Abstract
Intraductal papillary epithelial neoplasms of the pancreatobiliary system (intraductal papillary neoplasm of the bile duct (IPNB) and intraductal papillary mucinous neoplasm (IPMN)) seem to share many clinicopathological features; however, IPNB has not been fully characterized. In order to understand the clinicopathological/immunohistochemical features of IPNB better, we compared 52 cases of IPNB with 42 cases of IPMNs with mural nodules. The IPNB cases were divided into two groups according to their histological similarity and according to five key histological findings. All IPNB and IPMN cases mainly affected middle-aged to elderly people, predominantly men. Mucin hypersecretion was less frequent in IPNB compared to IPMN. Group 2 IPNB more frequently had a higher histopathological grade and more extensive stromal invasion than IPMN. Group 1 IPNB and IPMN were further classified into four subtypes (gastric, intestinal, pancreatobiliary, and oncocytic). Although each subtype of IPNB and IPMN showed similar histology, the immunohistochemical results were different. The gastric type of IPNB was less frequently positive for CDX2, and intestinal IPNB was more frequently positive for MUC1 and less frequently positive for MUC2, MUC5AC, and CDX2 compared to each subtype of IPMN, respectively. In conclusion, IPNB and IPMN have some clinicopathological features in common, but mucin hypersecretion was less frequent both in IPNBs than in IPMN. Group 2 IPNB differed from IPMN in several parameters of tumor aggressiveness. Additional clinicopathological and molecular studies should be performed with respect to the subtypes of IPNB and IPMN.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, much attention has been paid to preinvasive neoplasms of the pancreatobiliary systems [1,2,3,4,5,6,7,8,9,10,11,12,13]. In the pancreatobiliary system, preinvasive neoplasms include intraductal papillary mucinous neoplasm (IPMN) and intraductal tubulopapillary neoplasm (ITPN) [6, 12]. In the biliary tree, grossly visible intraductal tumors with predominantly papillary or tubular architectures similar to pancreatic IPMN or ITPN are occasionally seen and are called “intraductal papillary neoplasm of the bile duct” (IPNB) and “biliary intraductal tubulopapillary neoplasms” (b-ITPN) [1,2,3,4, 14]. IPMN/ITPN and IPNB/b-ITPN are thought to be preinvasive biliary and pancreatic ductal neoplasms, and they sometimes progress to an invasive carcinoma [1, 6, 12].
In order to understand IPNB better, several studies have been conducted on some genes and proteins that had been found to be involved in IPMNs [15,16,17,18]. Some studies have also reported clinicopathological similarities and differences between IPNB and IPMN; however, those results are somewhat controversial [7, 8, 11, 15]. Although histological subtypes are known to be closely related to the clinicopathological behavior of IPMNs, few studies have been conducted on IPNBs’ clinicopathological behavior by histological subtype. Some IPNBs have a similar histology to IPMN, and the others have a very different histology.
Hence, in this study, in order to understand IPNB better, clinicopathological/immunohistochemical features of IPNB and IPMN were compared by dividing IPNB into two groups (one group with histology similar to IPMN and the other dissimilar, and IPMN by histology).
Materials and methods
Case selection and tissue preparation
The study population consisted of 52 patients with IPNB and 42 patients with IPMN of the pancreas with mural nodules. All IPNB cases were surgically resected at the Shizuoka Cancer Center between 1999 and 2015. Tumors that predominantly showed intraductal growth, filling ducts with papillary or villous papillary neoplasms covering delicate fibrovascular stalks, were selected [1]. IPNB at the intrahepatic large bile duct (IB), hilar bile duct (HB) (right and left bile ducts and their convergence), and extrahepatic (superior, middle, and inferior) bile duct (EB) were included. Tumors of the gallbladder and ampulla of Vater were excluded from this study. All IPMN cases were surgically resected at Juntendo University Hospital or its branches (listed in the “Acknowledgements” section) between 1990 and 2015. While 59 IPMNs were surgically resected in this period, 17 cases were excluded from this study because they were composed only of tumor cells with flat to low papillary growth; only IPMN cases with mural nodules were examined in this study.
