Abstract
We report a unique case of adenoid cystic carcinoma (ACC) of the maxillary sinus, with gradual histologic transformation from lower-grade ACC (cribriform and tubular types) to high-grade adenocarcinoma (HGA) showing a sequential histologic spectrum via solid-type ACC. A 74-year-old man presented with swelling and mild pain of the right cheek. CT scan showed a mass measuring approximately 4 cm, with marked bone destruction in the right maxillary sinus. A surgically resected specimen revealed that the tumor was comprised of three different components: HGA and solid-type ACC in the central portion and lower-grade ACC in the periphery. The tumor was discriminated from a dedifferentiated carcinoma or hybrid tumor. Autopsy specimens also demonstrated both solid-type ACC and HGA components in the lung and spleen. Immunohistochemically, positive staining of p53 protein was detected on both solid-type ACC and HGA cells, but cyclin D1 and HER2/neu was only seen in HGA cells. Solid-type ACC cells were immunoreactive for CD117 (c-kit), but lower-grade ACC and HGA cells were negative. This case suggests that the overexpression of CD117, p53 protein, cyclin D1, and HER2/neu might be involved in the progression from lower-grade ACC to solid-type ACC and HGA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adenoid cystic carcinoma (ACC) is a specific type of adenocarcinoma, with a characteristic cribriform appearance that commonly occurs in the major and minor salivary glands. Patey and Thackray first reported that ACC with a solid growth pattern was associated with a worse prognosis than that of the cribriform type [12]. Perzin et al. described a more differentiated type of ACC, the tubular form, which is associated with a favorable prognosis [13]. Accordingly, ACCs are histologically classified into three subtypes: tubular, cribriform, and solid. The grading scheme that categorizes ACCs into three histologic grades is as follows: Grade 1—tubular and cribriform patterns without solid components, Grade 2—pure cribriform pattern or mixed with less than 30% of solid areas, and Grade 3—predominantly a solid pattern, and it is closely correlated with prognosis [15]. Therefore, the tubular- and cribriform-type ACCs are lower-grade, whereas solid-type ACC is higher grade.
Recently, cases have been reported of dedifferentiated ACC of the salivary gland and upper respiratory tract, which are rare variants of ACC [1, 2, 7, 9–11]. Dedifferentiation is defined as clonal evolution of a poorly differentiated or high-grade component arising within a low-grade carcinoma without histologic transition or spectrum from the original carcinoma [18], namely, as an abrupt transformation [11]. In all reported cases of dedifferentiated ACC, the low-grade component was tubular- or cribriform-type ACC, and high-grade areas demonstrated poorly differentiated adenocarcinoma or undifferentiated carcinoma. The two elements are clearly demarcated, although there is a transitional zone in some cases.
Although histologic progression from lower-grade ACC to solid-type ACC has previously been reported [16], there are no reports in the literature of transformation from solid-type ACC to high-grade adenocarcinoma (HGA) or undifferentiated carcinoma. The histologic appearance of solid-type ACC is variably sized solid islands of monomorphous tumor cells, which have small nuclei with fine chromatin and indistinct nucleoli, and scant cytoplasm [3]. Immunohistochemical staining for cytokeratin of solid-type ACC demonstrated disproportionate ductal-cell proliferation. The HGA showed a more pleomorphic appearance, large nuclei, and infiltrative growth pattern. We present here a case of ACC with gradual transformation from lower-grade ACC to HGA with a continuous histologic spectrum via solid-type ACC. The immunohistochemical characteristics are also described and reviewed as compared with those of dedifferentiated ACC and hybrid carcinoma.
Clinical history
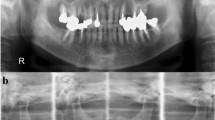
A 74-year-old man presented with swelling and mild pain of the right cheek, anosmia, and nasal bleeding for 6 months. CT scan demonstrated a mass approximately 4 cm in diameter, with marked bone destruction in the right maxillary sinus (Fig. 1). A needle biopsy specimen was diagnosed as poorly differentiated adenocarcinoma, and the mass was excised. Pulmonary and hepatic metastases were identified at follow-up 1 year later after the surgery. The patient died within 4 months following the development of metastases without any effect of chemotherapy. Autopsy was then performed.
