Abstract
L-type amino acid transporter 1 (LAT1) is a Na+-independent neutral amino acid transporter that plays a key role in cancer cell growth and survival. To determine the significance of LAT1 in prognosis and resistance to chemotherapy in ovarian carcinoma, we investigated the LAT1 expression in 245 ovarian carcinoma patients by immunohistochemistry using tissue microarray. High expression of LAT1 was detected in 85 (34.7%) patients. The ratio of high expression of LAT1 was significantly high in clear cell carcinoma and low in serous carcinoma compared to other histological types (P < 0.0001). High expression of LAT1 in ovarian carcinoma was associated with poorer prognosis as per log-rank test (P = 0.008). Cox’s univariate and multivariate analysis revealed that high expression of LAT1 is an independent marker indicating poor prognosis (hazard ratio = 2.810, P < 0.0001) as well as the FIGO stage III/IV (vs. I/II) and suboptimal surgery. High LAT1 expression was also found to be associated with resistance to chemotherapy (P = 0.016) notably in clear cell carcinoma. In conclusion, we demonstrate that LAT1 is not only associated with poor prognosis of ovarian carcinoma, but also associated with chemoresistance in ovarian carcinoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ovarian carcinoma is the most lethal gynecological cancer. The prognosis for patients with advanced-stage ovarian carcinoma remains poor despite aggressive surgical treatments and recent advances in chemotherapy [1, 2]. To improve the outcome of the patients with ovarian carcinoma, it is important to evaluate the potentiality of molecular markers for predicting prognosis and their efficacy to chemotherapies. However, there have been no established clinical markers that can be correlated with precise prognosis and therapeutic efficacy in ovarian carcinoma patients.
L-type amino acid transporter 1 (LAT1) belongs to system L, a Na+-independent carrier that transports large neutral amino acids such as leucine, isoleucine, valine, phenylalanine, tyrosine, tryptophan, methionine, and histidine [3, 4]. LAT1 requires covalent association with the heavy chain of 4F2 cell-surface antigen (CD98) for its functional expression in the plasma membrane [3, 4]. Previous studies have shown that LAT1 is highly expressed in many human cancers and is typically associated with more aggressive cancer biology [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19]. LAT1 provides cancer cells with the essential amino acids required for protein synthesis and to stimulate growth of cancer cells via the mammalian target of rapamycin (mTOR) pathway [20,21,22,23]. Recently, it has been suggested that system L inhibitors such as 2-aminobicyclio-(2,2,1)-heptane-2-carboxylic acid (BCH) may be useful as an adjuvant therapeutic in multiple cancers [21,22,23,24]. In addition, membrane transporters and channels (collectively called the transportome) including LAT1 have been drawing attention for their role in cancer chemosensitivity and chemoresistance [25].
Previous studies have indicated that LAT1 expression in ovarian carcinoma is associated with cancer cell proliferation [17] and poor prognosis of patients [18]. However, the relationship between LAT1 expression and resistance to chemotherapy has not been established. In this study, we evaluated the expression of LAT1 in ovarian carcinomas by immunohistochemistry and elucidated its significance in prognosis and response to chemotherapy.