Surgical specimens of IPNB and IPMN were fixed in formalin, dissected at 5-mm intervals, and paraffin embedded. These sections were prepared for histologic examination, and all hematoxylin and eosin (H&E)-stained sections were studied. One to three sections with the most abundant intraductal proliferation were chosen from each IPNB and IPMN specimen for immunohistochemical and histochemical (di-PAS) analyses. The study protocol was approved by the Institutional Review Board of the Shizuoka Cancer Center.
Clinical data
Information on age at the time of surgery, sex, the location of the tumor, the mucin hypersecretion of IPNB and IPMN, and IPMN duct type was obtained from clinical records. The location of the main tumor(s) in all IPNB cases was categorized according to biliary anatomy (IB, HB, or EB). When IPNB involved more than two locations of the three anatomical compartments, such case(s) was additionally recorded as extensive (EXT). The main tumor of one IPNB case was observed at the anastomosing site of the bile duct and jejunum, and it was recorded as other (O). The anatomical location of IPMN cases was divided into the pancreatic head and body and/or the tail. When IPMN involved both the pancreatic head and body-tail, it was additionally recorded as extensive (EXT). The mucin hypersecretion was assessed using preoperative endoscopy and/or the gross image of the tumor surface of the surgically resected specimen and the content of the affected bile duct(s). The duct type for IPMN was divided into the main pancreatic duct type, branch duct type, or combined duct type using preoperative imaging studies.
Pathological data
The histological grade of the main tumor, extent of invasion at the bile duct wall and periductal tissue (stromal invasion), histological type of the invasive tumor, maximum diameter of the dilated duct, maximum height of the papillary growth of the main tumor(s), width of the stroma of the intraductal tumorous nodule (“narrow” for cases with almost no collagenous tissue in the stroma, “intermediate” for cases with delicate but collagenous stroma, and “mixed narrow and intermediate” for cases containing both narrow and intermediate stroma), and ratio of tubular components among the papillary components of the intraductal main tumor were recorded. The histological grades of IPNB and IPMN were categorized into low- or intermediate-grade (Low/Int), high-grade (High), or high-grade associated with invasive carcinoma (High + Inv), according to the Armed Forces Institute of Pathology (AFIP) criteria of pancreatic IPMN and the WHO criteria for IPNB and IPMN [1, 6, 12]. The cases in which invasive tubular carcinoma, colloid carcinoma, or acellular stromal mucin (mucin leakage without evident tumor cells) were observed in connection with the selected ducts were graded as high-grade associated with invasive carcinoma (High + Inv), and the invasive component was also recorded.
Immunohistochemistry
Deparaffinized 4-μm sections from each paraffin block were exposed to 0.3% hydrogen peroxide for 15 min to block endogenous peroxide activity. Details of the primary antibodies used in this study are summarized in Table 1, and immunohistochemical results using these antibodies were interpreted as negative (0) when none to only a few tumor cells were positive, scatteredly positive (1+) when more than a few but less than 10% of the tumor cells were positive, partly positive (2+) when more than 10% but less than 30% of tumor cells were positive, and diffusely positive (3+) when more than 30% of the tumor cells were positive.
MUC1 is a pan-epithelial membrane mucin usually expressed in tumors originating in the pancreatobiliary system [18]. MUC2 is the principal secretory mucin in the colorectum, specific to goblet cells [19]. MUC5AC is a secreted gel-forming mucin, expressed in the surface mucous epithelium of normal gastric mucosa [20]. CDX2 is a caudal-type homeobox gene, encoding a transcription factor that plays an important role in proliferation and differentiation of intestinal epithelial cells [21]. The monoclonal antibody CDX2-88 has been shown to be a highly sensitive and specific marker of tumors of intestinal origin [22]. Positivity for MUC2 and CDX2 indicates cells differentiating towards the intestinal epithelium, and positivity for MUC5AC indicates cells differentiating towards the gastric foveolar epithelium. MUC6 is a secretory protein of gastric pyloric glands.
Histological assessment of histological typicalness/atypicalness of IPNB and subtyping of IPNB and IPMN
First, IPNB cases were divided into two groups according to the histological similarity with pancreatic IPMNs; group 1 for the IPNB cases with typical histology for IPMN and group 2 for the cases with atypical histology. Additionally, group 1 of the IPNB and IPMN cases was classified into gastric (G), intestinal (I), pancreatobiliary (PB), and oncocytic (O) subtypes.
