banff “ci” score for interstitial fibrosis both correlated significantly with graft survival. The stereology-based measurement was evaluated in an attempt to objectify the amount of interstitial tissue. The two principally different methods for estimation, the quantitative measurement contra the semi-quantitative score, led to comparable results regarding graft survival. Optimization of assessment of fibrosis is ongoing [40], and proposed in implantation biopsies as well [29]; automatization might also be implemented in future diagnostic practice.
The range of the cortical interstitial tissue measurements at baseline was 0.13 to 0.38, with a mean value of 0.24. A comparable variation has been reported [20, 41]. The result present a broad range for kidneys clinically regarded as normal. Different definitions of interstitial tissue and interstitial fibrosis might explain some of the variation [42]. We did not evaluate inflammation as it was very sparse at baseline. A former study found that total inflammation in 6-week transplant biopsies did not predict progression of fibrosis at 1 year [43].
Glomerular volume and the fraction of occluded glomerular profiles may reflect two stages or pathways of glomerular affection [44]. Both factors correlated with graft survival in our study; but only the unbiased estimate of glomerular volume also correlated with long-term renal function.
Baseline glomerular area evaluated by a maximal planar area method has been reported as a predictor of serum creatinine and creatinine clearance, with a follow-up of 7.5 years [22].
Nankivell et al. evaluated sequential graft biopsies up to 10 years after transplantation and reported that severe arteriolar hyalinosis resulted in greater glomerulosclerosis on sequential biopsies [45]. A prospective study by Wavamunno et al. used quantitative methods for ultrastructural parameters; the study was performed with surveillance biopsies in 15 patients in a 5-year ultrastructural follow-up [26]. They did not report on baseline findings, but found that ultrastructural changes were detectable early, and light microscopy changes regarding transplant glomerulopathy could be detected 2.3 years later.
Podocyte depletion has been shown to contribute to allograft failure [46]. The Ann Arbor group further reported increasing glomerular volume in biopsies with late transplant glomerulopathy; the glomerular volume was estimated in biopsies with at least 8 tuft profiles, and was based on the average radius of all tuft profiles in one section. Glomerular volume estimated from two-dimensional measurements has also been reported by the Mayo group [44]; based on one PAS section, biopsy section adequacy was defined as at least 2 mm2 of cortex and 4 glomeruli per section. They report that larger cortical nephron size, subclinical nephrosclerosis, and arteriolar hyalinosis modestly predict death censored graft failure; the mean follow-up was for 6.3 ± 3.8 years. The two-dimensional Weibel–based techniques for glomerular volume estimates are less work-demanding, compared to the unbiased “gold standard” method applied in our study; estimates from average profile area usually require correction factors (for shape, size distribution, and shrinkage) and a certain amount of glomerular profiles are needed [35]. There may indeed be a future role for these techniques in automated morphometry as suggested by Issa et al. [44], and perhaps in diagnostic routine. A prospective long-term follow-up cohort study with regard to baseline glomerular volume based on the Cavalieri estimator has not been performed before.
We found the Banff 97 score for mesangium hard to apply to the baseline biopsies, and we estimated the volume fraction of mesangium by electron microscopy. The basement membrane thickness of the glomerular capillaries was also estimated by electron microscopy. These factors did not correlate with graft survival.
The strength of the study is the prospective and consecutive inclusion of all baseline biopsies from a cohort of kidney transplant patients from a single center. Donor kidneys were not refused due to pre-implantation biopsies, which might have prevented a selection bias; standards for pre-implantation biopsies are evolving [47]. We used needle biopsies [47, 48], which were paraffin- or Epon-embedded, and we applied predefined inclusion criteria for evaluation of the biopsies and the measurements of structural parameters. All histological diagnoses and Banff scores were established by one experienced renal pathologist and quantitative measurements were conducted by one person. The scoring and measurements and the statistical evaluations were performed anonymized. The long observation period is a strength but also a weakness of the study; the patients dying with functioning grafts contributed to a reduction in the number of patients for long-term follow-up; the fraction is comparable to Issa et al. [44]. The number of biopsies fulfilling the predefined strict inclusion criteria also affected the groups for final evaluation. Despite limitations, the results of our cohort study show that existing changes within the donor kidney have extraordinary long-term implications.
