Abstract
Gastric carcinomas (GCs) with high microsatellite instability (MSI) or an Epstein–Barr virus (EBV) infection are prevalently poorly differentiated adenocarcinomas with abundant lymphoid infiltration. The aims of the study were to clarify (1) if tumour-infiltrating lymphocytes (TILs) and cytotoxic-activated TILs are associated with a better clinical outcome in patients with GCs characterised for the presence of MSI and EBV; (2) if the nature and the activation status of TILs are involved in tumour cell apoptosis, evaluated using the M30 antibody, directed against a fragment of cytokeratin-18 caspase-cleaved during early steps of epithelial cell apoptosis. The immunophenotype of TILs and the tumour cell apoptosis were analysed with immunohistochemistry in 96 GCs, including 35 MSI GCs, and 61 GCs without MSI [microsatellite stable (MSS)], 17 of which were EBV+. MSI and MSS/EBV+ GCs displayed a significantly higher mean number of cytotoxic-activated TILs and apoptotic tumour cells than MSS/EBV− GCs (CD8+ TILs/HPF, 21.7 and 69.6 vs 6.4; T-cell intracellular antigen (TIA)-1+ TILs/HPF, 16.7 and 32.05 vs 5.2; granzyme B+ TILs/HPF, 7.5 and 8.6 vs 0.8; perforin+ TILs/HPF, 5.9 and 9.2 vs 0.9; and M30 IR tumour cells, 5.9 and 2.9 vs 2.3%). In addition to the most reliable clinico-pathological parameters (lymph node status, depth of tumour invasion and tumour stage), a univariate analysis showed that the presence of CD3+ TILs higher than 14.9 (p=0.01), CD8+ TILs higher than 9.5 (p<0.05) and MSI (p=0.02) were associated with better overall patient survival. Using a Cox regression model, only a high number of CD3+ TILs (p=0.02) and a low tumour stage (p=0.00001) were identified as independent prognostic factors. In conclusion, our study demonstrates that a high number of CD3+ and CD8+ TILs is a characteristic of MSI- and EBV-associated GCs and represents a favourable prognostic factor, independently of the pathogenesis of GCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past 10 years, accumulating evidence has indicated that a pronounced intratumour inflammatory reaction plays a crucial role in host response to cancer. Various inflammatory elements (dendritic cells, macrophages, granulocytes and lymphocytes) have been found in the tumour micro-environment, both in supporting stroma and among epithelial cells in tumour nests.
The presence of tumour-infiltrating lymphocytes (TILs) has been shown to correlate with a favourable prognosis in several human malignancies such as melanoma and ovarian, prostatic, renal-cell, breast and colorectal carcinomas [12, 25, 27, 38, 42]. The presence of abundant lymphocytic tumour infiltration is a peculiar histological feature common to gastric carcinomas (GCs), both with Epstein–Barr virus (EBV) infection [4, 28, 37] and with a high level of microsatellite instability (MSI) [4, 34].
The immune response to cancer cells may be mediated by non-specific natural killer (NK) cells [30] or by cancer-specific lymphocytes, which are usually CD8-positive [2, 31].
In GCs, a high level of NK infiltration was significantly associated with a better prognosis of disease [14].
In MSI colorectal carcinomas, the intratumour lymphocytes have been demonstrated to be prevalently cytotoxic-activated T cells [7]. Their presence could be responsible for the increased apoptosis of tumour cells observed in these tumours, although Michael-Robinson et al. [23] demonstrated that TILs and apoptosis are independent characteristics of MSI tumours.
In the present investigation, we studied a series of GCs characterised for MSI status and EBV infection with the aims of clarifying whether TILs are associated with a better clinical outcome in GC patients and of defining the nature and the activation status of TILs in the different subtypes of GCs.
Materials and methods
Case selection
The study included 96 cases of sporadic advanced GCs (Table 1) operated on at the University Hospital of Varese between January 1980 and June 1999. The majority of the tumours were from a series of 185 cases which had previously been examined for the presence of high MSI, expression of hMSH2 and hMLH1 proteins and EBV infection with in situ hybridisation for EBER-1 [4]. In particular, 35 MSI and 31 microsatellite stable (MSS) GCs were selected for the presence of abundant lymphoid infiltration, while 20 cases, showing a less abundant lymphoid infiltrate, were selected from the remaining consecutive MSS glandular carcinomas. Ten other cases were added to the series because they were known to be EBV-positive. Altogether, 35 cases were MSI, and 61 were MSS carcinomas, 17 of which were positive for EBV.