The histological similarities were assessed by referring to pancreatic IPMN by four pathologists under a multi-headed microscope by blind observation of several representative histologic sections. When assessing, the configuration of the gland/papilla/villous structure, tumor stroma, color/amount of cytoplasmic mucin, shape of individual tumor cells, and nuclear features of tumor cells was evaluated, and the cases with the features (1) to (5) were judged “atypical” since such features are not present in IPMN series: (1) no mucinous/oncocytic cytoplasm for most tumor cells, (2) marked heterogeneous histology or different histological structures (villous/tubular, straight/branching, non-mucinous/mucinous) next to each other, (3) existence of necrosis inside/outside tumor tubules, (4) non-edematous but collagenous stroma with fibroblasts of more than a minimal amount, and (5) existence of true cribriform features.
Group 1 IPNB and IPMN cases were histologically subtyped referring to the WHO 2010 classification of tumors of the digestive system and the previous reports [12, 23,24,25,26]. The subtyping was conducted mainly with H&E-stained sections, and in cases where H&E histologies were not clearly a particular subtype, we relied on immunostaining. Cases showing positivity (>2+) for MUC2 or CDX2 were classified into “IPNB-O/IPMN-O”; the cases showing positivity (>2+) for MUC1 but 0 or 1+ for MUC2 and CDX2 were classified into “IPNB-PB/IPNB-PB”; and the cases showing positivity (>2+) for MUC6 but not for MUC2/CDX2/MUC1 were classified into “IPNB-G/O/IPNb-G/O,” according to their cytoplasmic features.
Statistics
Clinicopathological/immunohistochemical data were compared between group 1IPNB and IPMN, group 2 and IPMN, and each subtype of group 1 IPNB and IPMN. Pearson’s chi-square test was used to analyze statistical differences between categorical data, such as mucin hypersecretion and histological grade, of the two groups. Student’s t test was used to determine the difference in continuously varying data such as age, nodule height, and duct dilatation. A p value <0.05 was considered statistically significant.
Results
Histological assessment of typicalness of IPNB and subtyping of IPNB/IPMN
Among 52 IPNB cases, 26 cases were judged/categorized into group 1 IPNB (with typical histologies) while 26 cases (50%) were categorized into group 2 IPNB (with atypical histologies), such as almost no mucinous/oncocytic cytoplasm for most tumor cells (15 cases), very heterologous histological structures (6 cases), existence of necrosis (5 cases), existence of collagenous stroma (19 cases), and/or existence of true cribriform structure (5 cases).
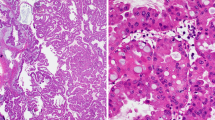
All cases of IPMN and 26 group 1 IPNB cases were classified into one of the four subtypes G, I, PB, or O. Not infrequently, more than two subtypes existed in the main tumor, and in such cases, the most predominant subtype was regarded as the subtype. However, in one IPNB case, neither of the two subtypes was predominant, and this case remained unclassified. The proportion of subtypes in group 1 IPNB and IPMN was not statistically different. Among the four subtypes, I was the most common (44.0% for group 1 IPNB and 45.2% for IPMN), followed by G (28.0% for group 1 IPNB and 35.7% for IPMN), while PB and O were relatively uncommon for both group 1 IPNB and IPMN. Representative cases of each subtype are shown in Fig. 1.