Materials and methods
The whole surgically resected specimen was cut into 12 tissue blocks 5 mm thick for histologic and immunohistochemical examination. For light microscopic evaluation, all thin sections were stained with hematoxylin and eosin. Immunohistochemistry was performed on the deparaffinized tissue sections using the primary antibodies listed in Table 1 and the avidin-biotin-peroxidase complex method of Hsu and co-workers [6].
Results
The surgically resected specimen, measuring 7.5×5×3 cm, was comprised of the right maxillary tumor mass, the overlying skin, and the subcutaneous tissue. Grossly, the tumor was solid with a white or gray-tan appearance and was approximately 4 cm in diameter, with an indistinct margin and destruction of the maxillary bone wall.
Microscopically, the tumor was comprised of three distinct components. In the central portion of the tumor, HGA and solid-type ACC were the main components. HGA was comprised of highly malignant tumor cells with pleomorphic vesicular nuclei and relatively abundant amphophilic cytoplasm proliferating irregularly with loose solid nests (Fig. 2a) or infiltratively in a small tubular and cord-like pattern (Fig. 2b). Solid ACC was comprised of either solid nests of small duct-like structures with occasional comedo-like necrosis (Fig. 2c) or solid nests of larger tumor cells with vesicular nuclei with some ductular structures. There were transitional areas between HGA and solid-type ACC (Fig. 2d). In the periphery of the tumor, a lower-grade ACC component was identified. Small monomorphous cells were infiltratively proliferated with a cribriform or bicellular tubular pattern. Perineural invasion was frequently observed in the area.
The postmortem examination revealed metastases in the lungs, liver, spleen, bone marrow in the lumbar vertebrae, and right pulmonary hilar lymph nodes. Histopathological findings in the metastatic lesions of the lung and spleen were solid-type ACC and HGA, which were similar to those of the central parts of the primary maxillary lesion.
Table 1 summarizes the immunohistochemical findings of the present case. Immunohistochemically, ductal cells stained positively for cytokeratin (AE1/AE3) in all components. All components showed a positive reaction for α-smooth muscle actin, markers of myoepithelial cell differentiation, and S-100 protein. Ductal cells in HGA and solid-type ACC areas expressed positive immunoreactivity for S-100 protein. CD117 (c-kit) immunostaining showed a positive reaction only in solid-type ACC, with no positive staining in lower-grade ACC or HGA (Fig. 3a). The staining for p53 protein was positive in more than 50% of carcinoma cells both in the HGA and solid-type ACC areas, whereas it was negative in the lower-grade ACC portion (Fig. 3b). Cyclin D1 overexpression was demonstrated in approximately 30% of the tumor cells in the HGA component, but was only weakly and focally reactive in the ACC component (Fig. 3c). Membranous staining for HER2/neu was detected only in the HGA component (Fig. 3d). The Ki-67 (MIB-1) labeling index in lower-grade ACC, solid-type ACC, and HGA areas was approximately 5, 15, and 30%, respectively.
Immunohistochemical findings of the tumor. ×200. a Solid-type adenoid cystic carcinoma cells with membranous expression for CD117 (left), which is negative in the high-grade adenocarcinoma cells (right). b Solid-type adenoid cystic carcinoma cells with nuclear expression for p53 protein (left), which is negative in the cribriform-type adenoid cystic carcinoma cells (right). c High-grade adenocarcinoma cells with nuclear expression for cyclin D1 (left), which is negative in the solid-type adenoid cystic carcinoma cells (right). d High-grade adenocarcinoma cells with membranous expression for HER2/neu (left), which is negative in the solid-type adenoid cystic carcinoma cells (right)
Discussion
The tumor in this case consisted of three different components: lower-grade (tubular and cribriform types) ACC, solid-type ACC, and HGA. We believe that gradual transformation from lower-grade ACC to HGA with a sequential histologic spectrum via solid-type ACC was involved in the tumor development. Discrimination from dedifferentiated carcinoma and hybrid carcinoma, which are organized with pleural carcinoma elements, is necessary in this unique case.