Prospective studies per se are historic. The clinical management might be different from current approaches. However, we present baseline structural factors in a cohort where no patient was lost to follow-up. In Denmark, we have access to all patient data due to a nationwide system with a specific “Personal identity number.” The clinical treatments are based on national guidelines, and the Health Service is without individual economical costs. We find that a strength, and it may make an extrapolation of the results realistic, also in a historic perspective.
This study should not cause a negative selection of donors [49]. We find that the early morphological signs, which point to later development of reduced graft function, should encourage the investigation of therapeutic targets [50] and introduction of further preventive therapies. Implementation of fibrosis-inhibiting drugs and renal protective treatments for risk groups of kidney graft recipients could be proposals to minimize the influence of the structural factors present at baseline, in an effort to delay the process of vascular damage and the glomerular and interstitial changes.
References
Zhu D, Everly MJ (2012) Deceased donor kidney transplantation in the United States from 1988 to 2011: an analysis of the OPTN/UNOS registry. Clin Transpl 1–12
Sund S, Reisaeter AV, Fauchald P, Bentdal O, Hall KS, Hovig T (1999) Living donor kidney transplants: a biopsy study 1 year after transplantation, compared with baseline changes and correlation to kidney function at 1 and 3 years. Nephrol Dial Transplant 14:2445–2454
Curschellas E, Landmann J, Durig M et al (1991) Morphologic findings in “zero-hour” biopsies of renal transplants. Clin Nephrol 36:215–222
Randhawa PS, Minervini MI, Lombardero M et al (2000) Biopsy of marginal donor kidneys: correlation of histologic findings with graft dysfunction. Transplantation 69:1352–1357
Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Allen RD, Chapman JR (2003) The natural history of chronic allograft nephropathy. N Engl J Med 349:2326–2333
El-Husseini A, Sabry A, Zahran A, Shoker A (2007) Can donor implantation renal biopsy predict long-term renal allograft outcome? Am J Nephrol 27:144–151
Almond PS, Matas A, Gillingham K et al (1993) Risk factors for chronic rejection in renal allograft recipients. Transplantation 55:752–756
Meier-Kriesche HU, Ojo AO, Hanson JA et al (2000) Increased impact of acute rejection on chronic allograft failure in recent era. Transplantation 70:1098–1100
Solez K, Axelsen RA, Benediktsson H et al (1993) International standardization of criteria for the histologic diagnosis of renal allograft rejection: the Banff working classification of kidney transplant pathology. Kidney Int 44:411–422
Racusen LC, Solez K, Colvin RB et al (1999) The Banff 97 working classification of renal allograft pathology. Kidney Int 55:713–723
Solez K, Colvin RB, Racusen LC et al (2007) Banff ′05 Meeting Report: differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (‘CAN’). Am J Transplant 7:518–526
Haas M (2014) Chronic allograft nephropathy or interstitial fibrosis and tubular atrophy: what is in a name? Curr Opin Nephrol Hypertens 23:245–250
Schweitzer EJ, Matas AJ, Gillingham KJ et al (1991) Causes of renal allograft loss. Progress in the 1980s, challenges for the 1990s. Ann Surg 214:679–688
Matas AJ, Payne WD, Sutherland DE et al (2001) 2,500 living donor kidney transplants: a single-center experience. Ann Surg 234:149–164
Matas AJ, Humar A, Gillingham KJ et al (2002) Five preventable causes of kidney graft loss in the 1990s: a single-center analysis. Kidney Int 62:704–714
Jepsen FL, Mortensen PB (1979) Interstitial fibrosis of the renal cortex in minimal change lesion and its correlation with renal function. A quantitative study. Virchows Arch A Pathol Anat Histol 383:265–270
