Abstract
Now that more than two decades have passed since the first reports of intraductal papillary-mucinous neoplasms (IPMNs), it has become clear that IPMN consists of a spectrum of neoplasms with both morphological and immunohistochemical variations. At a meeting of international experts on pancreatic precursor lesions held in 2003, it was agreed that a consensus classification of IPMN subtypes should be established to enable a more detailed analysis of the clinicopathological significance of the variations. Based on our experience and on information from the literature, we selected representative histological examples of IPMNs and defined a consensus nomenclature and criteria for classifying variants as distinctive IPMN subtypes including gastric type, intestinal type, pancreatobiliary type, and oncocytic type. These definitions can be used for further analyses of the clinicopathological significance of the variations of IPMN.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The first cases of intraductal papillary-mucinous neoplasm (IPMN) were reported in the 1970s and 1980s [17, 23]. In the 1990s, the term IPMN was coined, and the tumor was established as a special entity among the pancreatic neoplasms [25]. It was also found that IPMN is related to invasive pancreatic adenocarcinoma because one third of IPMNs have an associated invasive adenocarcinoma and some patients with a noninvasive IPMN subsequently develop either invasive colloid (mucinous noncystic) carcinoma or invasive ductal adenocarcinoma [5, 9, 10]. During the more than two decades that have passed since its first description, a number of reports have been published, and it has become clear that IPMNs include a spectrum of neoplasms with both morphological and immunohistochemical variations. These variations include neoplasms with tall columnar cells arranged in relatively short papillae without significant atypia, those with villous projections lined by cells with significant atypia, those with complex branching papillae containing cells with marked atypia, and those with oncocytic cells with complex papillae [2, 4, 8, 22, 30]. Several authors have proposed classification systems for the spectrum of morphologies seen in IPMNs [4, 8, 22, 30]. One such system divides IPMNs into clear-, dark-, and compact-cell types according to the density of the cytoplasm, shapes of the epithelial cells, and expression patterns of glycoproteins contained in mucin (MUCs), while another system separates IPMNs into intestinal, pancreatobiliary, and oncocytic subtypes primarily based on their architecture and cytology [4, 22, 30].
At a meeting of international experts on pancreatic precursor lesions held at the Johns Hopkins Hospital from August 18 to 19, 2003, a basic definition of an IPMN that is employed in the current study (Table 1) was worked out, and it was agreed that a consensus classification of IPMN subtypes should be established to make it easier to compare studies from different institutions and to improve patient care [14]. The purpose of this report is to establish consensus criteria for the classification of IPMN subtypes based on histological features and immunohistochemical reactivities with antibodies to specific types of mucin (MUCs).
Materials and methods
Review of slides
Representative slides of candidate subtypes of IPMNs were selected from the pathology files of Tohoku University Hospital. They were chosen so as to represent a broad spectrum of the morphological variations of IPMN, based on discussions at the Baltimore meeting and including information from the literature and personal communications. The slide set consisted of ten cases. Each case consisted of four slides, one stained with hematoxylin and eosin and one each labeled for MUC1, MUC2, and MUC5AC. The slides were reviewed by the authors independently and subsequently discussed at an international meeting in Sendai, Japan (July 2004). The goal of this meeting was to establish an international consensus on the classification of IPMNs.