a IPNB with atypical histology (IPNB group 2). In this case, most tumor cells had non-mucinous cytoplasm. There were a few foci of coagulative necrosis inside (arrow) and outside (arrow head) of tumor tubules, and collagenous stroma were sometimes seen with a relatively large numbers of fibroblasts (※). b IPNB with atypical histology (IPNB group 2). In this case, most tumor cells had non-mucinous cytoplasm. The collagenous stroma with a relatively large number of fibroblasts (※) and cribriform structures (arrow heads) were sometimes seen. c Gastric subtype of IPNB (G-IPNB) with typical histology (IPNB group 1). The tumor cells are tall columnar cells with basally oriented nuclei and abundant pale mucinous cytoplasm, reminiscent of gastric foveolar epithelium. Pyloric gland-like structures are also seen at the periphery. d–g Intestinal subtype of IPNB (I-IPNB) with typical histology (IPNB group 1). The tumor cells are columnar cells with pseudostratified, cigar-shaped nuclei, and basophilic cytoplasm with variable amounts of apical mucin, reminiscent of colonic villous adenoma (d). Most tumor cells are negative for MUC5AC (e), some tumor cells were positive for MUC2 (f), and most tumor cells are weakly positive for CDX2 (g). h–k Intestinal subtype of IPNB (I-IPNB) with typical histology (IPNB group 1). The tumor cells are columnar cells with pseudostratified, cigar-shaped nuclei, and basophilic cytoplasm with variable amounts of apical mucin, reminiscent of colonic villous adenoma (h). This case showed diffuse positivity for MUC5AC (i) and partial positivity for MUC2 (j), and was almost negative for CDX2 (k). l Pancreatobiliary subtype of IPNB (PB-IPNB) with typical histology (IPNB group 1). The tumor forms thin, branching papillae, and the neoplastic cells are cuboidal with a less mucinous cytoplasm (arrows). m Oncocytic subtype of IPNB (O-IPNB) with typical histology (IPNB group 1). The tumor forms complex papilla with edematous stroma. Tubule formation is often seen. The tumor cells show abundant eosinophilic cytoplasm
Clinicopathological features of group 1 IPNB, group 2 IPNB, and IPMN
The main data of group 1 IPNB, group 2 IPNB, and IPMN cases are shown in Table 2 and Fig. 2. The patient ages of group 1 IPNB, IPMN, and group 2 IPNB were all similar (range 42–84 years, mean 71.2 years; range 34–83 years, mean 67.1 years; and range 37–82 years, mean 69.9 years, respectively). All three tumor series showed a male predominance. The main papillary tumors in group 1 IPNB were located at IB (12 cases), HB (11 cases), and EB (2 cases); in group 2 IPNB, they were located at IB (1 case), HB (8 case), and EB (17 cases); and in IPMN, more than half of the cases (27 of 42) were located at the head of the pancreas with the rest located in the body-tail. IPNB and IPMN frequently showed grossly visible mucin hypersecretion, although the frequency of mucin hypersecretion was higher in IPMN (83%) compared to group 1 IPNB (50.0%) (p < 0.01) and group 2 IPNB (15.3%) (p < 0.001). Both group 1 and group 2 IPNB showed extensive intraductal spreading (13 of 26 and 12 of 26 cases) more frequently than IPMN (1 of 42 cases) according to their individual criteria (p < 0.0001 for both). The dilatation of the affected bile duct for both group 1 and group 2 IPNB was greater compared to IPMN (each p < 0.001).
Group 1 and 2 IPNB and IPMN exhibited variable histological grades in different proportions. The majority of group 1 IPNB cases (50.0%) and group 2 IPNB cases (80.8%) were of high grade with invasion, while 15 (38.1%) and 8 (23.8%) of IPMN cases were of Low/Int and High grades without invasion, respectively (p < 0.001 for both). The proportion of cases with wide (>20 mm) stromal invasion was higher in group 1 IPNB (19.2%) and group 2 IPNB (46.2%) compared to IPMN (4.8%) (p < 0.001 for both). For the histological type of invasive carcinoma, the proportion of mucinous subtype (colloid or acellular stromal mucin) was not different between group 1 IPNB (4 out of 13 invasive cases, 30.8%) compared to IPMN (7 out of 17 invasive cases, 41.2%), but no mucinous type of invasive carcinoma was seen in group 2 IPNB. The width of the intraductal papillary tumor stroma was narrow in all cases of IPMN and in all but one group 1 IPNB, but narrow only in 17 cases (65.4%) of group 2 IPNB with others being of intermediate (8 cases) and mixed width (1 cases) (p < 0.001). The ratio of tubular components among the papillary components of the intraductal main tumor was lower in group 1 IPNB than in IPMN, while no statistical difference was seen between group 2 IPNB and IPMN.
The immunohistochemical data of group 1 IPNB, group 2 IPNB, and IPMN cases are shown in Fig. 3. The frequency of MUC1 positivity was slightly higher both in group 1/group 2 IPNB compared to IPMN, while that of MUC2 and MUC5AC positivity was lower both in group 1/group 2 IPNB compared to IPMN. The frequency of MUC6 positivity was slightly lower both in group 1/group 2 IPNB compared to IPMN. That of CDX2 was lower in group 1 IPNB but similar in group 2 IPNB compared to IPMN.
