Abstract
Giant cell tumour of bone (GCTB) is a primary tumour of bone that may rarely, in the absence of malignant cytological features, produce metastatic lesions, most commonly in the lungs. Whether these lung nodules represent true neoplastic secondaries or implants derived from the primary tumour is not certain. In this study, we have analysed the morphological and immunophenotypic features of 19 conventional GCTBs and corresponding lung nodules for expression of macrophage, osteoclast, proliferation and tumour-associated markers. A striking morphological feature of all GCTBs that produced lung secondaries was the presence of large areas of haemorrhage and thrombus formation; mononuclear and multinucleated cells of GCTB were frequently found within these areas of haemorrhage and thrombus. A similar pattern of CD14, CD33, HLA-DR and CD51 expression was seen in macrophages and giant cells in primary and secondary tumours. Smooth muscle actin expression was frequently noted in primary GCTBs that recurred and metastasised. No difference was seen in the expression of p53, p63, Ki-67, cyclin D1 or Bcl-2 in primary and secondary tumours. Our findings suggest that most lung nodules associated with primary conventional GCTBs are implants derived from tumour emboli formed in areas of haemorrhage and thrombus formation within the primary tumour.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Giant cell tumour of bone (GCTB) is a primary tumour of bone characterised by the presence of numerous osteoclast-like giant cells and a mononuclear component that includes proliferating mononuclear stromal cells and infiltrating macrophages [1, 2]. GCTB has a variable and unpredictable course, in most cases behaving as a benign, but progressively enlarging lesion which can extend through the bone cortex into surrounding soft tissues. GCTB frequently recurs following curettage and can produce metastatic lesions, most commonly in the lungs in approximately 2–3% of patients [3–8].
Lung metastases of conventional GCTB are reported to grow slowly and show some morphological resemblance to the original primary tumour [1–3, 5, 6]. These tumours are distinct from lung secondaries of a primary GCTB which has undergone sarcomatous transformation and contains malignant tumour cells. Metastatic lung nodules associated with conventional GCTB can present at the time of initial surgery or develop many years after the appearance of the primary tumour. Although these lung nodules do not exhibit cytological features of malignancy, it is not certain whether they represent tumour implants rather than true neoplastic secondaries.
Radiologically aggressive (Enneking stage III) GCTBs are more prone to develop lung metastases, and these lung nodules develop more commonly in patients who have been treated for recurrent tumours [4–6, 9]. As a consequence, it has been suggested that the lung nodules could represent tumour emboli induced by repeated surgery [10]. Vascular invasion by GCTB has been reported in up to 30% of cases, although its precise incidence is unclear [2, 6, 10, 11], and no clear association has been established between vascular invasion in primary conventional GCTB and the development of lung nodules [2, 6, 9, 10].
In this study, we have analysed the morphological and immunophenotypic features of a large series of primary conventional GCTBs that metastasised to the lung, as well as corresponding features in the metastatic lung nodules. We have examined the expression of macrophage, osteoclast and stromal cell markers as well as expression of proliferation, apoptosis and tumour-associated antigens in these tumours. These findings have also been compared to a series of primary (non-recurrent/non-metastatic) GCTBs. Our aim has been to determine whether identification of specific morphological and immunophenotypic features in primary and metastatic GCTBs may provide a clue as to the pathogenetic mechanism which leads to lung metastasis in these tumours.
Materials and methods
Tumour specimens were obtained from Istituto Ortopedico Rizzoli, Bologna, Nuffield Orthopaedic Centre, Oxford and Royal Orthopaedic Hospital, Birmingham; ethical approval for analysis of the tissues from all these cases was obtained. Clinical details of these cases are shown in Table 1.
Morphological findings in haematoxylin–eosin-stained sections of 19 primary cases of conventional giant cell tumour which metastasised to the lung were examined. In 17 of these cases, histology of the lung nodules derived from these tumours was also available for examination. A tissue microarray (TMA) containing 70 sample cores (2 mm) of these primary and secondary GCTBs was made using a computer-guided instrument TMA Master (3DHistech Ltd, Budapest, Hungary), and sections of the TMA were cut and processed for immunohistochemistry. A TMA containing 80 sample cores of 40 primary GCTBs which had neither recurred nor metastasised was similarly made and processed.