Immunohistochemistry
The primary antibodies employed were a monoclonal antibody to MUC1-core (clone Ma552, Novocastra Laboratories Ltd., Newcastle upon Tyne, UK, 1:100), a monoclonal antibody to MUC2 (clone Ccp58, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA, 1:600), and a monoclonal antibody to MUC5AC (clone CLH2, Chemicon International Inc., Temecula, CA, USA, 1:1000). Paraffin sections cut at 4 μm were dewaxed with xylene and hydrated with serial immersing in 100, 90, 70, and 50% ethanol solutions and distilled water. Antigen retrieval was performed by incubating slides in boiled citric acid buffer (0.18 mM citric acid, 0.82 mM sodium citrate) for 15 min in a microwave oven. Indirect immunohistochemical labeling was performed as follows: The slides were incubated with PBS containing 0.3% hydrogen peroxide for 30 min to block the endogenous peroxidase activity. After washing with PBS, the slides were incubated with the primary antibody diluted in PBS containing 10% rabbit serum at 4°C overnight. After washing with PBS, the slides were incubated with the biotinylated rabbit anti-mouse IgG antibody solution (Nichirei, Tokyo, Japan) for 30 min at room temperature. The slides were then washed with PBS and incubated with streptavidin-horseradish peroxidase solution (Nichirei) for 30 min at room temperature. After washing with PBS, the slides were incubated with 3, 3′-diaminobenzidine tetrahydrochloride (DAB) diluted in PBS containing 0.05% hydrogen peroxide at a final concentration in 0.2 mg/ml. The slides were washed with running tap water and counterstained with hematoxylin.
Results
All participants agreed that the IPMN cases provided covered representative histological variations of IPMN and that they can be subclassified into four types. In the following, the consensus criteria for the classification of IPMN subtypes based on morphological features and the immunohistochemical reactivity for MUCs (Table 2) are presented.
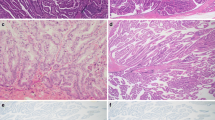
The gastric-type IPMN consisted of cells resembling gastric foveolae and usually showed low-grade atypia corresponding to a diagnosis of intraductal papillary-mucinous adenoma (Fig. 1a). They expressed MUC5AC but were negative for MUC1 and for MUC2 (Fig. 1b–d). The gastric-type IPMN corresponded to the previously reported null type or the clear-cell type of IPMN [6, 22, 30]. The gastric-type IPMN often presented as a small cystic lesion involving branch ducts of the pancreas. This type of IPMN sometimes merged with other subtypes of IPMNs (Fig. 2c, d).
Representative images of the subtypes of intraductal papillary-mucinous neoplasm of the pancreas. a–d The gastric type; e–h the intestinal type; i–l the pancreatobiliary type; m–p the oncocytic type. Hematoxylin and eosin staining (a, e, i, m) and immunohistochemical stainings of MUC1 (b, f, j, n), MUC2 (c, g, k, o), and MUC5AC (d, h, l, p)
Variation of the intestinal type mimicking goblet cells (hematoxylin and eosin) (a) and its immunohistochemical staining of MUC2 (b). Examples of mixed areas combining the gastric type and the intestinal type (c) and the gastric type and the pancreatobiliary type (d). Images show immunohistochemical stainings of MUC2 (c) and MUC1 (d)
The intestinal-type IPMN resembled intestinal villous neoplasms with tall columnar epithelial cells that usually showed moderate- or severe/high-grade atypia corresponding to borderline or in situ carcinoma (Fig. 1e). It corresponded to the previously reported dark-cell type of IPMN [22, 30]. The intestinal-type IPMN usually showed large cystic lesions involving the main duct and several branch ducts. Occasionally, there were small papillae with goblet-cell-like appearance with low-grade atypia (Fig. 2a, b). The neoplastic cells consistently expressed MUC2 and MUC5AC but were negative for MUC1 (Fig. 1f–h).
The pancreatobiliary type of IPMN consisted of cells resembling cholangiopapillary neoplasms and showed complex, thin, branching papillae with severe/high-grade atypia corresponding to carcinoma in situ (Fig. 1i). The lesion was at least focally positive for MUC1 and consistently expressed MUC5AC but not MUC2 (Fig. 1j–l).
The oncocytic type of IPMN consisted of cells with abundant, intensely eosinophilic cytoplasm and showed complex thick papillae with intraepithelial lumina and severe/high-grade atypia corresponding to carcinoma in situ (Fig. 1m). The oncocytic type of IPMN corresponded to the previously reported compact-cell type IPMN [22]. This IPMN subtype expressed MUC5AC consistently and MUC1 focally but was negative for MUC2 (Fig. 1n–p).