The tumours were classified, according to the criteria outlined by Carneiro et al. [3], as glandular, solid or mixed types and according to the Lauren criteria [19]. The tumour stage was assessed using the tumour node metastases (TNM) system defined by the International Union Against Cancer [35]. The clinico-pathological data of the GCs, characterised for MSI status and EBV infection, are summarised in Table 1.
All patients were followed-up either until death or for a median period of 150 months (range 42–238 months); information was obtained from the Lombardy Tumour Register and from the record offices of local authorities. The mean overall follow-up was 64.3 months (range 0–238).
Immunohistochemical study
The characterisation of TILs was performed on formalin-fixed, paraffin-embedded materials using the avidin–biotin–peroxidase complex (ABC) methods. Briefly, sections were deparaffinised, rehydrated and, for selected antigens, pre-treated with different antigen-retrieval solutions in a domestic 750-kW microwave oven (see Table 2). Endogenous peroxidase activity was quenched in 3% H2O2 in water for 10 min. Primary antibodies (listed in Table 2) were applied overnight at 4°C. Sections were then incubated with biotinylated anti-mouse immunoglobulins and with ABC peroxidase complex, each for 1 h at room temperature.
Immunoreactive intraepithelial lymphocytes were counted at 400× (Leitz, Laborlux K; field area 0.173 mm2) in ten consecutive fields, selecting areas containing the maximal number of neoplastic cells with minimal reactive stroma and necrosis. The mean value of immunoreactive TILs per high-power field was reported. Only the immunoreactive lymphocytes in direct contact with tumour cells were included in the count.
The apoptotic index was expressed as the percentage of M30 immunoreactive cells per 2,000 tumour cells counted in the ten most positive fields at 200×. Areas of intraglandular or superficial necrosis were not considered for the evaluation of the apoptotic index.
Statistical analysis
The statistical significance of the results was evaluated using the Wilcoxon rank-sum test for unpaired data, chi-square test and Fisher's Exact Test. The correlation of patient survival with the immunohistochemical and clinico-pathological data was estimated using the Kaplan–Meier product limit method, and statistical differences were tested using the log-rank test. A multivariate analysis was performed with the Cox proportional-hazard regression model.
Results
Clinical and pathological features
The clinico-pathological data of the GCs are summarised in Table 1. There were slightly more males (male to female ratio 2.4:1) with MSS/EBV+ cancers compared to MSI and MSS/EBV− cancers (male to female ratios 1.5:1 and 1.2:1, respectively).
Both MSI and MSS/EBV− carcinomas were more frequent in the antrum (77 and 68%, respectively), whereas MSS/EBV+ carcinomas were prevalently located in the gastric fundus (47%) and in the gastric stump (29%) (p<0.001).
Tumour size varied in diameter from 20 to 100 mm. The mean diameters were 58.6, 60.3 and 39.7 mm for MSI, MSS/EBV+ and MSS/EBV− carcinomas, respectively.
Histologically, 64% of all tumours were glandular carcinomas. In particular, 74% of MSI and all the MSS/EBV+ cases were poorly differentiated, whereas 61% of MSS/EBV− were moderately differentiated carcinomas (Fig. 1). According to Lauren's criteria [19], 80% of tumours were intestinal, and only 4% were diffuse GCs; 16% of cases were unclassified.
At diagnosis, MSS/EBV− and MSS/EBV+ carcinomas showed lymph node metastases in 31 of 43 (72%) and 14 of 17 (82%) cases, whereas they were present in only 18 of 35 (51%) MSI carcinomas (p<0.05).
Fifty (52%) patients died of disease after an average time of 32.5 months (range 2–181), including 14 (40%) patients with MSI, 27 (62%) with MSS/EBV− and 9 (53%) with MSS/EBV+ carcinomas.
Thirty (31%) patients died of other causes (mean follow up 46.5 months, range 0–212), and only 16 patients (17%) were still alive at the last follow-up after a mean time of 150 months (range 42–238).
The survival curves (Fig. 2) showed a significantly different prognosis in the three groups (p<0.05). In particular, the overall survival was significantly higher for patients with MSI than with MSS/EBV− GCs (p=0.01). The mean 5-year survival was 59, 46 and 32% for MSI, MSS/EBV+ and MSS EBV− GCs, respectively.