The immunophenotypic features of primary and secondary tumours were studied using a panel of monoclonal antibodies directed against macrophage (CD14, human leucocyte antigen-DR (HLA-DR)), osteoclast (CD51), blood vessel endothelial cell (CD31, CD34, factor 8), lymphatic endothelial cell (podoplanin and LYVE-1), smooth muscle actin (SMA) and proliferation (Ki-67), apoptosis (bcl-2) and tumour-associated (cyclin D1 p53, p63,) markers. Immunohistochemistry was carried out using a NovoLink (Leica-NovoCastra) polymer peroxidise detection kit. Monoclonal antibodies used in this study are shown in Table 2. Immunostained TMA slides were taken to whole-slide scanning using a Mirax Scan (3DHistech), and spots as digital slides linked with patient data were scored and analysed. All markers were scored as weak (+), moderate (++) and strong (+++), depending on the number of positive mononuclear cells and their intensity, setting up threshold values as <5%, 5–20% and >20%, respectively (Table 3).
Results
Morphological findings in primary and secondary GCTBs
Morphologically, all primary and recurrent GCTBs which produced lung nodules contained both giant cell and mononuclear cell components typical of GCTB. Morphological features varied not only between tumours but also in different areas of the primary tumour. In general, osteoclast-like cells were numerous and widely scattered throughout the tumour. Focally, areas of spindle-shaped mononuclear stromal cells predominated and were associated with a variable degree of collagen formation; in some areas, proliferating stromal cells were disposed in a storiform pattern. Osteoid and/or woven bone formation by mononuclear stromal cells was seen, and spindle-shaped mononuclear cells showed variable, occasionally frequent typical mitotic activity (one to 15 mitotic figures per 10 high-power fields (HPF)); none of the tumours showed marked cytological atypia or features suggestive of malignancy. Aneurysmal bone cyst formation was seen in five of the tumours. The tumours were highly vascular and frequently contained dilated thin-walled blood vessels.
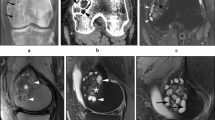
A prominent feature in all primary GCTBs which produced lung nodules was the presence of large areas of haemorrhage with formation of thrombus that in some cases was undergoing organisation and contained both giant cell and mononuclear components of the tumour (Fig. 1a, b). Vascular invasion at the periphery of the tumour was seen in three of the 19 tumours.
GCTB lung nodules were generally small. They contained a variable number of osteoclast-like giant cells and a proliferation of mononuclear cells which showed typical mitotic activity but no nuclear atypia (Fig. 1c). In general, the number of giant cells was fewer than in the primary tumours. Cytologically, the metastatic lung nodules were relatively bland, and there was no marked nuclear atypia. None of the lung nodules exhibited a high mitotic rate or showed cytological features of malignancy.
In the TMAs of the control group of 40 primary GCTBs which neither recurred nor metastasised, osteoclast-like giant cells were generally numerous, and there was a variable proliferation of mononuclear stromal cells and collagen within these tumours. The number of mitotic figures per 10 HPF was highly variable, with some tumours containing up to 10 mitotic figures per HPF. Small areas of haemorrhage were seen in 16 of these tumours, but none of the tumours contained large areas of haemorrhage; no evidence of thrombus formation was seen in any of the control group tumours.
Immunophenotypic features of primary and secondary GCTBs
Results of immunohistochemical studies are shown in Table 3. Mononuclear stromal cells in both primary and secondary GCTBs strongly expressed HLA-DR and SMA (Figs. 2 and 3). SMA expression by mononuclear cells was variable in primary GCTBs, but in some tumours, particularly recurrent GCTBs, SMA was strongly expressed. Frequently, the same degree of HLA-DR and SMA expression was seen in both primary tumours and lung nodules (Fig. 3). Expression of the proliferation-associated marker Ki67 and the proto-oncogene cyclin D1 was noted in mononuclear cells in both primary and secondary tumours. No difference was seen in the expression of p53, p63 and bcl-2 in primary and secondary tumours; no appreciable Her2 membrane staining for the epidermal growth factor receptor type 2 was noted in any of the cases tested.