Comparison of histological and immunohistochemical features by subtypes of group 1 IPNB and IPMN
Gastric subtype
There were no differences in the histological grade, extent of stromal invasion, or width of the stroma between this subtype of group 1 IPNB and that of IPMN. The tubular component was more abundant in IPMN-G. No differences were seen in the expression of MUCs between IPNB-G and IPMN-G; however, CDX2 tended to be more often expressed by IPMN-G cells (Table 3).
Intestinal subtype
There were no differences in the histological grade, extent of stromal invasion, or width of the stroma or the ratio of tubular component between IPNB-I and IPMN (Table 4). Immunohistochemically, the expression of MUC1was more frequent for IPNB-I, while that of MUC2, MUC5AC, and CDX2 was less frequent for IPNB-I than for IPMN-I. Six cases (54.5%) were negative or scatteredly positive for MUC2 but partly or diffusely positive for CDX2 (Fig. 1d–g), and four cases (36.4%) were negative or scatteredly positive for CDX2 but partly or diffuse positivity for MUC2 (Fig. 1h–k).
PB and oncocytic subtype
The histological features and expression of MUCs/CDX2 were similar between IPNB-PB/IPNB-O and IPMN-PB/IPMN-O, respectively (Tables 5 and 6).
Discussion
The findings of this study are summarized as follows: (i) Approximately half of the IPNB cases were categorized into group 1 and group 2 IPNB, that is, IPNB with typical histology and IPNB with atypical histology. (ii) Both groups of IPNB and non-flat IPMN exhibited similar clincopathological features including the age and sex of the patients and histologically the height of papillary growth. (iii) Both group 1 and group 2 IPNB had different clinicopathological features, namely, less frequent mucin hypersecretion compared to IPMN, more extensive lateral spreading of the tumor, and more dilatation of the associated ducts. (iv) The different clinicopathological features of group 2 IPNB, but not group 1 IPNB, included a more frequent aggressive histological grade, greater extent of stromal invasion, a different histological type of invasion, and greater width of the tumor stroma compared to IPMN. (v) IPNB and IPMN showed immunohistochemical differences. IPNB was more frequently MUC1 positive and less frequently MUC2/MUC5AC positive than IPMN. Compared to IPMN, group 1 but not group 2 IPNB was less frequently CDX2 positive. (vii) The proportion of four histological subtypes among group 1 IPNBs and IPMNs did not differ and the intestinal subtype was the most common in both. (viii) By subtype, IPNB-G was less frequently CDX2 positive compared to IPMN-G. IPNB-I was less frequently MUC2/MUC5AC/CDX2 positive and more frequently MUC1 positive compared to IPMN-I.
In the present study, group 2 IPNB, which showed very dissimilar histology to IPMN in that there was less mucinous cytoplasm in most tumor cells, marked heterogeneous histology among some tumors, and tumor cells with high-grade atypia and necrosis, had non-edematous but fibrous stroma with a lot of fibroblasts, or contained true cribriforming, comprised half of the IPNB cases studied. In these cases, histological subtyping was difficult, since subtyping is conducted referring to histological features of each subtype of IPMN.
In this study, five histological parameters were used for dividing IPNB into group 1 and group 2. These five parameters were selected through discussion by four pathologists referring to pancreatic IPMN. In IPMN, most tumor cells have intracellular mucin in the cytoplasm, relatively homogeneous histology can be recognized for a certain area, and the stroma of the tumor papilla is variously edematous with almost no to a limited amount of fibroblasts. Necrotic debris is usually not detectable inside the tumor tubules or on the tumor papilla. Although pseudo-cribriform-like structures are often observed in IPMN, particularly in IPMN-O, true cribriform, that is, dense gland formation in back-to-back structures, is not seen in IPMN.
When the IPNBs were divided into two groups in this way, several differences were detected between the two; the tumor location of group 1 IPNB was mostly intrahepatic or hepatic hilus, and there was more mucin hypersecretion and a lower histological grade/less extensive stromal invasion. Although a comparison between group 1 and group 2 IPNB is not within the scope of this study, more studies including molecular analyses are necessary in order to understand the difference and similarities of group 1 IPNB and group 2 IPNB better.