Materials and methods
Patient data
Formalin-fixed paraffin-embedded tissue blocks of patients with ovarian carcinoma who were surgically treated at the National Defense Medical College Hospital between 1984 and 2008 were collected. A total of 245 patients were enrolled in this investigation who met the following inclusion criteria: (i) patients who had received no prior chemotherapy before the surgical therapy; (ii) patients who were diagnosed with ovarian carcinoma by pathological evaluation; (iii) patients identified with histological type as serous, endometrioid, mucinous, or clear cell type ovarian carcinoma; and (iv) patients whose medical information and tissue blocks were available. To exclude metastasis from gastrointestinal cancer, we routinely performed endoscopic examination on all patients before surgery. Metastatic carcinomas have not been included in this study. Patient characteristics are summarized in Table 1. The investigated tissue blocks included 98 serous carcinomas (SC), 49 mucinous carcinomas (MC), 37 endometrioid carcinomas (EC), and 61 clear cell carcinomas (CCC). The patient age group was between 16 and 82 years with the median age of 53 years. The progression of cancer in each patient was determined according to the International Federation of Gynecology and Obstetrics (FIGO) system. There were 99 (40.4%) cases in FIGO stage I, 32 (13.1%) in stage II, 82 (33.5%) in stage III, and 32 (13.1%) in stage IV. Optimal surgery (if residual tumor ≤ 1 cm) was performed in 150 cases (61.2%), and suboptimal surgery (if residual tumor > 1 cm) was performed in 95 cases (38.8%). Of the 222 patients (91.0%), 112 cases experienced postoperative chemotherapy with cyclophosphamide, adriamycin, and cisplatin (CAP), 54 cases with paclitaxel and carboplatin (TC), 23 cases with irinotecan and cisplatin (CPT-P), 12 cases with etoposide and cisplatin (EP), 8 cases with docetaxel and carboplatin (DC), while 13 cases by other procedures. Patients who received neoadjuvant chemotherapy were excluded from the study. The research protocol was approved by the Institutional Ethical Review Board Committee of the National Defense Medical College, Tokorozawa, Japan. Informed consent was obtained from all the individuals included in this study.
Tissue microarray construction
From each tumor tissue block, two 1.5 mm cores were punched. These cores were arranged on a tray and tissue microarray (TMA) blocks were constructed. All TMA blocks were sectioned into 4 μm thick slices to make slides for immunohistochemical (IHC) staining.
Immunohistochemistry
For IHC staining, we used a rabbit polyclonal antibody for LAT1 (dilution 1:800; NB100–734, Novus Biologicals, Littleton, CO, USA). Tissue microarray slides were deparaffinized in xylene and hydrated in gradients of alcohol. Antigen retrieval was performed by incubating slides with 10 mM sodium citrate buffer (pH 6) for 60 min at 98 °C. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide (H2O2) solution. Slides were incubated with primary antibodies overnight at 4 °C and exposed to the Dako EnVision+ System-HRP labeled polymer anti-rabbit antibody for 60 min at 20 °C. Specific antigen-antibody reactions were visualized with the DAB substrate and counterstained with the Mayer hematoxylin. We used human testicular tissue as positive control. Only phosphate buffered saline (PBS) incubated without primary antibody was used as negative control.
A semi-quantitative evaluation of the LAT1 immunoreactivity was assigned as follows: score 1 for ≤ 10% of tumor area stained, score 2 for 11–25% of area stained, score 3 for 26–50% of area stained, and score 4 for ≥ 51% of area stained. The staining intensity of LAT1 expression was not considered during staining assessment. If the immunoreactivity score was 3 or 4, then tissue was defined as high expression. Tissue with a score of 1 or 2 was defined as low expression.
Statistical analysis
The JMP Pro software version 14 (SAS Institution Inc., Cary, NC, USA) was used for statistical analysis. Overall survival (OS) was defined as the interval between the diagnosis or the start of treatment and death due to any cause. Progression-free survival (PFS) was defined as the interval between the completion of upfront treatment until death or disease progression. Response rate was evaluated using response evaluation criteria in solid tumors (RECIST) guidelines [26]. Optimal surgery was defined as the cytoreductive surgery achieving residual tumor less than 1 cm in diameter, while suboptimal surgery was defined as the surgery with residual tumor equal to or more than 1 cm in diameter. The χ2 test and Fisher’s exact test were used to evaluate differences in the correlation between the expression of LAT1 and clinicopathological parameters. OS and PFS curves were generated using the Kaplan-Meier method. Comparison of the survival distribution was performed with the log-rank test. Univariate and multivariate analyses were performed using Cox’s proportional hazards model to evaluate the risk factors for cancer-related mortality. We performed logistic regression analysis with odds ratio and 95% confidence intervals (CIs) for evaluating the risk factors for chemoresistance. Statistical significance was defined as P < 0.05.