As previously noted [12, 13], macrophage-like mononuclear cells in primary GCTB expressed CD14, CD33 and HLA-DR and the osteoclast-like giant cells in GCTB expressed CD51 and CD33 but did not express CD14 or HLA-DR. Fewer mononuclear cells expressed CD51 compared with CD14 and CD33. Mononuclear and multinucleated cells in lung nodules showed a similar pattern of expression of macrophage and osteoclast markers (Figs. 3 and 4).
Both primary and secondary tumours were highly vascular and contained numerous small blood vessels which were lined by endothelial cells that expressed CD31 and CD34. There was no expression of the lymphatic endothelial cell markers, LYVE1 or podoplanin in primary GCTBs.
An identical pattern of expression of HLA-DR, SMA, p53, p63 and bcl2 was seen in mononuclear stromal cells in the control group of 40 primary (non-metastatic, non-recurrent) GCTBs. Giant cells in these tumours, as expected, expressed an osteoclast phenotype (CD51+/CD33+/CD14−/HLA-DR−). A similar pattern of vascular endothelial marker expression was also noted in these tumours.
Discussion
It is known that the pulmonary metastases that develop in patients with conventional GCTBs are usually solitary or few in number and that these tumours grow slowly and have a long doubling time [4–7]. Surgically unresected lung metastases in such cases have been reported to remain stationary or even spontaneously regress without therapy [5, 6, 14]. These observations have been interpreted by some investigators to support the concept of “benign metastasis” whereby the lung nodules are thought to result from implantation of tumour emboli that have seeded to the lung from a primary GCTB [7, 10, 11]. The morphological and immunophenotypic findings in the primary GCTBs and corresponding lung nodules that we examined in this study would be in keeping with this pathogenetic mechanism of metastasis from a conventional GCTB.
A striking morphological feature of all the primary GCTBs which were associated with lung secondaries was the presence of numerous large areas of haemorrhage within the tumour. Morphologically, in all the cases of primary GCTB that we examined, there were areas of haemorrhage and thrombus formation; these thrombi were at various stages of organisation; in some early thrombi, there was prominent ingrowth of fibrous and granulation tissue into the thrombus, and both mononuclear stromal cells and giant cells were commonly seen in or around small vessels within this reparative tissue. These areas of haemorrhage and thrombus formation were distinct from blood-filled spaces associated with aneurysmal bone cyst change, which was a prominent feature in only a minority of the primary GCTBs we examined. In contrast to the group of primary GCTBs which had metastasised, the control group of primary (non-metastatic, non-recurrent) GCTBs did not show thrombus formation. There has been no systematic analysis of haemorrhage and thrombus formation in GCTB. Secondary changes, including areas of haemorrhage and ABC change, have been reported by some observers to be frequent and noted in up to 15% of cases [15].
Pathogenetic mechanisms which have been proposed for the formation of lung nodules that develop in the context of conventional GCTB include proliferation of a subpopulation of neoplastic cells which have metastatic potential in an otherwise benign or locally aggressive tumour and origin from tumour emboli derived from the primary GCTB [9–11, 14]. It has been noted that most metastatic GCTBs are detected a year or more after initial surgery to the primary [4, 6, 7, 14]. As surgery would result in haemorrhage and thrombus formation within the bone, it has been proposed that any residual GCTB not removed at the time of surgery could grow into this thrombus and then break off, in this way leading to an embolic metastasis in the lungs [10, 11].
