Abstract
Sarcomatoid transformation was observed in 4.5% of autopsied cases of intrahepatic cholangiocarcinoma. Here, we report a case of intrahepatic sarcomatoid cholangiocarcinoma with round cell feature, extremely rare variant. An 87-year-old man was incidentally found to have a tumor in the left lobe of the liver by abdominal computed tomography scans. The patient was clinically diagnosed to have intrahepatic cholangiocarcinoma and received palliative care without specific treatment. He died of hepatic insufficiency 3 months after the diagnosis. The autopsied liver specimens showed a reddish gray tumor of 4.0×2.8 cm in size. Histologically, the tumor was centrally hemorrhagic and necrotic and was composed of tubular adenocarcinoma and a round cell component, which has an eccentrically located nucleus and eosinophilic cytoplasm without mucin production. Immunohistochemically, the adenocarcinoma cells expressed cytokeratin 19 and β-catenin in their cytoplasm, with E-cadherin and CD44s at the plasma membrane. In the round cells, cytokeratin 19 and vimentin was detected in their cytoplasm and CD44s at the plasma membrane. E-cadherin immunoreactivity was weakly present in their cytoplasm and β-catenin was negative. Loss or reduction of the E-cadherin and β-catenin expressions and overexpression of CD44s in the round cells are suggested to be contributed to the high propensity for lymphatic permeation and poor prognosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sarcomatoid transformation has been observed in carcinomas in various organs. In the liver, sarcomatoid transformation was present in 3.9% (14 of 355) to 9.4% (55 of 579) of hepatocellular carcinoma (HCC) autopsy cases [10, 11] and 4.5% (7 of 155) of cholangiocarcinoma cases [17]. The sarcomatoid components usually show spindle-shaped cells, pleomorphic cells or multinucleated giant cells in previously reported cases of intrahepatic sarcomatoid cholangiocarcinoma (ISC) [5, 6, 8, 9, 14, 16, 17, 20, 21] and combined hepatocellular and cholangiocellular carcinoma with sarcomatoid changes [5, 15, 18]. However, intrahepatic cholangiocarcinoma (ICC) with round cell feature is extremely rare [16].
Cell adhesion molecules are considered to be involved in tumor invasion and metastasis. Cadherins are Ca2+-dependent cell–cell adhesion molecules that interact with catenins, which are submembranous cytoplasmic proteins termed α-, β-, and γ-catenin [19]. Reduced expressions of E-cadherin, which are constantly expressed in epithelial cells, and catenins have been reported in many cancers. In cholangiocarcinoma, down-regulated expressions of E-cadherin and β-catenin have been reported to be significantly correlated with high grade tumors but not with vascular invasion and metastasis [2].
CD44 standard (CD44s) is a cell adhesion molecule, specifically a transmembrane glycoprotein that functions as a hyaluronate receptor, which may modulate cell–cell or cell–matrix interactions [23]. Aberrant expressions of CD44 have been observed in a variety of carcinomas [23]. In the bile duct, normal epithelial cells never express CD44s, even weakly or focally, in a nonspecific manner [2, 23, 24]. There have been a few reports of aberrant CD44s expressions in bile duct carcinoma cells, although no relationships of the CD44s immunoreactivity with the tumor grade, vascular invasion, and metastasis were noted [2, 24].
To date, no studies have investigated the above-described molecules in cholangiocarcinoma with sarcomatoid transformation. Here, we described a case of ISC of round cell variant and examined the differences between the immunohistochemical profiles of the tubular adenocarcinoma and the round cells, with particular focus on E-cadherin, β-catenin, and CD44s.
Clinical history
An 87-year-old man was incidentally found to have elevated ductal enzyme levels. His family history was unremarkable. He had never suffered from liver disease. Laboratory data revealed a moderately elevated serum level of carcinoembryonic antigen (16.2 ng/ml) and a highly elevated level of CA19-9 (2,894 U/ml). The serum level of alpha-fetoprotein was within the normal range. Hepatitis B surface antigen and anti-hepatitis C virus antibody in the serum were negative. Abdominal computed tomography (CT) scans showed a tumor in the left lobe of the liver. The patient was clinically diagnosed to have ICC. He received palliative care without surgery or chemotherapy and died of hepatic insufficiency and bronchopneumonia 3 months after the diagnosis. An autopsy was performed 1 h after death.