Results
Expression of LAT1 in ovarian carcinomas
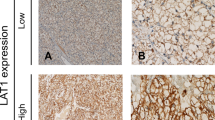
High LAT1 expression was detected in 85 cases (34.7%) and low LAT1 expression was detected in 160 cases (65.3%) of the investigated ovarian carcinoma resected tissues. LAT1 was observed predominantly in the plasma membrane with occasional cytoplasmic appearance in high LAT1-expressing cancer cells (Fig. 1a–d).
Representative IHC staining of high and low expression of LAT1 in tissue microarray-based samples of ovarian carcinoma (× 400). a High LAT1 expression in serous carcinoma (SC). b High LAT1 expression in mucinous carcinoma (MC). c High LAT1 expression in endometrioid carcinoma (EC). d High LAT1 expression in clear cell carcinoma (CCC). e Low LAT1 expression in SC. f Low LAT1 expression in CCC
Relationship between LAT1 expression and histological type
Relationships between LAT1 expression and clinicopathological characteristics are summarized in Table 2. Our analysis of LAT1 expression in each histological type of ovarian carcinoma revealed that high LAT1 expression was statistically predominant in CCC (34/61, 55.7%) and less frequent in SC (13/98, 13.3%) than other histological types, MC (23/49, 46.9%), and EC (15/37, 40.5%) (P < 0.0001). High LAT1 expression was also significantly associated with younger age (≤ 50 years) compared to the expression in > 50 years age group, (P = 0.040). LAT1 expression was not substantially associated with the FIGO stage of cancer (stages I–II vs. stages III–IV) and lymph node metastasis (positive vs. negative). However, the rates of LAT1 expression were observed to be lower in stages III–IV of ovarian carcinoma with positive lymph node metastasis (P = 0.082 and P = 0.070, respectively). In 27 cases with lymph node metastasis, 5 cases (1 case of SC, 1 of MC, and 3 of CCC) showed high LAT1 expression.
Relationship between LAT1 expression and prognosis
OS curves showed statistical difference between the patient groups with high LAT1 expression and low LAT1 ovarian carcinomas (P = 0.008, Fig. 2a). Overall survival rates spanning 5 years after initial treatment were 55.6% in the high LAT1 group and 72.0% in the low LAT1 group. When ovarian carcinomas were arranged according to their histological type, it was observed that high LAT1 expression was significantly associated with poorer prognosis in SC (P = 0.034, Fig. 2b), EC (P = 0.039, Fig. 2c), and CCC (P = 0.034, Fig. 2d). Overall survival curves also illustrated to be different between the high and low LAT1 expression groups in MC. However, the difference was not statistically significant (P = 0.078, Fig. 2e). There was no statistical difference in the PFS curves between the high and low LAT1 expression groups in the overall cases (Fig. 3a) and SC (Fig. 3b), but statistical significance in EC (P = 0.028) and slight significance in CCC were observed (P = 0.052, Fig. 3c, d). In MC, LAT1 was not correlated with PFS (Fig. 3e).
Analysis of all ovarian carcinomas using Cox’s univariate proportional hazard model revealed that FIGO stage III/IV, suboptimal surgery, and high LAT1 were significant indicators of poorer prognosis. Cox’s multivariate analysis including these three parameters revealed that they were independent indicators of poor prognosis (hazard ratio = 3.915, P < 0.0001; hazard ratio = 2.179, P = 0.002; hazard ratio = 2.810, P < 0.0001 respectively; Table 3). In a subset analysis of CCC, Cox’s multivariate analysis revealed that only FIGO stage III/IV was an independent prognostic factor (hazard ratio = 4.463, P = 0.013) although suboptimal surgery (hazard ratio = 2.712, P = 0.068) and high LAT1 expression (hazard ratio = 2.587, P = 0.052) had nearly significant impact (Table 4).