Characterisation of lymphoid infiltration
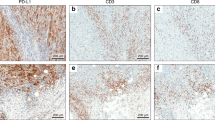
Lymphocytes were present both within tumour-cell nests and in peritumoural stroma. CD3+ intraepithelial TILs varied in number from 0 to 254 in a high-power microscopic field (mean value 31.6, median value 14.9).
The mean number of CD3+ TILs was 30.7 (range 1.6–77), 6.6 (range 0–30.4) and 100 (range 43.3–254) in MSI, MSS/EBV− and MSS/EBV+, respectively, and the differences were statistically significant (p<0.001; Fig. 3).
The nature and the activation status of TILs were analysed in a subset of GCs, selected because sufficient material was available for study, and the immunohistochemical results are summarised in Table 3. The majority of TILs showed an intense CD8 immunoreactivity. CD8+ TILs varied from 0.3 to 118.7 cells per field, the mean value being 25.5 and the median value being 14.6. The mean numbers of CD8+ TILs in MSI (21.7; range 2.1–56) and in MSS/EBV+ (69.6; range 26–118.7) GCs were significantly higher (p<0.001) than those found in MSS/EBV− cases (6.4; range 0.3–23.7).
A minimal number of TILs was composed of NK cells. The number of CD57+ TILs was significantly higher (p<0.01) in MSS/EBV+ (mean value 4.2) than in MSS/EBV− (mean value 0.8) or in MSI (mean value 3.1) GCs.
The presence of cytotoxic cells was confirmed by the expression of T-cell intracellular antigen (TIA-1) in the cytoplasmic granules in a considerable proportion of TILs. The mean number of TIA-1+ TILs was significantly higher in MSS/EBV+ (32.05; range 3–84.6) than that observed in MSS/EBV− (5.2; range 0–29.1; p<0.001) and MSI (16.7; range 0.4–59.5; p<0.05) GCs.
The activation status of TILs was assessed on the basis of immunoreactivity for granzyme B and perforin. The number of granzyme B+ TILs (Fig. 4) was significantly higher (p<0.001) in MSI and in MSS/EBV+ than in MSS/EBV− GCs (mean values 7.5 and 8.6, respectively, vs 0.8). Perforin+ TILs were also significantly more numerous in MSI GCs and in MSS/EBV+ than in MSS/EBV− tumours (mean values 5.9 and 9.2, respectively, vs 0.9; p<0.001 and p<0.05).
Apoptosis of tumour cells
To assess the apoptotic index of tumour cells, GCs were immunostained with the M30 CytoDEATH antibody that recognises a fragment of cytokeratin-18 cleaved by a caspase at the beginning of the apoptotic process. Tumour cells showed an intense and variable cytoplasmic M30 immunoreactivity in relation to the apoptotic stage of the cell (Fig. 5). At the beginning of the apoptotic process, in cells which were still histologically normal, the immunoreactivity was distributed throughout the cytoplasm, whereas in damaged cells, the immunoreactivity was localised in granules, which varied in size and number, within the cytoplasm.
The distribution of M30 immunoreactive tumour cells differed among the various tumours; in some cases, M30+ cells were dispersed throughout the tumour, while in other cases, they were localised near necrotic areas or within the glandular lumens. The mean percentage of M30+ cells varied from 0.05 to 29.2% (Table 3). M30+ cells were more frequent in MSI and in MSS/EBV+ than in MSS/EBV− GCs (mean percentages 5.9 and 2.9, respectively, vs 2.3; p<0.01).
Necrotic areas and intraglandular abscesses, frequently observed in MSS/EBV+ GCs, were always M30-negative.
Correlation with survival
A univariate analysis (Table 4) revealed that the low-tumour stage (p<0.0001), the absence of node metastases (pN, p<0.0001), the low depth of tumour invasion (pT, p<0.0003), the intestinal type (p=0.04), the presence of MSI (p=0.02) and high numbers of CD3+ TILs and CD8+ TILs correlated with survival. In particular, carcinomas with CD3+ TILs higher than 14.9 (Fig. 6) and CD8+ TILs higher than 9.5 (Fig. 7) were associated with a significantly improved survival (p=0.01 and p<0.05, respectively).
The presence of intratumoural CD8+-activated lymphocytes (perforin >1.5 and granzyme B >1.75) was also statistically significant (p<0.01).