Dedifferentiation is recognized when a lower-grade malignant neoplasm abruptly transforms to a high-grade [11, 17], in which the high-grade component is juxtaposed to the original lesion or the original line of differentiation is lost [2]. This concept should be distinguished from malignancy arising from a benign tumor such as carcinoma ex pleomorphic adenoma, hybrid tumors, and transformation within a high-grade carcinoma to another high-grade pattern [18]. There are no previously reported cases of dedifferentiated ACC of the salivary glands and upper respiratory tract where a solid-type ACC area was mentioned [1, 2, 7, 9, 10], or accounted for only as a scant portion [11], which is consistent with the definition. The present case is unique in that the histologic features indicated a gradually successive transition from lower-grade to solid-type ACC and HGA. Histologic transformation from solid-type ACC to HGA corresponds to a transition from high-grade carcinoma to another high-grade carcinoma, which should discriminate from dedifferentiation.
Hybrid tumors are composed of two histologically distinct categories of tumor, each of which corresponds to an exactly defined tumor entity and an identical origin [14]. In the present case, the tumor components were lower-grade ACC, solid-type ACC, and HGA. Some of the tumor nests of the HGA component with comedo-like necrosis were superficially analogous to salivary duct carcinoma. However, dystrophic calcification, apocrine-like eosinophilic cytoplasm, coarse chromatin, and distinct single nucleolus, which are characteristic features of salivary duct carcinoma [18], were not observed. Immunoreactivity was not detected for CEA, GCDFP-15, androgen receptor, and prostate-specific antigen, which are frequently expressed in salivary duct carcinoma. Therefore, the HGA component was not assigned to a specific carcinoma entity and should be classified into adenocarcinoma NOS (not otherwise specified). The tumor in the present case could be differentiated from hybrid carcinoma.
Histologic transition from solid-type ACC to HGA is a characteristic finding in this case. Comedo-like necrosis and mitoses have appeared to a variable extent in solid-type ACC [3, 4] and were identified in the present case. In the HGA component, there were distinctive irregularly sized solid islands of highly pleomorphous tumor cells with vesicular large nuclei. There were transitional features between solid-type ACC and HGA. These morphological findings suggest that the increase in cellular atypism and pleomorphism in solid-type ACC might be involved in the initial stage of histologic transformation to HGA. In the autopsy specimens of the lung and spleen, histopathological findings of metastatic lesions showed the same pattern as the central portion of the surgical samples, i.e., combination of solid-type ACC and HGA, showing that solid-type ACC metastasized and then transformed to HGA. These findings also suggest a gradual successive transformation of tumor cells in this case.
Immunohistochemical studies and genetic analysis have been carried out for oncoprotein in dedifferentiated ACC and solid-type ACC. The Ki-67 labeling index was higher in dedifferentiated ACC than in that of lower-grade ACC [1, 2, 11]. The Ki-67 labeling index of solid-type ACC in the present case was between lower-grade ACC and HGA, demonstrating that proliferation activity increased in proportion to histologic progression from lower-grade ACC to solid-type ACC and HGA. CD117 (c-kit) expression was detected in ACC, which was intense in solid-type ACC [5]. Overexpression of p53 protein was detected by immunohistochemistry in six of nine cases (67%) [1, 2, 7, 9, 11], and that of cyclin D1 was detected in five of seven cases (71%) in previously reported dedifferentiated ACC cases [1, 2, 11]. Nagao et al. and Chau et al. reported dedifferentiated ACC cases with a p53 gene mutation [1, 11]. Immunohistochemical positive staining of HER2/neu has been demonstrated in three of five cases (60%) [1, 8, 10] of dedifferentiated ACC. Immunohistochemical expression of p53 protein [8] and p53 gene alterations [17] were markedly more frequent in solid-type ACC than lower-grade ACC. In our case, positivity for p53 protein was detected in both solid-type ACC and HGA cells, but immunoreactivity for cyclin D1 and HER2/neu were detected only in HGA cells. The molecular mechanisms of malignant transformation of ACC have not been clearly elucidated. Our case suggests that overexpression of CD117 (c-kit), p53 protein, cyclin D1, and HER2/neu are involved in the transformation from lower-grade ACC to solid-type ACC and HGA.