Materials and methods
The autopsy liver specimens were fixed in 10% neutral-buffered formalin and embedded in paraffin. Tissue sections, 4 μm in thickness, were used for hematoxylin and eosin (HE) staining, special staining, and immunohistochemical examination. Primary antibodies used in this study are listed in Table 1. Detection was achieved with automated immunostainer (Ventana Medical System, Tucson, AZ, USA).
Ultrastructural analysis was performed for autopsied liver specimens which is fixed in 10% neutral-buffered formalin, postfixed in 2% osmium tetroxide, dehydrated, and embedded in epoxy resin. Ultrathin sections were examined under electron microscope (JEM1200EXII, Japan Electron Optics Laboratory, Tokyo, Japan).
DNA extracted from submicrodissected paraffin-embedded tissue was analyzed by polymerase chain reaction-single strand conformational polymorphism (PCR-SSCP) to screen for mutations in exons 5–8 of the p53 gene. The primers used for PCR amplification were as follows: exon 5 (5′-TGTTCACTTGTGCCCTGACT-3′ and 5′-CAGCCCTGTCGTCTCTCCAG-3′), exon 6 (5′-CCCAGGCCTCTGATTCCTCA-3′ and 5′-CAACCACCCTTAACCCCTCC-3′), exon 7 (5′-ACTGGCCTCATCTTGGGCCT-3′and 5′-TGTGCAGGGTGGCAAGTGGC-3′), and exon 8 (5′-TAAATGGGACAGGTAGGACC-3′and 5′-TCCACCGCTTCTTGTCCTGC-3′), respectively.
Results
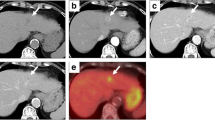
At autopsy, the gross appearance of the tumor, which measured 4.0×2.8 cm, was reddish gray to tan in color with massive hemorrhagic necrosis (Fig. 1). Microscopically, the tumor was mostly necrotic and viable tumor cells were only identified in the periphery. The tumor was composed of two different components: moderately differentiated tubular adenocarcinoma and round cell elements (Fig. 2a). In the former portion, conglutinated atypical glands had proliferated with a fibrous stroma (Fig. 2b). In the latter portion, noncohesive round cells showed sarcomatoid appearances with eccentrically located nuclei and abundant eosinophilic cytoplasm (Fig. 2c). Multinucleated giant cells were scattered throughout the area (Fig. 2d). Round cells showed numerous lymphatic invasions into the liver and direct invasion into the gallbladder wall. No HCC component was detected. Lymph node metastasis, which was detected at the hepatic hilum and around the common bile duct, had resulted in obstructive jaundice. Metastatic nodules of round cells were identified in the bilateral adrenal glands. There was no evidence of cirrhosis, hepatolithiasis, or sclerosing cholangitis in the background liver. Mucin production of round cells was not detected in periodic acid Schiff (PAS), mucicarmine, and alcian blue stainings.