Both group 1 and group 2 IPNB had some clinicopathological features in common but not with the other features of IPMN. There were several clinicopathological parameters in which group 2 but not group 1 IPNB differed from IPMN: frequency of histological grade, the extent of invasion, type of invasion, and width of the tumor stroma. Although most IPNBs have been reported to be of high-grade histology, the histological aggressiveness was similar between group 1 IPNBs and IPMN. Hence, it may be important to treat group 1 and group 2 IPNB differently, if there are situations where presurgical diagnosis of group 1 or group 2 IPNB was obtained.
In the present study, the proportion of four histological subtypes among group 1 IPNBs and IPMNs did not differ and the intestinal subtype was the most common in both. However, it should be noted that the relative frequency of “gastric IPMN” is highly dependent on the resection criteria and the way the population is studied. For example, several years ago, when most BD-IPMNs were being resected, gastric IPMNs were more frequent, but nowadays, since many of the BD-IPMNs are watched, the frequency of gastric IPMNs is changing, just based on the selection criteria.
In this study, immunohistochemical data of MUCs and CDX2 were collected and compared by each subtype and overall. Although study cases were relatively small in number, their immunohistochemical features were very different between IPNB and IPMN, particularly between IPNB-I and IPMN-I; MUC5AC was often expressed in a very limited way for IPNB-I, although the IPMN-I cases that were very analogous in histology with IPNB-I were positive for MUC5AC diffusely. The lower frequency of MUC2/CDX2 in IPNB-I was somewhat surprising, since these proteins are very important to group IPNB/IPMN into the intestinal subtype. More studies are needed to determine if different immunohistochemical methods (different antigen retrieval, different dilution, and so on) are more suitable for IPNB. Also, a quantitative study of mRNA for these proteins may be necessary to support their expression. Meanwhile, it is important to understand these immunohistochemical differences between IPNB and IPMN in order to avoid confusion in the practical situation of IPNB subtyping.
Some other studies have reported on the similarities and differences of IPNB and IPMN. For tumor behavior, Kloek et al. [8] and Minagawa et al. [7] reported that IPNB was associated with a higher malignancy rate compared to IPMN, while Wang et al. [11] showed a similar proportion of malignancy between IPNB and IPMN. Since the former two studies included more IPNBs of distal bile ducts and the latter study included more IPNBs of intrahepatic/hilar bile ducts, the controversial results of these studies may be due to study materials; the former two studies might include more “Group 2 IPNB” and the latter more “Group 1 IPNB” as defined in the present study. According to the molecular studies by Schlitter et al., IPNB harbors KRAS and/or GNAS mutations with much less frequency compared to pancreatic IPMN, suggesting differences in the molecular pathogenesis as well as in the clinicopathological/immunohistochemical characteristics of the two entities [15]. Therefore, it is highly recommended to analyze similarities and differences of IPNB and IPMN by dividing IPNBs into two groups and also dividing group 1 IPNB into four subtypes.
In conclusion, half of 52 cases of IPNB showed typical histology (group 1) and the remaining half showed atypical histology (group 2) in reference to IPMN. Group 1 IPNB/group 2 IPNB and IPMN are classified as intraductal neoplasms of the pancreatobiliary system, sharing many clinicopathological/histopathological features in common, although group 2 IPNB shows more aggressive features clinicopathologically, and the immunoprofile for MUCs and CDX2 of both groups of IPNB and IPMN are different. Group 1 IPNB could be easily subtyped into the four subtypes, and each subtype of group 1 IPNB and IPMN is very similar histologically but not immunohistochemically. In order to evaluate the different features of IPNB and IPMN with respect to their subtypes and developmental processes, analyzing genetic and molecular features separately for each subtype of group 1 IPNB as well as group 2 IPNB is imperative.