On the basis of a Cox regression analysis, only a low tumour stage (p<0.00001) and a high number of CD3+ TILs (p=0.02) were identified as independent prognostic factors (Table 5).
Discussion
Abundant TILs seem to be associated with a more favourable prognosis in various malignancies, including melanomas and breast, renal, colorectal and GCs [12, 25, 27, 38, 42]. Tumour-associated lymphocytes show oligoclonal expansion [13], exhibit tumour-specific cytotoxic activity in vitro [32] and recognise tumour-specific antigens [17, 29, 33].
The prognostic significance of lymphocytic infiltration in gastric cancer was first emphasised by MacCarty and Mahle [21]. In 1976 Watanabe and colleagues [39] observed a high survival rate among patients with GCs with lymphoid stroma. With the aim of evaluating the prognostic significance of TILs, we selected, from a series of consecutive GCs, those showing abundant lymphoid infiltration. The MSS/EBV− and MSS/EBV+ groups were enriched in number to be better comparable. Our study, with the limit that our cases were partly selected, seems to indicate that the presence of intratumoural T cells correlates with the clinical outcome of advanced gastric cancer after apparently radical surgery. In a series of 96 patients with advanced GCs, cancer-specific survival of patients appeared to be increased by a factor of approximately 2 if more than 14.9 CD3+ TILs or more than 9.5 CD8+ TILs were present per high-power microscopic field. A univariate analysis showed that, in addition to clinico-pathological parameters including stage, lymph node status, depth of tumour invasion, histological type and MSI status, TIL count was significantly correlated with patient survival. A Cox regression analysis confirmed TILs as an independent prognostic factor together with stage.
Two types of GCs are characterised by a large number of TILs: the MSI- and the EBV-associated GCs [16, 28, 34, 37].
Like patients with sporadic MSI colorectal cancers [8, 11, 40], patients with sporadic MSI GCs apparently have a better survival rate than patients with MSS tumours [34, 41]. EBV-positive GCs have sometimes been associated with a survival advantage [5, 16, 36]. However, in 1994, Nakamura et al. [26] observed that, among GCs with lymphoid stroma, there was no significant difference in the 5-year survival rate between cases with EBV-positive and EBV-negative GCs.
The molecular characteristics of tumours that elicit an enhanced immune response are unclear, but the increased production of abnormal peptides seems to be the more probable hypothesis to explain this phenomenon. In patients with MSI colorectal carcinomas with abundant T-cell infiltration, Ishikawa et al. [15] demonstrated the production of specific antibodies against an abnormal fragment of CDX2 protein found in tumour tissue. The aberrant protein is produced by a frameshift mutation in the microsatellite sequence of the CDX2 coding region. This study seems to support the hypothesis that, in MSI tumours, the alteration of the mismatch repair system is responsible for the production, by tumour cells, of abnormal tumour-specific peptides which recruit lymphocytes in the tumour and induce an immune response.
A similar mechanism can operate in EBV-positive tumours, where the recruitment of TILs could be the consequence of the production by a virus, via tumour cells, of abnormal peptides, although this hypothesis has not been demonstrated yet. As a matter of fact, in their paper, Kuzushima and colleagues [18] observed that CD8-infiltrating lymphocytes did not recognise EBNA−1 and BRAF antigens produced by tumour cells.
The nature and the activation status of TILs in our cases of GCs seem to be similar to that observed by Dolcetti et al. [7] in MSI colorectal carcinomas. In both MSS/EBV+ and MSI GCs, the majority of TILs was represented by cytotoxic CD8+ cells. A minor component of TILs was represented by NK CD57+ cells. Cytotoxic effector CD8+ and NK cells are characterised by the presence of TIA-1 immunoreactive granules in their cytoplasms, independently of their activation status. TIA-1 protein is crucial for DNA fragmentation and the apoptotic process [1]. In our study, the mean value was significantly higher in MSS/EBV+ and MSI than in MSS/EBV− GCs.
The production and secretion of granzyme B and perforin are indispensable for the activation of cytotoxic and NK lymphocytes. In the presence of Ca ++, the monomers of perforin are released by the killer lymphocytes and inserted into the membrane of target cells, where they form membrane pores which promote the entry of cytolytic enzymes, in particular, TIA-1 and granzyme B [20].Granzyme B is a granule-associated serine protease that triggers the apoptotic cascade, probably by activating caspases 10, 3 and 7 [9].