Bohle A, Grund KE, Mackensen S, Tolon M (1977) Correlations between renal interstitium and level of serum creatinine. Morphometric investigations of biopsies in perimembranous glomerulonephritis. Virchows Arch A Pathol Anat Histol 373:15–22
Isoniemi HM, Krogerus L, von Willebrand E, Taskinen E, Ahonen J, Hayry P (1992) Histopathological findings in well-functioning, long-term renal allografts. Kidney Int 41:155–160
Nickerson P, Jeffery J, Gough J et al (1998) Identification of clinical and histopathologic risk factors for diminished renal function 2 years posttransplant. J Am Soc Nephrol 9:482–487
Nicholson ML, McCulloch TA, Harper SJ et al (1996) Early measurement of interstitial fibrosis predicts long-term renal function and graft survival in renal transplantation. Br J Surg 83:1082–1085
Nankivell BJ, Fenton-Lee CA, Kuypers DR et al (2001) Effect of histological damage on long-term kidney transplant outcome. Transplantation 71:515–523
Abdi R, Slakey D, Kittur D, Burdick J, Racusen L (1998) Baseline glomerular size as a predictor of function in human renal transplantation. Transplantation 66:329–333
Gaber LW, Moore LW, Alloway RR, Amiri MH, Vera SR, Gaber AO (1995) Glomerulosclerosis as a determinant of posttransplant function of older donor renal allografts. Transplantation 60:334–339
Wavamunno MD, O'Connell PJ, Vitalone M et al (2007) Transplant glomerulopathy: ultrastructural abnormalities occur early in longitudinal analysis of protocol biopsies. Am J Transplant 7:2757–2768
Woestenburg AT, Verpooten GA, Ysebaert DK, Van Marck EA, Verbeelen D, Bosmans JL (2009) Fibrous intimal thickening at implantation adversely affects long-term kidney allograft function. Transplantation 87:72–78
Pokorna E, Vitko S, Chadimova M, Schuck O (2000) Adverse effect of donor arteriolosclerosis on graft outcome after renal transplantation. Nephrol Dial Transplant 15:705–710
Mengel M, Sis B, Haas M et al (2012) Banff 2011 Meeting report: new concepts in antibody-mediated rejection. Am J Transplant 12:563–570
Haas M, Sis B, Racusen LC et al (2014) Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant 14:272–283
Sethi S, D'Agati VD, Nast CC et al (2017) A proposal for standardized grading of chronic changes in native kidney biopsy specimens. Kidney Int 91:787–789
Gundersen HJ, Bendtsen TF, Korbo L et al (1988) Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS 1988 96:379–394
Ellingsen AR (2002) Prognostic factors in baseline biopsies from human kidney transplants – with special emphasis on chronic allograft nephropathy. PhD thesis, Faculty of Health Sciences, University of Aarhus, Denmark, pp 1–60
European best practice guidelines for renal transplantation (2002) Section IV: Long-term management of the transplant recipient. IV.13 Analysis of patient and graft survival. Nephrol Dial Transplant 17(Suppl 4):60–67
Ellingsen AR, Nyengaard JR, Østerby R, Jørgensen KA, Petersen SE, Marcussen N (2002) Measurements of cortical interstitium in biopsies from human kidney grafts: how representative and how reproducible? Nephrol Dial Transplant 17:788–792
Macleod JM, White KE, Tate H, Bilous RW (2000) Measurement of glomerular volume in needle biopsy specimens. The ESPRIT Study Group (European Study of the Progression of Renal Disease in Type 1 Diabetes). Nephrol Dial Transplant 15:239–243
Osterby R (1995) Research methodologies related to renal complications: structural changes. In: Research methodologies in human diabetes - Part 2. Walter de Gruyter, Berlin, pp 289–309
Mueller TF, Solez K, Mas V (2011) Assessment of kidney organ quality and prediction of outcome at time of transplantation. Semin Immunopathol 33:185–199