Relationship between LAT1 expression and response to chemotherapy
Of the 222 patients who received chemotherapy after surgery, 130 patients were evaluated for chemotherapeutic effect. When we defined complete/partial response as chemosensitive while stable/progressive disease as chemoresistant, 88 cases were found to be chemosensitive and 42 cases as chemoresistant (Table 1). Expression of LAT1 and response to chemotherapy in total ovarian carcinoma and each histological type was summarized in Table 5. High LAT1 expression was significantly associated with chemoresistance among total ovarian carcinoma cases (P = 0.016) and the CCC subset (P = 0.019). In other histological types, LAT1 expression was not correlated with chemosensitivity. Univariate and multivariate logistic regression analyses revealed that high LAT1 expression was an independent factor for chemoresistance in the total ovarian carcinoma cases (odds ratio = 2.837, P = 0.011, Table 6) and in the subset of CCC (odds ratio = 15.86, P = 0.013, Table 7).
Discussion
In the present study, we examined the expression of LAT1 in 245 ovarian carcinoma resected tissues using immunohistochemical analysis. Statistical analysis revealed that high expression of LAT1 was significantly associated with poorer prognosis and chemoresistance in patients with ovarian carcinoma. Cox’s multivariate analysis confirmed that the high LAT1 expression was an independent prognostic factor for predicting poor OS. When categorized according to the histological type, high LAT1 expression was significantly associated with worse prognosis in SC, EC, and CCC with chemoresistance observed specifically in CCC.
The distribution of types of ovarian carcinoma is unusual in this study. The incidence of SC is relatively low, and the incidence of non-serous carcinomas such as CCC and MC is relatively high. The high incidence of CCC is acceptable in Japan [27, 28]. The precise reason for the high incidence of MC in this series is uncertain. At least, we routinely performed endoscopic examination to exclude gastrointestinal cancer on all patients before surgery. It seems that it happened by chance that the frequency of MC was high in the single institute during the limited observation period. The high incidence of stage I cases in this study may also be due to the high incidence of non-serous carcinomas that were treated in stage I.
LAT1 is widely expressed in primary human cancers and several cancer cell lines where it has been shown to play essential roles in growth and survival of cancer cells [20]. Clinically, LAT1 is overexpressed in various malignant tumors and is associated with tumor proliferation, angiogenesis, and poor survival rate of the patient [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19]. It has been shown that LAT1 provides cancer cells with the essential amino acids for protein synthesis as well as to stimulate growth of cancer cells via the mTOR pathway [20,21,22,23].
With respect to ovarian carcinoma, Kaji et al. demonstrated that LAT1 plays significant roles in nutrition, proliferation, and migration of ovarian cancer [17]. They also showed that LAT1 inhibition by BCH can be utilized as an anticancer therapy in suppressing tumor growth without affecting normal healthy tissues [17]. Kaira et al. demonstrated that LAT1 is highly expressed in ovarian carcinomas and a positive LAT1 expression can serve as a significant independent factor for predicting a poor overall survival in patients with ovarian carcinoma [18]. Our results support the findings of the previous studies and suggest that LAT1 inhibition may be a useful therapy for ovarian carcinoma. Unexpectedly, the ratio of the cases highly expressing LAT1 was lower in SC compared to other histological types despite their poorer prognosis. Kaira et al. also reported that lower LAT1 expression is observed in SC compared to CCC [18]. They found that the reason for the low rate of high LAT1 expression cases in SC was uncertain, but other types of transporters might be providing essential amino acids to SC cells. The high expression of LAT1 in CCC was compatible with the previous studies and so LAT1 can be more related to the metabolism of CCC compared to other histological types. Our results also showed that lower expression of LAT1 was associated with FIGO stage III/IV and positive lymph node metastasis cases, although it was not statistically significant. It is an interesting phenomenon that high LAT1 expression is associated with poorer prognosis. Further studies are needed to elucidate the reason of lower expression of LAT1 in SC, advanced FIGO stage, and positive lymph node metastatic cases.