While it was the consensus of the group that the classification of IPMNs into these well-defined subtypes will help standardize the reporting of IPMNs, it was recognized that IPMNs often are composed of a combination of more than one cell type. The group therefore proposed that each IPMN should be subclassified by the dominant component and that any other significant subtypes present should be documented. In case of an invasive carcinoma associated with IPMN, the subtypes of the intraductal component should be evaluated and noted, especially in areas associated with the invasive component.
Discussion
The diagnostic term IPMN encompasses a spectrum of intraductal mucin-producing neoplasms composed of a variety of cell types showing distinctive architectural and histochemical features [19, 25]. An international panel of experts was assembled to establish a nomenclature for classifying the subtypes of IPMNs. Based on the experience of the panel, information from the literature, and a review of a selected series of IPMNs, consensus criteria for the classification of subtypes of IPMNs were agreed upon. These definitions should facilitate further analyses of the clinicopathological significance of the variations of IPMN.
The panel of experts agreed unanimously that four types of IPMN can be distinguished among the series of cases that had been studied. These IPMN types were classified as gastric, intestinal, pancreatobiliary, or oncocytic type.
According to the currently available data, the gastric-type IPMN usually presents as relatively small cystic lesions in peripheral branch ducts with low-grade atypia, mostly corresponding to intraductal papillary-mucinous adenoma, while the other subtypes of IPMN usually present as large lesions involving the main duct and connecting branch ducts with marked atypia, mostly corresponding to intraductal papillary-mucinous neoplasm in the borderline category or intraductal papillary-mucinous carcinoma [4, 28]. These latter types of IPMN are often associated with an invasive adenocarcinoma [10, 18, 28].
We present this subclassification of IPMN to aid in the comparison of studies from different institutions. One interpretation of our findings is that the types of IPMN described here may simply be a manifestation of a spectrum in the differentiation and progression of a single lineage of IPMN and that these morphologies may, in part, reflect the degree of dysplasia. IPMN may be a progressive neoplastic lesion, in which a small cystic lesion with low-grade atypia may progress to large multicystic ductal lesions with severe atypia and complex histological architecture and, eventually, to invasive cancer. Areas with gastric-type epithelium can be associated with the other subtypes, implying that the gastric type might be a common precursor of the other types [6], although we feel that such a conclusion is premature. Whether the IPMN types we defined are truly distinctive lineages or variations of a single progressive neoplastic lineage should be further clarified by clinicopathological and molecular studies based on the definitions provided. One study on 51 pancreata with IPMNs that compared the various subtypes of IPMN and aberrations of tumor suppressor genes, including CDKN2A, TP53, SMAD4, and DUSP6, revealed no significant association [11]. It is hoped that such studies will lead to an understanding of the mechanisms by which IPMNs develop and progress.
Pancreatic intraductal neoplasms consisting mainly of tubular glands of pyloric-gland type have been reported and designated as “intraductal tubular adenoma” [7, 15]. We did not include examples with predominantly tubular growth in the current study. However, because focal tubule formation is often observed in IPMNs, especially those of the gastric type or the pancreatobiliary type, the authors would assume that tubular growth may occur in IPMNs and that neoplasms with a predominantly tubular pattern may correspond to intraductal tubular adenoma, pyloric-gland type. The coexistence of papillae and tubules in both IPMNs and intraductal tubular adenoma suggests that they may be closely related, albeit distinctive, morphological features.