References
Nakanuma Y, Curado M-P, Franceschi S, Gores G, Paradis V, Sripa B (2010) Intrahepatic cholangiocarcinoma. In: Bosman F, Carmeiro F, Hruban RH, Theise ND (eds) WHO classification of tumours of the digestive system, 4th edn. International Agency for Research on Cancer, Lyon, pp 217–224
Klöppel G, Kosmahl M (2006) Is the intraductal papillary mucinous neoplasia of the biliary tract a counterpart of pancreatic papillary mucinous neoplasm? J Hepatol 44:249–250
Chen TC, Nakanuma Y, Zen Y, Chen MF, Jan YY, Yeh TS, Chiu CT, Kuo TT, Kamiya J, Oda K, Hamaguchi M, Ohno Y, Hsieh LL, Nimura Y (2001) Intraductal papillary neoplasia of the liver associated with hepatolithiasis. Hepatology 34:651–658
Shibahara H, Tamada S, Goto M, Oda K, Nagino M, Nagasaka T, Batra SK, Hollingsworth MA, Imai K, Nimura Y, Yonezawa S (2004) Pathologic features of mucin-producing bile duct tumors. Two histopathologic categories as counterparts of pancreatic intraductal papillary-mucinous neoplasms. Am J Surg Pathol 28:327–338
Ohtsuka M, Shimizu H, Kato A, Yoshitomi H, Furukawa K, Tsuyuguchi T, Sakai Y, Yokosuka O, Miyazaki M (2014) Intraductal papillary neoplasms of the bile duct. Int J Hepatol. doi:10.1155/2014/45909
Hruban RH, Pitman MB, Klimstra DS (2007) Intraductal neoplasms. In: AFIP ATLAS OF TUMOR PATHOLOGY Series 4. Tumors of the pancreas. American Registry of Pathology and Armed Forces Institute of Pathology, Washington, DC, pp 75–110
Minagawa N, Sato N, Mori Y, Tamura T, Higure A, Yamaguchi K (2013) A comparison between intraductal papillary neoplasms of the biliary tract (BT-IPMNs) and intraductal papillary mucinous neoplasms of the pancreas (P-IPMNs) reveals distinct clinical manifestations and outcomes. Eur J Surg Oncol 39:554–558
Kloek JJ, van der Gaag NA, Erdogan D, Rauws EA, Busch OR, Gouma DJ, ten Kate FJ, van Gulik TM (2011) A comparative study of intraductal papillary neoplasia of the biliary tract and pancreas. Hum Pathol 42:824–832
Kubota K, Nakanuma Y, Kondo F, Hachiya H, Miyazaki M, Nagino M, Yamamoto M, Isayama H, Tabata M, Kinoshita H, Kamisawa T, Inui K (2014) Clinicopathological features and prognosis of mucin-producing bile duct tumor and mucinous cystic tumor of the liver: a multi-institutional study by the Japan Biliary Association. J Hepatobiliary Pancreat Sci 21:176–185
Nakanuma Y (2010) A novel approach to biliary tract pathology based on similarities to pancreatic counterparts: is the biliary tract an incomplete pancreas? Pathol Int 60:419–429
Wang M, Deng BY, Wen TF, Peng W, Li C, Trishul NM (2016) An observational and comparative study on intraductal papillary mucinous neoplasm of the biliary tract and the cohort. Clin Res Hepatol Gastroenterol 40:161–168
Adsay NV, Fukushima N, Furukawa T, Hruban RH, Klimstra DS, Klöppel G, Offerhaus GJA, Pitman MB, Shimizu M, Zamboni G (2010) Intraductal neoplasms of the pancreas. In: Bosman F, Carmeiro F, Hruban RH, Theise ND (eds) WHO classification of tumours of the digestive system, 4th edn. International Agency for Research on Cancer, Lyon, pp 304–313
Zen Y, Fujii T, Itatsu K, Nakamura K, Minato H, Kasashima S, Kurumaya H, Katayanagi K, Kawashima A, Masuda S, Niwa H, Mitsui T, Asada Y, Miura S, Ohta T, Nakanuma Y (2006) Biliary papillary tumors share pathological features with intraductal papillary mucinous neoplasm of the pancreas. Hepatology 44:1333–1343
Schlitter AM, Jang KT, Klöppel G, Saka B, Hong SM, Choi H, Offerhaus GJ, Hruban RH, Zen Y, Konukiewitz B, Regel I, Allgauer M, Balci S, Basturk O, Reid MD, Esposito I, Adsay V (2015) Intraductal tubulopapillary neoplasms of the bile ducts: clinicopathologic, immunohistochemical, and molecular analysis of 20 cases. Modern Pathol 28:1249–1264