Some support for this concept of benign metastasising GCTBs, initially proposed by Huvos [10], is provided by our immunophenotypic findings in which we found that the immunophenotype of mononuclear stromal cells in lung metastases of GCTB recapitulated that of the primary tumours from which they were presumably derived. Mononuclear stromal cells in both primary and secondary GCTBs showed a similar expression of SMA and HLA-DR. We also found no significant difference in the expression of proliferation, apoptosis and tumour-associated markers by mononuclear cells in primary GCTBs and the corresponding lung nodules which developed in these patients. p63 has been proposed as a specific marker for GCTB [16], and our findings show that it was expressed weakly by some but not all mononuclear cells in primary and secondary GCTBs. Most primary and secondary GCTBs are diploid [17]; only a minority of GCTB cases have been found to show cytogenetic abnormalities, and no clear consistent genetic abnormality has been identified in conventional GCTBs associated with lung metastasis [17–19]. Telomeric associations can be identified in most cases of GCTBs [20], and it has been proposed that disturbed telomere maintenance may play a role in the pathogenesis and behaviour of GCTBs. It has been concluded on the basis of an analysis of doubling time and flow cytometry data that most cases of GCTB and their pulmonary metastases are of spontaneous regression or growth cessation type. These metastatic tumours show less expression of the proliferation marker Ki-67 than continuous slow-growing and rapidly growing metastases. Other studies, however, have reported no consistent difference in the mitotic index [1, 2, 15] or in expression of proliferation and other tumour markers in primary and recurrent GCTBs associated with lung metastasis [21–25]. We noted that the immunophenotype of giant cells in primary (non-metastatic and metastatic) GCTBs and lung nodules was identical, indicating that the osteoclasts are essentially reactive cells recruited by receptor activator of nuclear factor-κB ligand-expressing mononuclear stromal cells [13]; the role of osteoclast specific proteases, such as cathepsin K [26], in lung nodule formation has not been determined, but it would be expected that this enzyme would be similarly expressed by osteoclasts in primary and secondary GCTBs.
The presence of organising thrombus and invasion of granulation tissue by mononuclear and giant cell components of GCTB was a consistent finding in the primary GCTBs which metastasised. This finding suggests that most lung nodules associated with primary conventional GCTB are implants derived from tumour emboli that originate from thrombi in areas of haemorrhage within the primary tumour. These tumour thrombi could propagate and grow into venous channels of small- to medium-sized arteries and thus account for the finding of tumour within vessels at the periphery of GCTBs. It is possible that, in some cases, thrombus-containing GCTB becomes detached and enters the circulation, resulting in one or more emboli that become implanted within the lungs. Such tumour implants would presumably form in the same way as they do in other benign/locally aggressive tumours that exhibit vascular invasion and are associated with lung nodule formation (e.g. chondroblastoma). No lymphatic vessels were found within primary tumours, thus indicating that lymphatic spread of GCTB does not occur.
Lung metastases of conventional GCTB have been shown to occur more commonly in aggressive tumours with soft tissue extension or local recurrence and are rarely seen at initial presentation, appearing on average 3.5 years after diagnosis [4, 5, 14]. True malignant GCTBs account for less than 5% of cases in most series and in 70% of cases are radiation-induced sarcomas [1–3]. Our finding that the lung nodules do not morphologically show malignant features and have the same immunophenotypic features as primary GCTBs accords with the suggestion of Huvos that these lesions may be iatrogenically produced following curettage [10]. Noting the presence of numerous large areas of haemorrhage within the primary tumour, in addition to the cytological features of the mononuclear cells, should be useful in the assessment of lung nodules that develop in GCTB.