References
Chau Y, Hongyo T, Aozasa K et al (2001) Dedifferentiation of adenoid cystic carcinoma: report of a case implicating p53 gene mutation. Human Pathol 32:1403–1407
Cheuk W, Chan JKC, Ngan RKC (1999) Dedifferentiation in adenoid cystic carcinoma of salivary gland: an uncommon complication associated with an accelerated clinical course. Am J Surg Pathol 23:465–472
Cleveland D, Abrams AM, Melrose RJ et al (1990) Solid adenoid cystic carcinoma of the maxilla. Oral Surg Oral Med Oral Pathol 69:470–478
Eby LS, Johnson DS, Baker HW (1972) Adenoid cystic carcinoma of the head and neck. Cancer 29:1160–1168
Holst VA, Marshall CE, Moskaluk CA, Frierson HF Jr (1999) KIT protein expression and analysis of c-kit gene mutation in adenoid cystic carcinoma. Mod Path 12:956–960
Hsu SM, Raine L, Fanger H (1981) Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 29:577–580
Ide F, Mishima K, Saito I (2003) Small foci of high-grade carcinoma cells in adenoid cystic carcinoma represent an incipient phase of dedifferentiation. Histopathology 43:604–606
Jia L, Esguerra RL, Tang X et al (2004) Prognostic value of apoptosis and apoptosis-associated proteins in salivary gland adenoid cystic carcinoma. Pathol Int 54:217–223
Kamio N, Tanaka Y, Mukai M et al (1997) A hybrid carcinoma: adenoid cystic carcinoma and salivary duct carcinoma of the salivary gland. An immunohistochemical study. Virchows Arch 430:495–500
Moles MA, Avila IR, Archilla AR (1999) Dedifferentiation occurring in adenoid cystic carcinoma of the tongue. Oral Surg Oral Med Oral Pathol Oral Radiol Endo 88:177–180
Nagao T, Gaffey TA, Serizawa H et al (2003) Dedifferentiated adenoid cystic carcinoma: a clinicopathologic study of 6 cases. Mod Path 16:1265–1272
Patey DH, Thackray AC (1958) The treatment of parotid tumors in the light of a pathological study of parotidectomy material. Br J Surg 45:477–487
Perzin KH, Gullane P, Clairmont AC (1978) Adenoid cystic carcinomas arising in salivary glands. A correlation of histologic features and clinical course. Cancer 42:265–282
Seifert G, Donath K (1996) Hybrid tumours of salivary glands. Definition and classification of five rare cases. Eur J Cancer B Oral Oncol 32:251–259
Szanto PA, Luna MA, Tortoledo ME et al (1984) Histologic grading of adenoid cystic carcinoma of the salivary glands. Cancer 54:1062–1069
Yamamoto Y, Saka T, Makimoto K et al (1992) Histological changes during progression of adenoid cystic carcinoma. J Laryngol Otol 106:1016–1020
Yamamoto Y, Virmani AK, Wistuba II et al (1996) Loss of heterozygosity and microsatellite alterations in p53 and RB genes in adenoid cystic carcinoma of the salivary gland. Human Pathol 27:1204–1210
Zarbo RJ (2002) Salivary gland neoplasia. A review for the practicing pathologist. Mod Path 15:298–323
Acknowledgements
We are grateful to Dr. Toshitaka Nagao, Department of Diagnostic Pathology, Tokyo Medical University, for providing useful comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sato, K., Ueda, Y., Sakurai, A. et al. Adenoid cystic carcinoma of the maxillary sinus with gradual histologic transformation to high-grade adenocarcinoma: a comparative report with dedifferentiated carcinoma. Virchows Arch 448, 204–208 (2006). https://doi.org/10.1007/s00428-005-0054-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-005-0054-8