MUCs are useful markers for evaluating subtypes of IPMN [13, 20–22, 29, 30]. MUC1 is a component of the membrane-bound type of mucin and is usually detected in intralobular ductal lumina in the normal pancreas [1]. MUC1 is expressed in invasive ductal adenocarcinoma of the pancreas, often showing extensive positivity [24]. Among the IPMN subtypes, the pancreatobiliary type usually shows positivity for MUC1, which parallels its association with invasive ductal adenocarcinoma of the conventional tubular type [6]. The oncocytic type expresses MUC1 focally, and while it has been reported to be associated with invasive oncocytic carcinoma, its association with invasive ductal carcinoma is unknown [2]. MUC2 consists of secreted mucin usually observed in intestinal glands and is considered to be a marker of intestinal differentiation [12]. The intestinal type of IPMN, of both villous and goblet-cell features, strongly expresses MUC2 [6, 13, 21, 22, 24, 30]. The intestinal differentiation of this subtype of IPMN was supported by the demonstration that they also express CDX2, a homeobox gene involved in intestinal development [6]. MUC5AC is expressed by mucous surface cells of the stomach. MUC5AC is not detected in the normal pancreas but is consistently detected by immunohistochemistry in all types of IPMN [13, 30]. It is also known that pancreatic intraepithelial neoplasia (PanIN), precursor lesions associated with invasive ductal adenocarcinoma, also consistently express MUC5AC [16, 21]. PanINs are usually negative for MUC2 but often express MUC1, especially in high-grade atypical lesions [5]. The differential diagnosis between PanIN and IPMN was discussed in detail in a previous publication [14].
The prognosis of patients with an IPMN has been shown to depend on the presence or absence of an associated invasive carcinoma and, if an invasive carcinoma is present, on the histological type of the associated invasive carcinoma [26]. Invasive carcinomas associated with IPMN largely consist of two distinct types, invasive carcinoma mimicking usual invasive ductal adenocarcinoma and invasive colloid (mucinous noncystic) carcinoma [5, 21]. Patients with an IPMN associated with an invasive colloid (mucinous noncystic) carcinoma usually have a better prognosis than do those with an associated invasive ductal adenocarcinoma [3, 5].
The classification proposed is based primarily on morphological features. The immunolabeling for MUCs may serve as a confirmatory marker for the classification. We believe our definitions may contribute to an understanding of the biologic behavior of IPMN and to better clinical management. For example, it would be valuable to determine whether a particular type of noninvasive IPMN is associated with a particular type of invasive adenocarcinoma. It will be also of interest to clarify the relationship of the intraductal tubular neoplasm of the pancreas [27] with the types of IPMN described herein.
References
Abe M, Kufe D (1993) Characterization of cis-acting elements regulating transcription of the human DF3 breast carcinoma-associated antigen (MUC1) gene. Proc Natl Acad Sci U S A 90:282–286
Adsay NV, Adair CF, Heffess CS, Klimstra DS (1996) Intraductal oncocytic papillary neoplasms of the pancreas. Am J Surg Pathol 20:980–994
Adsay NV, Pierson C, Sarkar F, Abrams J, Weaver D, Conlon KC, Brennan MF, Klimstra DS (2001) Colloid (mucinous noncystic) carcinoma of the pancreas. Am J Surg Pathol 25:26–42
Adsay NV, Conlon KC, Zee SY, Brennan MF, Klimstra DS (2002) Intraductal papillary-mucinous neoplasms of the pancreas: an analysis of in situ and invasive carcinomas in 28 patients. Cancer 94:62–77