Histological appearances of the tumor (Hematoxylin and eosin staining). a Tubular adenocarcinoma (lower right) and sarcomatoid components with round feature (upper left) are observed in the periphery, ×100. b Tubular adenocarcinoma composed of small atypical glands, ×200. c Sarcomatoid round cells are noncohesive and have eccentric large vesicular nuclei, prominent nucleoli, and abundant paranuclear eosinophilic cytoplasm, ×400. d Multinucleated giant cells are shown, ×400
The immunohistochemical profiles of the two types of tumor cells are summarized in Table 1. The round cells were positive for cytokeratin (CK) 19 (Fig. 3a) and vimentin, but CK 7 expression was reduced. E-cadherin expression was lost at the membrane and weakly found in the cytoplasm (Fig. 3b), while no immunoreactivity for β-catenin was detected (Fig. 3c). The round cells in the lymphatic vessels and stromal invasive sites demonstrated marked CD44s expression at the membrane (Fig. 3d). The tubular adenocarcinoma cells expressed CK19 (Fig. 3e) and β-catenin (Fig. 3g) in their cytoplasm, with E-cadherin (Fig. 3f) and CD44s (Fig. 3h) at the plasma membrane. Round cells and multinucleated giant cells presented immnoreactivity for mitochondria densely in the paranuclear cytoplasm. Nuclear expression of p53 protein was found in the adenocarcinoma cells. No specific expression of rhabdomyogenic markers was found for desmin, myoglobin, myogenin, or MyoD1. Electron micrographs revealed accumulation of large mitochondria in the cytoplasm of the round cells and multinucleated giant cells (Fig. 4). No band shifting was detected in exons 5–8 of the p53 gene by PCR-SSCP analysis in both adenocarcinoma and round cell components.
Immunohistochemical findings of the tumor cells, ×400. The round cells express CK19 in the cytoplasm (a) and E-cadherin weakly in the cytoplasm (b), but no β-catenin expression is detected (c). The round cells in the lymph vessels express CD44s at the plasma membrane (d). The tubular adenocarcinoma cells express CK19 in the cytoplasm (e), E-cadherin at the membrane around the cell (f), β-catenin weakly in the cytoplasm (g) and CD44s at the lateral plasma membrane (h)
Discussion
ISC is an uncommon variant of ICC and sarcomatoid component exhibited spindle-shaped cells in almost previously reported cases [5, 8, 15, 16, 20, 21]. Furthermore, ISC of round cell feature is very rare. Nakajima et al. [16] report a case of ICC comprised papillary carcinoma and round cells. Sasaki et al. [20] described a case of ISC composed of adenosquamous carcinoma, spindle-shaped cells, multinucleated giant cells, and round cells resembling rhabdomyosarcoma cells. Because the round cells of the present case apparently seemed to be rhabdomyosarcoma cells, displayed no mucin in their cytoplasm, and expressed both cytokeratins and vimentin, we considered this tumor as a sarcomatoid carcinoma. However, the round cells were not rhabdomyosarcoma or rhabdoid cells, as myogenic markers were not expressed by immunohistochemistry, and a whorl of intermediate filaments was not confirmed by ultrastructural study. Electron micrographs confirmed that the accumulation of mitochondria was attributable to the eosinophilic paranuclear cytoplasm. ICC with rhabdoid phenotype was reported by Honda et al. [7] and Lim et al. [12] but made no mention of ultrastructural findings.
Sarcomatous transformation occasionally appears in HCC cases treated with anticancer therapy, particularly, transcatheter arterial embolization (TAE) [11]. The present tumor was distinguished from HCC due to its negative immunoreactivity for an anti-hepatocyte antibody (OCH1E5). There have been no previous reports concerning the relationship between ISC and anticancer therapy. The previously reported cases of a round cell variant ICC and the present case had received neither TAE nor chemotherapy. It is, therefore, reasonable to consider that sarcomatoid transformation is a biologically natural course of tumor progression rather than a secondary event due to artificial treatment.
In ISC, sarcomatoid component usually coexists with cholangiocarcinoma component. The previously reported cases of ISC showed cytokeratin expressions in both the adenocarcinoma and sarcomatoid components [5–7, 9, 15, 20, 21]. In the present case, immunohistochemical positivity for CK19, which is restricted to the biliary epithelium within the liver, was detected in both the adenocarcinoma component and round cells. Therefore, the round cell component can reasonably be considered to have transformed from the cholangiocarcinoma cells. Differences in the immunoreactivities for CK7, vimentin, and CA19-9 between adenocarcinoma and round cells suggest that alterations in genetic expressions and phenotypic changes occur during sarcomatoid transformation. By immunohistochemistry, the expression of p53 protein was different between adenocarcinoma and round cells, but no mutation was detected in exons 5–8 of the p53 gene in both components.