Schlitter AM, Born D, Bettstetter M, Specht K, Kim-Fuchs C, Riener MO, Jeliazkova P, Sipos B, Siveke JT, Terris B, Zen Y, Schuster T, Hofler H, Perren A, Kloppel G, Esposito I (2014) Intraductal papillary neoplasms of the bile duct: stepwise progression to carcinoma involves common molecular pathways. Modern Pathol 27:73–86
Tsai JH, Yuan RH, Chen YL, Liau JY, Jeng YM (2013) GNAS is frequently mutated in a specific subgroup of intraductal papillary neoplasms of the bile duct. Am J Surg Pathol 37:1862–1870
Sasaki M, Matsubara T, Nitta T, Sato Y, Nakanuma Y (2013) GNAS and KRAS mutations are common in intraductal papillary neoplasms of the bile duct. PLoS One 8:e81706
Matthaei H, Wu J, Dal Molin M, Debeljak M, Lingohr P, Katabi N, Klimstra DS, Adsay NV, Eshleman JR, Schulick RD, Kinzler KW, Vogelstein B, Hruban RH, Maitra A (2012) GNAS codon 201 mutations are uncommon in intraductal papillary neoplasms of the bile duct. HPB 14:677–683
Ajioka Y, Watanabe H, Jass JR (1997) MUC1 and MUC2 mucins in flat and polypoid colorectal adenomas. Clin Pathol 50:417–421
Yonezawa S, Higashi M, Yamada N, Yokoyama S, Kitamoto S, Kitajima S, Goto M (2011) Mucins in human neoplasms: clinical pathology, gene expression and diagnostic application. Pathol Int 61:697–716
Drummond F, Putt W, Fox M, Edwards YH (1997) Cloning and chromosome assignment of the human CDX2 gene. Ann Hum Genet 61:393–400
Werling RW, Yaziji H, Bacchi CE, Gown AM (2003) CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin. An immunohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol 27:303–310
Reid MD, Stallworth CR, Lewis MM, Akkas G, Memis B, Bastrurk O, Adsay NV (2016) Cytopathologic diagnosis of oncocytic type intraductal papillary mucinous neoplasm: criteria and clinical implications of accurate diagnosis. Cancer Cytopathol 124:122–134
Adsay NV, Adair DF, Heffess CS, Klimstra DS (1996) Intraductal oncocytic papillary neoplasms of the pancreas. Am J Surg Pathol 20:980–994
Marchegiani G, Mino-Kenudson M, Ferrone CR, Warshaw AL, Lillemore KD, Fernandez-del Castillo C (2015) Oncocytic-type intraductal papillary mucinous neoplasms: a unique malignant pancreatic tumor with good long-term prognosis. J Am Coll Surg 220:839–844
Basturk O, Khayyata S, Klimstra DS, Hruban RH, Zamboni G, Coban I, Adsay NV (2010) Preferential expression of MUC6 in oncocytic and pancreatobiliary types of intraductal papillary neoplasms highlights a pyloropancreatic pathway, in pancreatic carcinogenesis. Am J Surg Pathol 34:364–370
Acknowledgements
The authors thank Dr. Katsuhiko Uesaka, Director of the Department of Hepatobiliary Surgery in Shizuoka Cancer Center, for his support on the clinicopathological analysis of biliary tract carcinoma cases in Shizuoka Cancer Center, and also Dr. Jo Matsuoka, Research Support Center, Juntendo University Graduate School of Medicine, Tokyo, Japan, for his advice on statistical methods. The authors also thank Dr. R Wada, Professor of Juntendo Shizuoka Hospital, Department of Clinical Pathology; Dr. N. Tomita, Professor of Juntendo Urayasu Hospital, Department of Clinical Pathology; and Dr. T. Matsumoto, Professor of Juntendo Nerima Hospital, Department of Clinical Pathology, for providing us the opportunity to analyze IPMN cases resected at their hospitals.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Institutional review board approval was obtained from Shizuoka Cancer Center.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
None.
Rights and permissions
About this article
Cite this article
Fukumura, Y., Nakanuma, Y., Kakuda, Y. et al. Clinicopathological features of intraductal papillary neoplasms of the bile duct: a comparison with intraductal papillary mucinous neoplasm of the pancreas with reference to subtypes. Virchows Arch 471, 65–76 (2017). https://doi.org/10.1007/s00428-017-2144-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-017-2144-9