Both perforin+ and granzyme B+ TILs were significantly more numerous in MSS/EBV+ and MSI than in MSS/EBV− GCs, suggesting that the activation of cytotoxic cells is a specific event priming the apoptotic process in MSS/EBV+ and MSI GCs.
The M30 monoclonal antibody identifies epithelial apoptotic cells and has been previously used by Michael-Robinson et al. [24] to evaluate the apoptotic index in MSI colorectal carcinomas. This antibody binds to the caspase-cleaved fragment of cytokeratin-18 during the early steps of epithelial cell apoptosis. The demonstration that apoptosis of neoplastic cells was significantly more frequent in MSI (5.87%) and in MSS/EBV+ (2.95%) than in MSS/EBV− (2.26%) GCs supports the hypothesis that, in these tumours, TILs play a crucial role in the increased apoptotic cell death of neoplastic cells.
As for the difference between MSI and MSS/EBV+ GCs, it is worth noting that, although CD3+, CD8+ and TIA-1+ TILs were significantly higher in MSS/EBV+ than in MSI GCs, the percentage of activated TILs, as well as the apoptotic index of tumour cells, were not significantly different between the two subsets of GCs. These data might explain the absence of a significant difference in prognosis between patients with MSS/EBV+ and MSI GCs.
In our study, the prognosis of MSS/EBV+ cases appears to be better than that of MSS/EBV− cases, although the difference is not statistically significant. In addition, the prognosis of patients with MSI+ GCs appears to be better than that of patients with MSS/EBV+ GCs. Previous studies examining the correlation between survival and EBV infection in GCs did not consider the MSI status [5, 16, 22] with the exception of that of Grogg et al. [10]. In this context, it is important to underline that in our study, all the MSI GCs are EBV-negative, in agreement with the findings of previous investigations [6, 10]. In addition, our results confirm that MSI GCs are associated with a significantly better prognosis than that of the MSS GCs, and particularly, of MSS/EBV− GCs.
In conclusion, our study demonstrates that the presence of a high number of CD3+ TILs is a favourable prognostic factor, independently of the pathogenesis of GCs. EBV-positive and MSI GCs are two subsets of tumours characterised by a similar intratumour lymphocytic infiltration prevalently composed of activated cytotoxic CD8+ cells. The relatively better prognosis of these subsets of GCs seems to be related to the increased host immune response against abnormal peptides, probably produced by the virus infection or by the presence of an altered mismatch repair system.
References
Anderson P, Nagler-Anderson C, O'Brein C, Levine H, Watkins S, Slayter HS, Blue ML, Schlossman SF (1990) A monoclonal antibody reactive with a 15-kDa cytoplasmic granule-associated protein defines a subpopulation of Cd8+ T lymphocytes. J Immunol 144:574–582
Boon T, Cerottini JC, Van den Eynde B, van der Bruggen P, Van Pel A (1994) Tumor antigens recognized by T lymphocytes. Annu Rev Immunol 12:337–365
Carneiro F, Seixas M, Sobrinho-Simoes M (1995) New elements for an updated classification of carcinomas of the stomach. Pathol Res Pract 191:571–584
Chiaravalli AM, Cornaggia M, Furlan D, Capella C, Fiocca R, Tagliabue G, Klersy C, Solcia E (2001) The role of histological investigation in prognostic evaluation of advanced gastric cancer. Analysis of histological structure and molecular changes compared with invasive pattern and stage. Virchows Arch 439:158–169
Cho MY, Kim TH, Yi SY, Jung WH, Park KH (2004) Relationship between Epstein–Barr virus encoded RNA expression, apoptosis and lymphocytic infiltration in gastric carcinoma with lymphoid-rich stroma. Med Princ Pract 13:353–360
Chong JM, Fukayama M, Hayashi Y, Takizawa T, Koike M, Konishi M, Kikuchi-Yanoshita R, Miyaki M (1994) Microsatellite instability in the progression of gastric carcinoma. Cancer Res 54:4595–4597