In this study, we revealed that high LAT1 expression was associated with resistance to chemotherapy in ovarian carcinoma. Logistic regression analysis revealed that LAT1 was an independent factor to determine chemoresistance in CCC. On the other hand, we were unable to evaluate the relationship between LAT1 expression and chemoresistance in other histological types. Relationship between LAT1 expression and chemoresistance has been reported in non-small cell lung cancer [29] and pancreatic ductal adenocarcinoma [30], but not in ovarian carcinoma. It is possible that LAT1 expression may be associated with amino acid transport necessary for protein synthesis supporting chemoresistance. However, little is known about the LAT1 expression and the proteins involved in chemoresistance. It is speculated that hypoxic environment of the tumor cells initiates the suppression of chemosensitive condition and hypoxic inducible factor-1α (HIF-1α) stimulates the expression of LAT1 [30]. Yamauchi et al. [21] reported that LAT1 inhibitor (BCH) enhances antitumor activity of cisplatin in a head and neck squamous cell carcinoma cell line (Hep-2). One explanatory mechanism of potentiating cytotoxicity by BCH treatment is that limited availability of essential amino acids that impairs DNA damage repair after cisplatin administration. Another possibility is the initiation or augmentation of apoptosis by reduced concentration of amino acids in the cell [21]. It is known that multiple transporters and ion channels are associated with cancer chemosensitivity as well as chemoresistance [25]. Our study revealed that high LAT1 expression was associated with poorer prognosis and chemoresistance in CCC, but could not explain the relationship between poorer prognosis and chemotherapeutic effect in other histological types based on LAT1 expression. The limitation of this study was to only consider immunohistochemistry analysis, which is retrospective. Although we believe that the semi-quantitative analysis using immunohistochemistry has significance as previously reported [7, 10, 12, 14, 18, 29], quantitative analysis should be performed for more reliable evaluation. When categorized according to the histological types, the sample size was too small to analyze.
In conclusion, LAT1 expression is associated with poorer prognosis and chemoresistance in ovarian carcinoma. It is promising to use LAT1 as a biomarker to predict prognosis and response to chemotherapy in ovarian carcinoma especially in clear cell carcinoma.
References
Cannistra SA (2004) Cancer of the ovary. N Engl J Med 351:2519–2529
Zhang XY, Zhang PY (2016) Recent perspectives of epithelial ovarian carcinoma. Oncol Lett 12:3055–3058
Kanai Y, Segawa H, Miyamoto K, Uchino H, Takeda E, Endou H (1998) Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98). J Biol Chem 273:23629–23632
Yanagida O, Kanai Y, Chairoungdua A, Kim DK, Segawa H, Nii T, Cha SH, Matsuo H, Fukushima JI, Fukasawa Y, Tani Y, Taketani Y, Uchino H, Kim JY, Inatomi J, Okayasu I, Miyamoto KI, Takeda E, Goya T, Endou H (2001) Human L-type amino acid transporter 1 (LAT 1): characterization of function and expression in tumor cell lines. Biochim Biophys Acta 1514:291–302
Nawashiro H, Otani N, Shinomiya N, Fukui S, Ooigawa H, Shima K, Matsuo H, Kanai Y, Endou H (2006) L-type amino acid transporter 1 as a potential molecular target in human astrocytic tumors. Int J Cancer 119:484–492
Nakanishi K, Ogata S, Matsuo H, Kanai Y, Endou H, Hiroi S, Tominaga S, Aida S, Kasamatsu H, Kawai T (2007) Expression of LAT1 predicts risk of progression of transitional cell carcinoma of the upper urinary tract. Virchows Arch 451:681–690