Adsay NV, Merati K, Andea A, Sarkar F, Hruban RH, Wilentz RE, Goggins M, Iocobuzio-Donahue C, Longnecker DS, Klimstra DS (2002) The dichotomy in the preinvasive neoplasia to invasive carcinoma sequence in the pancreas: differential expression of MUC1 and MUC2 supports the existence of two separate pathways of carcinogenesis. Mod Pathol 15:1087–1095
Adsay NV, Merati K, Basturk O, Iacobuzio-Donahue C, Levi E, Cheng JD, Sarkar FH, Hruban RH, Klimstra DS (2004) Pathologically and biologically distinct types of epithelium in intraductal papillary mucinous neoplasms: delineation of an “intestinal” pathway of carcinogenesis in the pancreas. Am J Surg Pathol 28:839–848
Albores-Saavedra J, Sheahan K, O'Riain C, Shukla D (2004) Intraductal tubular adenoma, pyloric type, of the pancreas: additional observations on a new type of pancreatic neoplasm. Am J Surg Pathol 28:233–238
Fukushima N, Mukai K, Kanai Y, Hasebe T, Shimada K, Ozaki H, Kinoshita T, Kosuge T (1997) Intraductal papillary tumors and mucinous cystic tumors of the pancreas: clinicopathologic study of 38 cases. Hum Pathol 28:1010–1017
Fukushima N, Mukai K, Sakamoto M, Hasebe T, Shimada K, Kosuge T, Kinoshita T, Hirohashi S (2001) Invasive carcinoma derived from intraductal papillary-mucinous carcinoma of the pancreas: clinicopathologic and immunohistochemical study of eight cases. Virchows Arch 439:6–13
Furukawa T, Takahashi T, Kobari M, Matsuno S (1992) The mucus-hypersecreting tumor of the pancreas. Development and extension visualized by three-dimensional computerized mapping. Cancer 70:1505–1513
Furukawa T, Fujisaki R, Yoshida Y, Kanai N, Sunamura M, Abe T, Takeda K, Matsuno S, Horii A (2005) Distinct progression pathways involving the dysfunction of DUSP6/MKP-3 in pancreatic intraepithelial neoplasia and intraductal papillary-mucinous neoplasms of the pancreas. Mod Pathol 18:1034–1042
Gum JR, Byrd JC, Hicks JW, Toribara NW, Lamport DT, Kim YS (1989) Molecular cloning of human intestinal mucin cDNAs. Sequence analysis and evidence for genetic polymorphism. J Biol Chem 264:6480–6487
Horinouchi M, Nagata K, Nakamura A, Goto M, Takao S, Sakamoto M, Fukushima N, Miwa A, Irimura T, Imai K, Sato E, Yonezawa S (2003) Expression of different glycoforms of membrane mucin (MUC1) and secretory mucin (MUC2, MUC5AC and MUC6) in pancreatic neoplasms. Acta Histochem Cytochem 36:443–453
Hruban RH, Takaori K, Klimstra DS, Adsay NV, Albores-Saavedra J, Biankin AV, Biankin SA, Compton C, Fukushima N, Furukawa T, Goggins M, Kato Y, Klöppel G, Longnecker DS, Lüttges J, Maitra A, Offerhaus GJ, Shimizu M, Yonezawa S (2004) An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol 28:977–987
Kato N, Akiyama S, Motoyama T (2002) Pyloric gland-type tubular adenoma superimposed on intraductal papillary mucinous tumor of the pancreas. Pyloric gland adenoma of the pancreas. Virchows Arch 440:205–208
Kim GE, Bae HI, Park HU, Kuan SF, Crawley SC, Ho JJ, Kim YS (2002) Aberrant expression of MUC5AC and MUC6 gastric mucins and sialyl Tn antigen in intraepithelial neoplasms of the pancreas. Gastroenterology 123:1052–1060
Klöppel G (1998) Clinicopathologic view of intraductal papillary-mucinous tumor of the pancreas. Hepatogastroenterology 45:1981–1985
Kobari M, Egawa S, Shibuya K, Shimamura H, Sunamura M, Takeda K, Matsuno S, Furukawa T (1999) Intraductal papillary mucinous tumors of the pancreas comprise 2 clinical subtypes: differences in clinical characteristics and surgical management. Arch Surg 134:1131–1136
Kosmahl M, Pauser U, Peters K, Sipos B, Lüttges J, Kremer B, Klöppel G (2004) Cystic neoplasms of the pancreas and tumor-like lesions with cystic features: a review of 418 cases and a classification proposal. Virchows Arch 445:168–178