Dolcetti R, Viel A, Doglioni C, Russo A, Guidoboni M, Capozzi E, Vecchiato N, Macrì E, Fornasarig M, Boiocchi M (1999) High prevalence of activated intraepithelial cytotoxic T lymphocytes and increased neoplastic cell apoptosis in colorectal carcinomas with microsatellite instability. Am J Pathol 154(6):1805–1813
Elsaleh H, Powell B, Soontrapornchai P, Joseph D, Goria F, Spry N, Iacopetta B (2000) p53 gene mutation, microsatellite instability and adjuvant chemotherapy: impact on survival of 388 patients with Duke's C colon carcinoma. Oncology 58:52–59
Froelich CJ, Dixit VM, Yang X (1998) Lymphocyte granule-mediated apoptosis: matter of viral mimicry and deadly proteases. Immunol Today 19:30–36
Grogg KL, Lohse CM, Shane Pankratz V, Halling KC, Smyrk TC (2003) Lymphocyte-rich gastric cancer: association with Epstein–Barr virus, microsatellite instability, histology, and survival. Mod Pathol 16 (7):641–651
Gryfe R, Kim H, Hsieh ETK, Aronson MD, Holowaty EJ, Bull SB, Redston M, Gallinger S (2000) Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med 342:69–77
Halpern AC, Schuchter LM (1997) Prognostic models in melanoma. Semin Oncol 24(Suppl 4):S2–S7
Hayashi K, Yonamine K, Masuko-Hongo K, Iida T, Yamamoto K, Nishioka K, Kato T (1999) Clonal expansion of T cells that are specific for autologous ovarian tumor among tumor-infiltrating T cells in humans. Gynecol Oncol 74:86–92
Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Che X, Iwashige H, Aridome K, Hokita S, Aikou T (2000) Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer 88:577–583
Ishikawa T, Fujita T, Suzuki Y, Okabe S, Yuasa Y, Iwai T, Kawakami Y (2003) Tumor-specific immunological recognition of frameshift mutated peptides in colon cancer with microsatellite instability. Cancer Res 63:5564–5572
Kijima Y, Ishigami S, Hokita S, Koriyama C, Akiba S, Eizuru Y, Aikou T (2003) The comparison of the prognosis between Epstein–Barr virus (EBV)-positive gastric carcinomas and EBV-negative ones. Cancer Lett 200:33–40
Kooi S, Freedman RS, Rodriguez-Villanueva J, Platsoucas CD (1993) Cytokine production by T-cell lines derived from tumor-infiltrating lymphocytes from patients with ovarian carcinoma: tumor-specific immune responses and inhibition of antigen-independent cytokine production by ovarian tumor cells. Lymphokine Cytokine Res 12:429–437
Kuzushima K, Nakamura S, Nakamura T, Yamamura N, Yokoyama N, Fujita M, Kiyono T, Tsurumi T (1999) Increased frequency of antigen-specific CD8+ cytotoxic lymphocytes infiltrating an Epstein–Barr virus associated gastric carcinoma. J Clin Invest 140:163–171
Lauren P (1965) The two histological main type of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. Acta Pathol Microbiol Scand 64:31–49
Liu B, Nicolaides NC, Markowitz S, Willson JK, Parsons RE, Jen J, Papadopulos N, Peltomaki P, de la Chapelle A, Hamilton SR, Kinzler KW, Vogelstein B (1995) Mismatch repair gene defects in sporadic colorectal cancers with microsatellite instability. Nat Genet 9:48–55
MacCarty WC, Mahle AE (1921) Relation of differentiation and lymphocytic infiltration to postoperative longevity in gastric carcinoma. J Lab Clin Med 6:473–480
Matsunou H, Konishi F, Hori H, Ikeda T, Sasaki K, Hirose Y, Yamamichi N (1996) Characteristics of Epstein–Barr virus-associated gastric carcinoma with lymphoid stroma in Japan. Cancer 77:1998–2004
Michael-Robinson JM, Biemer-Huttmann A-E, Purdie DM, Walsh M, Simms LA, Biden KG, Young JP, Leggett BA, Jass JR, Radford-Smith GL (2001) Tumor infiltrating lymphocytes and apoptosis are independent features in colorectal cancer stratified according to microsatellite instability status. Gut 48:360–366
Michael-Robinson JM, Reid LE, Purdie DM, Biemer-Huttmann A-E, Walsh MD, Pandeya N, Simms LA, Young JP, Leggett BA, Jass JR, Radford-Smith GL (2001) Proliferation, apoptosis and survival in high-level microsatellite instability sporadic colorectal cancer. Clin Cancer Res 7:2347–2356