Wang CJ, Wetmore JB, Crary GS, Kasiske BL (2015) The donor kidney biopsy and its implications in predicting graft outcomes: a systematic review. Am J Transplant 15:1903–1914
Naesens M, Kuypers DR, De Vusser K et al (2013) Chronic histological damage in early indication biopsies is an independent risk factor for late renal allograft failure. Am J Transplant 13:86–99
De Vusser K, Lerut E, Kuypers D et al (2013) The predictive value of kidney allograft baseline biopsies for long-term graft survival. J Am Soc Nephrol 24:1913–1923
Farris AB, Chan S, Climenhaga J et al (2014) Banff fibrosis study: multicenter visual assessment and computerized analysis of interstitial fibrosis in kidney biopsies. Am J Transplant 14:897–907
Seron D, Carrera M, Grino JM et al (1993) Relationship between donor renal interstitial surface and post-transplant function. Nephrol Dial Transplant 8:539–543
Farris AB, Alpers CE (2014) What is the best way to measure renal fibrosis?: a pathologist’s perspective. Kidney Int Suppl 4:9–15
Dorje C, Reisaeter AV, Dahle DO et al (2016) Total inflammation in early protocol kidney graft biopsies does not predict progression of fibrosis at one year post-transplant. Clin Transpl 30:802–809
Issa N, Lopez CL, Denic A et al (2020) Kidney structural features from living donors predict graft failure in the recipient. J Am Soc Neprol 31:415–423
Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Allen RD, Chapman JR (2004) Evolution and pathophysiology of renal-transplant glomerulosclerosis. Transplantation 78:461–468
Yang Y, Hodgin JB, Afshinnia F et al (2015) The two kidney to one kidney transition and transplant glomerulopathy: a podocyte perspective. J Am Soc Nephrol 26:1450–1465
Liapis H, Gaut JP, Klein C et al (2017) Banff histopathological consensus criteria for preimplantation kidney biopsies. Am J Transplant 17:140–150
Nickeleit V (2013) Foretelling the future: predicting graft outcome by evaluating kidney baseline transplant biopsies. J Am Soc Nephrol 24:1716–1719
Mohan S, Campenot E, Chiles MC et al (2017) Association between reperfusion renal allograft biopsy findings and transplant outcomes. J Am Soc Nephrol 28:3109–3117
Boor P, Floege J (2015) Renal allograft fibrosis: biology and therapeutic targets. Am J Transplant 15:863–886
Acknowledgments
The article is dedicated to Melvin Madsen (1950–2006), former Director of Scandiatransplant and former Consultant at Department of Nephrology, Aarhus University Hospital, Denmark.
Funding
The project received non-commercial financial support from Danish foundations and institutions. See Online Resource Material 6.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Anne Ringer Ellingsen, Kaj Anker Jørgensen, Ruth Østerby, Steffen Ellebæk Petersen, Svend Juul, Niels Marcussen, and Jens Randel Nyengaard.
The first draft of the manuscript was written by Anne Ringer Ellingsen and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
The study was approved by the Central Denmark Region Committees on Health Research Ethics and the Danish Data Protection Agency 1-16-02-97-12 / 1-16-02-420-12.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Data repository
Data repository is not available due to the permission from the Central Denmark Region Committees on Health Research Ethics.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Quality in Pathology
Electronic supplementary material
ESM 1
(PDF 464 kb)
Rights and permissions
About this article
Cite this article
Ellingsen, A.R., Jørgensen, K.A., Østerby, R. et al. Human kidney graft survival correlates with structural parameters in baseline biopsies: a quantitative observational cohort study with more than 14 years’ follow-up. Virchows Arch 478, 659–668 (2021). https://doi.org/10.1007/s00428-020-02924-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-020-02924-3