Normal bile duct epithelial cells show immunoreactivities for E-cadherin and β-catenin on the plasma membrane [2]. Reductions of these molecules were detected in cholangiocarcinoma and correlated with high-grade character [2]. The expressions of E-cadherin and β-catenin contribute to the maintenance of the normal morphology and polarity of bile ducts [2]. In the present case, immunohistochemical staining for E-cadherin revealed diffuse membranous expression in the adenocarcinoma component and weak cytoplasmic expression in the round cell component. The β-catenin immunoreactivity was weakly positive in the adenocarcinoma component and negative in the round cells. Loss of the membranous expression and cytoplasmic localization of E-cadherin have been reported to be associated with functional and structural disorders [22]. Loss of the membranous expressions of E-cadherin and β-catenin may play an important role in disruption of the histologic architecture and aggressive behavior for invasion and metastasis of the round cell component.
CD44s is aberrantly expressed at the membrane of cholangiocarcinoma cells [2]. However, its roles in tumor invasion and lymph–vascular penetration have not been fully elucidated. In HCC, upregulation of CD44 was related to vascular invasion [13]. In contrast, reduced or loss of CD44 expression is associated with lymph-vascular permeation in invasive micropapillary carcinoma of the breast, endometrial carcinoma, and colon carcinoma [1, 3, 4]. In the present case, the round cells showed a distinct membranous expression of CD44s. The CD44s immunoreactivity was more intense in the round cells than in the adenocarcinoma cells. The results suggest that aberrant overexpression of CD44s may be involved in marked lymph–vascular invasion by sarcomatoid cholangiocarcinoma cells as has been reported for HCC.
In conclusion, ISC with a round cell phenotype is an aggressive variant of ICC. Loss or reduction of the membranous expressions of E-cadherin and β-catenin and upregulation of CD44s in the round cell component may be associated with the high propensity for lymph–vascular permeation.
References
Asao T, Nakamura J, Shitara Y, Tsutsumi S, Mochiki E, Shimura T, Takenoshita S, Kuwano H (2000) Loss of standard type of CD44 expression in invaded area as a good indicator of lymph-node metastasis in colorectal carcinoma. Dis Colon Rectum 43:1250–1254
Ashida K, Terada T, Kitamura Y, Kaibara N (1998) Expression of E-cadherin, alpha-catenin, beta-catenin, and CD44 (standard and variant isoforms) in human cholangiocarcinoma: an immunohistochemical study. Hepatology 27:974–982
Fujita N, Yaegashi N, Ide Y, Sato S, Nakamura M, Ishiwata I, Yajima A (1994) Expression of CD44 in normal human versus tumor endometrial tissues: possible implication of reduced expression of CD44 in lymph-vascular space involvement of cancer cells. Cancer Res 54:3922–3928
Gong Y, Sun X, Huo L, Wiley EL, Rao MS (2005) Expression of cell adhesion molecules, CD44s and E-cadherin, and microvessel density in invasive micropapillary carcinoma of the breast. Histopathology 46:24–30
Haratake J, Horie A (1991) An immunohistochemical study of sarcomatoid liver carcinomas. Cancer 68:93–97
Haratake J, Yamada H, Horie A, Inokuma T (1992) Giant cell tumor-like cholangiocarcinoma associated with systemic cholelithiasis. Cancer 69:2444–2448
Honda M, Enjoji M, Sakai H, Yamamoto I, Tsuneyoshi M, Nawata H (1996) Case report: intrahepatic cholangiocarcinoma with rhabdoid transformation. J Gastroenterol Hepatol 11:771–774