Kaira K, Oriuchi N, Imai H, Shimizu K, Yanagitani N, Sunaga N, Hisada T, Tanaka S, Ishizuka T, Kanai Y, Endou H, Nakajima T, Mori M (2008) Prognostic significance of L-type amino acid transporter 1 expression in resectable stage I-III nonsmall cell lung cancer. Br J Cancer 98:742–748
Sakata T, Ferdous G, Tsuruta T, Satoh T, Baba S, Muto T, Ueno A, Kanai Y, Endou H, Okayasu I (2009) L-type amino-acid transporter 1 as a novel biomarker for high-grade malignancy in prostate cancer. Pathol Int 59:7–18
Liang Z, Cho HT, Williams L, Zhu A, Liang K, Huang K, Wu H, Jiang C, Hong S, Crowe R, Goodman MM, Shim H (2011) Potential biomarker of L-type amino acid transporter 1 in breast cancer progression. Nucl Med Mol Imaging 45:93–102
Ichinoe M, Mikami T, Yoshida T, Igawa I, Tsuruta T, Nakada N, Anzai N, Suzuki Y, Endou H, Okayasu I (2011) High expression of L-type amino-acid transporter 1 (LAT1) in gastric carcinomas: comparison with non-cancerous lesions. Pathol Int 61:281–289
Uno K, Kuwabara H, Terado Y, Kojima K, Kawakami T, Kamma H, Sakurai H, Sakamoto A, Kurata A (2011) Divergent expression of L-type amino acid transporter 1 during uterine cervical carcinogenesis. Hum Pathol 42:1660–1666
Kaira K, Sunose Y, Arakawa K, Ogawa T, Sunaga N, Shimizu K, Tominaga H, Oriuchi N, Itoh H, Nagamori S, Kanai Y, Segawa A, Furuya M, Mori M, Oyama T, Takeyoshi I (2012) Prognostic significance of L-type amino-acid transporter 1 expression in surgically resected pancreatic cancer. Br J Cancer 107:632–638
Kaira K, Sunose Y, Ohshima Y, Ishioka NS, Arakawa K, Ogawa T, Sunaga N, Shimizu K, Tominaga H, Oriuchi N, Itoh H, Nagamori S, Kanai Y, Yamaguchi A, Segawa A, Ide M, Mori M, Oyama T, Takeyoshi I (2013) Clinical significance of L-type amino acid transporter 1 expression as a prognostic marker and potential of new targeting therapy in biliary tract cancer. BMC Cancer 13:482
Toyoda M, Kaira K, Ohshima Y, Ishioka NS, Shino M, Sakakura K, Takayasu Y, Takahashi K, Tominaga H, Oriuchi N, Nagamori S, Kanai Y, Oyama T, Chikamatsu K (2014) Prognostic significance of amino-acid transporter expression (LAT1, ASCT2, and xCT) in surgically resected tongue cancer. Br J Cancer 110:2506–2513
Isoda A, Kaira K, Iwashina M, Oriuchi N, Tominaga H, Nagamori S, Kanai Y, Oyama T, Asao T, Matsumoto M, Sawamura M (2014) Expression of L-type amino acid transporter 1 (LAT1) as a prognostic and therapeutic indicator in multiple myeloma. Cancer Sci 105:1496–1502
Hayase S, Kumamoto K, Saito K, Kofunato Y, Sato Y, Okayama H, Miyamoto K, Ohki S, Takenoshita S (2017) L-type amino acid transporter 1 expression is upregulated and associated with cellular proliferation in colorectal cancer. Oncol Lett 14:7410–7416
Kaji M, Kabir-Salmani M, Anzai N, Jin CJ, Akimoto Y, Horita A, Sakamoto A, Kanai Y, Sakurai H, Iwashita M (2010) Properties of L-type amino acid transporter 1 in epidermal ovarian cancer. Int J Gynecol Cancer 20:329–336
Kaira K, Nakamura K, Hirakawa T, Imai H, Tominaga H, Oriuchi N, Nagamori S, Kanai Y, Tsukamoto N, Oyama T, Asao T, Minegishi T (2015) Prognostic significance of L-type amino acid transporter 1 (LAT1) expression in patients with ovarian tumors. Am J Transl Res 7:1161–1171
Betsunoh H, Fukuda T, Anzai N, Nishihara D, Mizuno T, Yuki H, Masuda A, Yamaguchi Y, Abe H, Yashi M, Fukabori Y, Yoshida KI, Kamai T (2013) Increased expression of system large amino acid transporter (LAT)-1 mRNA is associated with invasive potential and unfavorable prognosis of human clear cell renal cell carcinoma. BMC Cancer 13:509