References
Reid R, Bareyce SS, Sciot R (2002) Giant cell tumour. In: Fletcher C, Unni K, Mertens F (eds) Pathology and genetics of tumours of soft tissue and bone. IARC, Lyon, pp 310–312
Unni K, Inwards C, Bridge J et al (2005) Tumours of the bones and joints. AFIP Atlas of Tumour Pathology Fourth Series, AFIP, pp 281–298
Geshickter CF, Copeland MM (1930) Recurrent and so-called metastatic giant cell tumour. Arch Surg 20(5):714–755
Turcotte RE (2006) Giant cell tumour of bone. Orthop Clin N Am 32:35–51
Bertoni F, Present D, Sudanese A et al (1988) Giant cell tumour of bone with pulmonary metastases. Clin Orthop 237:275–285
Rock MG, Pritchard DJ, Unni KK (1984) Metastases from histologically benign giant-cell tumour of bone. J Bone Joint Surg 66N:269–273
Tubbs WS, Brown LR, Beabout JW et al (1992) Benign giant-cell tumour of bone with pulmonary metastases. AJR 158:331–334
Faisham WI, Zulmi W, Saim AH et al (2004) Pulmonary metastases of giant cell tumour of the bone. Med J Malaysia 59(Suppl F):78–81
Maloney WJ, Vaughan LM, Jones HH et al (1989) Benign metastasizing giant cell tumour of bone. Clin Orthop 243:208–215
Huvos AG (1991) Bone tumours: diagnosis, treatment and prognosis. Saunders, Philadelphia, pp 429–467
Mirra JM, Ulich T, Magidson J et al (1982) A case of probable benign pulmonary “metastases” or implants arising from a giant cell tumour of bone. Clin Orthop 162:245–254
Forsyth RG, De Boeck G, Baeide JJ et al (2009) CD33+ CD14− phenotype is characteristic of multinuclear osteoclast-like cells in giant cell tumour of bone. J Bone Miner Res 24:70–77
Lau YS, Sabokbar A, Gibbons CL et al (2005) Phenotypic and molecular studies of giant-cell tumours of bone and soft tissue. Hum Pathol 36:945–954
Bertoni F, Present D, Enneking WF (1985) Giant-cell tumour of bone with pulmonary metastases. J Bone Joint Surg 67A:890–900
Forest M (1988) Orthopaedic surgical pathology: diagnosis of tumours and pseudotumoral lesions of bone and joint. Churchill Livingstone, New York, pp 423–428
Dickson BC, Li SQ, Wunder JS et al (2008) Gene expression profiling identifies p63 as a diagnostic marker for giant cell tumour of the bone. Mod Pathol 21:531–539
Ladanyi M, Traganos F, Huvos AG (1989) Benign metastasizing giant cell tumours of bone. A DNA flow cytometric study. Cancer 64:1521–1526
Osaka S, Toriyama M, Taira K et al (1997) Analysis of giant cell tumour of bone with pulmonary metastases. Clin Orthop 335:253–261
Osaka S, Sugita H, Osaka E et al (2004) Clinical and immunohistochemical characteristics of benign giant cell tumour of bone with pulmonary metastases: case series. Orthop Surg 12:55–62
Forsyth RG, De Boeck G, Bekaert S et al (2008) Telomere biology in giant cell tumour of bone. J Pathol 214:555–563
de Souza PE, Paim JF, Carvalhais JN et al (1999) Immunohistochemical expression of p53, MDM2, Ki-67 and PCNA in central giant cell granuloma and giant cell tumour. J Oral Pathol Med 28:54–58
Ueda Y, Dockhorn-Dworniczak B, Blasius S et al (1993) Analysis of mutant p53 protein in osteosarcomas and other malignant and benign lesions of bone. J Cancer Res Clin Oncol 119:172–178
Rao UN, Goodman M, Chung WW et al (2005) Molecular analysis of primary and recurrent giant cell tumours of bone. Cancer Genet Cytogenet 158:126–136
Masui F, Ushigome S, Fujii K (1998) Giant cell tumour of bone: a clinicopathologic study of prognostic factors. Pathol Int 48:723–729
Sulh MA, Greco MA, Jiang T et al (1996) Proliferation index and vascular density of giant cell tumours of bone: are they prognostic markers? Cancer 77:2044–2051
Lindeman JH, Hanemaaijer R, Mulder A et al (2004) Cathepsin K is the principal protease in giant cell tumour of bone. Am J Pathol 165:593–600
Acknowledgement
The authors are members of EuroBoNet, a European Network of Excellence studying the pathology and genetics of bone tumours. The authors thank Chris Lowe for typing the manuscript.
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alberghini, M., Kliskey, K., Krenacs, T. et al. Morphological and immunophenotypic features of primary and metastatic giant cell tumour of bone. Virchows Arch 456, 97–103 (2010). https://doi.org/10.1007/s00428-009-0863-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-009-0863-2