Imazu H, Ochiai M, Funabiki T (1995) Intrahepatic sarcomatous cholangiocarcinoma. J Gastroenterol 30:677–682
Kaibori M, Kawaguchi Y, Yokoigawa N, Yanagida H, Takai S, Kwon AH, Uemura Y, Kamiyama Y (2003) Intrahepatic sarcomatoid cholangiocarcinoma. J Gastroenterol 38:1097–1101
Kakizoe S, Kojiro M, Nakashima T (1987) Hepatocellular carcinoma with sarcomatous change. Clinicopathologic and immunohistochemical studies of 14 autopsy cases. Cancer 59:310–316
Kojiro M, Sugihara S, Kakizoe S, Nakashima O, Kiyomatsu K (1989) Hepatocellular carcinoma with sarcomatous change: a special reference to the relationship with anticancer therapy. Cancer Chemother Pharmacol 23 Suppl:S4–S8
Lim BJ, Kim KS, Lim JS, Kim MJ, Park C, Park YN (2004) Rhabdoid cholangiocarcinoma: a variant of cholangiocarcinoma with aggressive behavior. Yonsei Med J 45:543–546
Mathew J, Hines JE, Obafunwa JO, Burr AW, Toole K, Burt AD (1996) CD44 is expressed in hepatocellular carcinomas showing vascular invasion. J Pathol 179:74–79
Matsuo S, Shinozaki T, Yamaguchi S, Takami Y, Obata S, Tsuda N, Kanematsu T (1999) Intrahepatic cholangiocarcinoma with extensive sarcomatous change: report of a case. Surg Today 29:560–563
Nakajima T, Kubosawa H, Kondo Y, Konno A, Iwama S (1988) Combined hepatocellular-cholangiocarcinoma with variable sarcomatous transformation. Am J Clin Pathol 90:309–312
Nakajima T, Kondo Y, Miyazaki M, Okui K (1988) A histopathologic study of 102 cases of intrahepatic cholangiocarcinoma: histologic classification and modes of spreading. Hum Pathol 19:1228–1234
Nakajima T, Tajima Y, Sugano I, Nagao K, Kondo Y, Wada K (1993) Intrahepatic cholangiocarcinoma with sarcomatous change. Clinicopathologic and immunohistochemical evaluation of seven cases. Cancer 72:1872–1877
Papotti M, Sambataro D, Marchesa P, Negro F (1997) A combined hepatocellular/cholangiocellular carcinoma with sarcomatoid features. Liver 17:47–52
Pignatelli M, Vessey CJ (1994) Adhesion molecules: novel molecular tools in tumor pathology. Hum Pathol 25:849–856
Sasaki M, Nakanuma Y, Nagai Y, Nonomura A (1991) Intrahepatic cholangiocarcinoma with sarcomatous transformation: an autopsy case. J Clin Gastroenterol 13:220–225
Shimada M, Takenaka K, Rikimaru T, Hamatsu T, Yamashita Y, Kajiyama K, Taguchi K, Shirabe K, Sugimachi K (2000) Characteristics of sarcomatous cholangiocarcinoma of the liver. Hepatogastroenterology 47:956–961
Shiozaki H, Tahara H, Oka H, Miyata M, Kobayashi K, Tamura S, Iihara K, Doki Y, Hirano S, Takeichi M, Mori T (1991) Expression of immunoreactive E-cadherin adhesion molecules in human cancers. Am J Pathol 139:17–23
Sneath RJ, Mangham DC (1998) The normal structure and function of CD44 and its role in neoplasia. Mol Pathol 51:191–200
Washington K, Telen MJ, Gottfried MR (1997) Expression of cell adhesion molecule CD44 in primary tumors of the liver: an immunohistochemical study. Liver 17:17–23
Acknowledgements
The authors thank Ms. Yumi Tsubata (Department of Pathology, Kanazawa Medical University Hospital) for immunohistochemistry and Ms. Yoshiiku Okanemasa (Department of Pathology, Kanazawa Medical University Hospital) for electron micrographs.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sato, K., Murai, H., Ueda, Y. et al. Intrahepatic sarcomatoid cholangiocarcinoma of round cell variant: a case report and immunohistochemical studies. Virchows Arch 449, 585–590 (2006). https://doi.org/10.1007/s00428-006-0291-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-006-0291-5