Fuchs BC, Bode BP (2005) Amino acid transporters ASCT2 and LAT1 in cancer: partners in crime? Semin Cancer Biol 15:254–266
Yamauchi K, Sakurai H, Kimura T, Wiriyasermkul P, Nagamori S, Kanai Y, Kohno N (2009) System L amino acid transporter inhibitor enhances anti-tumor activity of cisplatin in a head and neck squamous cell carcinoma cell line. Cancer Lett 276:95–101
Imai H, Kaira K, Oriuchi N, Shimizu K, Tominaga H, Yanagitani N, Sunaga N, Ishizuka T, Nagamori S, Promchan K, Nakajima T, Yamamoto N, Mori M, Kanai Y (2010) Inhibition of L-type amino acid transporter 1 has antitumor activity in non-small cell lung cancer. Anticancer Res 30:4819–4828
Wang Q, Holst J (2015) L-type amino acid transport and cancer: targeting the mTORC1 pathway to inhibit neoplasia. Am J Cancer Res 5:1281–1294
Fan X, Ross DD, Arakawa H, Ganapathy V, Tamai I, Nakanishi T (2010) Impact of system L amino acid transporter 1 (LAT1) on proliferation of human ovarian cancer cells: a possible target for combination therapy with anti-proliferative aminopeptidase inhibitors. Biochem Pharmacol 80:811–818
Huang Y, Anderle P, Bussey KJ, Barbacioru C, Shankavaram U, Dai Z, Reinhold WC, Papp A, Weinstein JN, Sadée W (2004) Membrane transporters and channels: role of the transportome in cancer chemosensitivity and chemoresistance. Cancer Res 64:4294–4301
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Itamochi H, Kigawa J, Terakawa N (2008) Mechanisms of chemoresistance and poor prognosis in ovarian clear cell carcinoma. Cancer Sci 99:653–658
Sugiyama T, Kamura T, Kigawa J, Terakawa N, Kikuchi Y, Kita T, Suzuki M, Sato I, Taguchi K (2000) Clinical characteristics of clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer 88:2584–2589
Kaira K, Takahashi T, Murakami H, Shukuya T, Kenmotsu H, Naito T, Oriuchi N, Kanai Y, Endo M, Kondo H, Nakajima T, Yamamoto N (2011) Relationship between LAT1 expression and response to platinum-based chemotherapy in non-small cell lung cancer patients with postoperative recurrence. Anticancer Res 31:3775–3782
Altan B, Kaira K, Watanabe A, Kubo N, Bao P, Dolgormaa G, Bilguun EO, Araki K, Kanai Y, Yokobori T, Oyama T, Nishiyama M, Kuwano H, Shirabe K (2018) Relationship between LAT1 expression and resistance to chemotherapy in pancreatic ductal adenocarcinoma. Cancer Chemother Pharmacol 81:141–153
Acknowledgments
The authors would appreciate Chinami Onuma for her excellent technical assistance, and Editage (www.editage.jp) for English language editing.
Author information
Authors and Affiliations
Contributions
Kimiya Sato, Morikazu Miyamoto, Masashi Takano, Kenichi Furuya, and Hitoshi Tsuda conceived and designed the study, and wrote, edited, and reviewed the manuscript. Kimiya Sato and Hitoshi Tsuda researched and analyzed data. All authors gave final approval for publication. Kimiya Sato takes full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript.
Corresponding author
Ethics declarations
The research protocol was approved by the Institutional Ethical Review Board Committee of the National Defense Medical College, Tokorozawa, Japan. Informed consent was obtained from all the individuals included in this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sato, K., Miyamoto, M., Takano, M. et al. Significant relationship between the LAT1 expression pattern and chemoresistance in ovarian clear cell carcinoma. Virchows Arch 474, 701–710 (2019). https://doi.org/10.1007/s00428-019-02520-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-019-02520-0