Levi E, Klimstra DS, Andea A, Basturk O, Adsay NV (2004) MUC1 and MUC2 in pancreatic neoplasia. J Clin Pathol 57:456–462
Lüttges J, Zamboni G, Longnecker D, Klöppel G (2001) The immunohistochemical mucin expression pattern distinguishes different types of intraductal papillary mucinous neoplasms of the pancreas and determines their relationship to mucinous noncystic carcinoma and ductal adenocarcinoma. Am J Surg Pathol 25:942–948
Nakamura A, Horinouchi M, Goto M, Nagata K, Sakoda K, Takao S, Imai K, Kim YS, Sato E, Yonezawa S (2002) New classification of pancreatic intraductal papillary-mucinous tumour by mucin expression: its relationship with potential for malignancy. J Pathol 197:201–210
Ohhashi K, Murakami Y, Maruyama M, Takekoshi T, Ohta H, Ohhashi I, Takagi K, Kato Y (1982) Four cases of mucous secreting pancreatic cancer. (in Japanese, with English abstract). Prog Digest Endosc 20:348–351
Osako M, Yonezawa S, Siddiki B, Huang J, Ho JJ, Kim YS, Sato E (1993) Immunohistochemical study of mucin carbohydrates and core proteins in human pancreatic tumors. Cancer 71:2191–2199
Sessa F, Solcia E, Capella C, Bonato M, Scarpa A, Zamboni G, Pellegata NS, Ranzani GN, Rickaert F, Klöppel G (1994) Intraductal papillary-mucinous tumours represent a distinct group of pancreatic neoplasms: an investigation of tumour cell differentiation and K-ras, p53 and c-erbB-2 abnormalities in 26 patients. Virchows Arch 425:357–367
Suzuki Y, Atomi Y, Sugiyama M, Isaji S, Inui K, Kimura W, Sunamura M, Furukawa T, Yanagisawa A, Ariyama J, Takada T, Watanabe H, Suda K (2004) Cystic neoplasm of the pancreas: a Japanese multiinstitutional study of intraductal papillary mucinous tumor and mucinous cystic tumor. Pancreas 28:241–246
Tajiri T, Tate G, Inagaki T, Kunimura T, Inoue K, Mitsuya T, Yoshiba M, Morohoshi T (2005) Intraductal tubular neoplasms of the pancreas: histogenesis and differentiation. Pancreas 30:115–121
Terris B, Ponsot P, Paye F, Hammel P, Sauvanet A, Molas G, Bernades P, Belghiti J, Ruszniewski P, Flejou JF (2000) Intraductal papillary mucinous tumors of the pancreas confined to secondary ducts show less aggressive pathologic features as compared with those involving the main pancreatic duct. Am J Surg Pathol 24:1372–1377
Terris B, Dubois S, Buisine MP, Sauvanet A, Ruszniewski P, Aubert JP, Porchet N, Couvelard A, Degott C, Flejou JF (2002) Mucin gene expression in intraductal papillary-mucinous pancreatic tumours and related lesions. J Pathol 197:632–637
Yonezawa S, Horinouchi M, Osako M, Kubo M, Takao S, Arimura Y, Nagata K, Tanaka S, Sakoda K, Aikou T, Sato E (1999) Gene expression of gastric type mucin (MUC5AC) in pancreatic tumors: its relationship with the biological behavior of the tumor. Pathol Int 49:45–54
Acknowledgements
We thank Ms. Naomi Kanai for technical assistance and Ms. Kay Dege for editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported in part by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan, the Pancreas Research Foundation of Japan, the Gonryo Medical Foundation, and NIH SPORE (Specialized Programs of Research Excellence) in Gastrointestinal Cancer Grant CA62924.
An erratum to this article can be found at http://dx.doi.org/10.1007/s00428-012-1260-9
Rights and permissions
About this article
Cite this article
Furukawa, T., Klöppel, G., Volkan Adsay, N. et al. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: a consensus study. Virchows Arch 447, 794–799 (2005). https://doi.org/10.1007/s00428-005-0039-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-005-0039-7