Naito Y, Saito K, Shiiba K, Ohuci A, Saigenji K, Nagura H, Ohtani H (1998) CD8+ T cells infiltrated within cancer cell nests as prognostic factor in human colorectal cancer. Cancer Res 58:3491–3494
Nakamura S, Ueki T, Yao T, Ueyama T, Tsuneyoshi M (1994) Epstein–Barr virus in gastric carcinoma with lymphoid stroma. Cancer 73:2239–2249
Nakano O, Sato M, Naito Y, Suzuki K, Orikasa S, Aizawa M, Suzuki Y, Shintaku I, Nagura H, Ohtani H (2001) Proliferative activity of intratumoral CD8(+) T-lymphocytes as prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res 61:5132–5136
Oda K, Tamaru J, Takenouchi T, Mikata A, Nunomura M, Saitoh N, Sarashina H, Nakajima N (1993) Association of Epstein–Barr virus with gastric carcinoma with lymphoid stroma. Am J Pathol 143:1063–1071
Peoples GE, Goedegebuure PS, Smith R, Linehan DC, Yoshino I, Eberlein TJ (1995) Breast and ovarian cancer-specific cytotoxic T lymphocytes recognize the same HER2/neu-derived peptide. Proc Natl Acad Sci U S A 17:432–436
Pross HF, Lotzova E (1993) Role of natural killer cells in cancer. Nat Immunol 12:279–292
Saeterdal I, Gjertsen MK, Straten P, Eriksen JA, Gaudernack G (2001) A TGRFβRII frameshift-mutation-derived CTL epitope recognised by HLA-A2-restricted CD8+ T cells. Cancer Immunol Immunother 50:469–476
Santin AD, Bellone S, Ravaggi A, Pecorelli S, Cannon MJ, Parham GP (2000) Induction of ovarian tumor-specific CD8+ cytotoxic T lymphocytes by acid-eluted peptide-pulsed autologous dendritic cells. Obstet Gynecol 96:422–30
Santin AD, Hermonat PL, Ravaggi A, Bellone S, Roman JJ, Smith CV, Pecorelli S, Radominska-Pandya A, Cannon MJ, Parham GP (2001) Phenotypic and functional analysis of tumor-infiltrating lymphocytes compared with tumor-associated lymphocytes from ascitic fluid and peripheral blood lymphocytes in patients with advanced ovarian cancer. Gynecol Obstet Invest 51:254–261
Seruca R, Santos NR, David L, Constancia M, Barroca H, Carneiro F, Seixas M, Peltomaki P, Lothe R, Sobrinho-Simoes M (1995) Sporadic gastric carcinomas with microsatellite instability display a particular clinicopathologic profile. Int J Cancer 64:32–36
Sobin LH, Wittekind C (eds) (2002) TNM classification of malignant tumors, 6th edn. Wiley-Liss, New York
Stracca-Pansa V, Menegon A, Donisi PM, Bozzola L, Fedeli F, Quarto F, Nappi O, Pettinato G (1995) Gastric carcinoma with osteoclast-like giant cells. Report of four cases. Am J Clin Pathol 103:453–459
Tokunaga M, Land CE, Uemura Y, Tokudome T, Tanaka S, Sato E (1993) Epstein–Barr virus in gastric carcinoma. Am J Pathol 143:1250–1254
Vesalainen S, Lipponen P, Talia M, Syrjanen K (1994) Histological grade, perineural infiltration, tumor-infiltrating lymphocytes and apoptosis as determinants of long-term prognosis in prostatic adenocarcinoma. Eur J Cancer 30A:1797–1803
Watanabe H, Enjoji M, Imai T (1976) Gastric carcinoma with lymphoid stroma. Cancer 38:232–243
Wright CM, Dent OF, Barker M, Newland RC, Chapuis PH, Bokey EL, Young JP, Leggett BA, Jass JR, Macdonald GA (2000) Prognostic significance of extensive microsatellite instability in sporadic clinicopathological stage C colorectal cancer. Br J Surg 87:1197–1202
Yamamoto H, Perez-Piteira J, Yoshida T, Terada M, Itoh F, Imai K, Perucho M (1999) Gastric cancers of the microsatellite mutator phenotype display characteristic genetic and clinical features. Gastroenterology 116:1348–1357
Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G (2003) Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 348:203–213
Acknowledgements
The authors thank Prof. E. Solcia for his helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chiaravalli, A.M., Feltri, M., Bertolini, V. et al. Intratumour T cells, their activation status and survival in gastric carcinomas characterised for microsatellite instability and Epstein–Barr virus infection. Virchows Arch 448, 344–353 (2006). https://doi.org/10.1007/s00428-005-0066-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-005-0066-4