Abstract
Several studies have shown that endometrial stromal neoplasms express estrogen and progesterone receptors (ER, PR). To our knowledge, the presence or absence of androgen receptors (AR) in these rare uterine neoplasms has not been investigated. Tumors (n=20)—3 endometrial stromal nodules, 14 low-grade endometrial stromal sarcomas (ESS, low grade), and 3 high-grade endometrial sarcomas (undifferentiated endometrial sarcoma, UES)—were studied. Immunohistochemical analyses for ER, PR, and AR were performed on formalin-fixed, paraffin-embedded archival material. Positive immunoreactions for ER and PR were observed in 14 (70%) and 17 (85%) cases, respectively. Furthermore, 9 cases (45%) were positive for AR. Among 17 ESS and UES cases, 7 (41%) revealed positivity for AR. Two of three benign stromal nodules were also positive for AR. Moreover, one of the three high-grade sarcomas (undifferentiated endometrial sarcoma) was negative for both ER and PR, but showed positive reaction for AR. In summary, ARs are expressed in 45% of endometrial stromal neoplasms. In addition to determination of ER and PR, the results of immunohistochemical examination of AR in these rare uterine tumors may have some impact on the postoperative management of the patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Uterine endometrial stromal tumors are among the rarest neoplasms in the female genital tract. Malignant mesenchymal tumors comprise less than 5% of primary uterine cancers, with endometrial stromal neoplasms accounting for less than 10% thereof [1, 11]. Endometrial stromal tumors are composed of cells resembling those of proliferative phase endometrial stroma. These tumors are subdivided into benign and malignant categories based on the type of tumor margin. Uterine endometrial stromal tumors with pushing margins are benign and designated endometrial stromal nodules (ESN). In contrast, endometrial stromal sarcomas (ESS) infiltrate the myometrium; they have been traditionally divided into low and high grades mainly based on mitotic count [16]. However, since high-grade endometrial sarcomas largely lack specific differentiation and hardly display histological resemblance to endometrial stroma, it has been proposed that they should be designated undifferentiated endometrial or uterine sarcomas [5]. Thus, the recent World Health Organization Classification of Tumors of the Breast and Female Genital Organs divides the uterine stromal neoplasms into three groups: (i) benign ESN, (ii) low-grade ESS, and (iii) undifferentiated endometrial sarcoma [27].
While low-grade ESS is a clinically indolent malignant neoplasm, which shows minimal cytological atypia, infrequent mitotic figures, and numerous thin-walled small arteriolar type (plexiform) vessels, the undifferentiated endometrial sarcoma is a highly aggressive tumor that lacks a plexiform vasculature, features severe cytological atypia, and has frequent and often atypical mitotic figures [4, 27].
Several previous biochemical and immunohistochemical studies [13, 21, 22, 29] have shown that uterine stromal neoplasms, particularly low-grade ESS, often express estrogen and progesterone receptors (ER, PR). Studies have also revealed that therapy with progestational agents is an important adjunct to surgical treatment in cases of low-grade ESS [7]. Many of the tumors that contain PR will, at least partially, respond to hormonal manipulation using progestins [10, 15]. In contrast to ER and PR, the status of androgen receptors (AR) in benign and malignant uterine stromal neoplasms (ESN, ESS) has not yet been investigated. In the present study, we analyzed the immunoexpressions of AR as well as ER and PR in a series of 20 primary endometrial stromal tumors.
Materials and methods
Cases (n=20) comprising 3 benign ESN, 14 low-grade ESS, and 3 undifferentiated endometrial sarcomas were retrieved from the files of the Department of Gynecologic and Breast Pathology, Armed Forces Institute of Pathology, Washington, DC, and the Department of Pathology, Medical University of Graz, Austria. Determination of tumor type and histopathological grade was performed according to the new World Health Organization classification on Tumors of the Breast and Female Genital Organs [27].
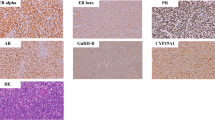
Formalin-fixed, paraffin-embedded tissue blocks were cut into 4-µm-thick serial sections, which were mounted on pre-coated slides. The sections were deparaffinized, rehydrated, and rinsed in distilled water. Immunohistochemical assays for AR, ER, and PR were performed on consecutive paraffin sections using standardized automated procedures (Ventana Medical System, Tucson, AZ and Dako, Glostrup, Denmark) (Table 1). Monoclonal mouse antihuman antibody clones 6F11 and 1A6 (Ventana Medical Systems) were used as primary antibodies for ER and PR, respectively. For determination of AR expression, the monoclonal mouse antihuman androgen receptor antibody (clone: AR441; Dako) was used. In brief, antigen retrieval was achieved with microwave treatment (ER, PR) or heating in a water bath (AR) (Table 1). A Ventana ES (Ventana Medical Systems) or Chem-Mate (Dako) autostainer was used in conjunction with an indirect streptavidin-biotin method. After incubation with the primary antibody, incubation with the secondary (link) biotinylated antibody was performed for 30 min. After washing, sections were incubated with streptavidin-peroxidase for 30 min. Finally, the enzyme was visualized with diaminobenzidine. Counterstaining was performed with hematoxylin. In each case, the intensity of immunoreaction (negative, 1+, 2+, 3+) and the percentage of tumor cells with positive nuclear reaction were evaluated. Samples were scored as positive when at least 10% of nuclei were immunoreactive. Internal positive controls included normal endomyometrial tissue surrounding the tumors (Fig. 1A). Negative controls included substitution of the primary antibody with normal sera or phosphate-buffered saline, omission of the secondary antibody, and incubation of the primary antibody solution with lymphoid tissue. All slides were evaluated independently by at least two investigators (F.M. and A.D.T.). The rare cases in which disagreement occurred were reevaluated using a multi-headed microscope; a final agreement was reached in all cases.
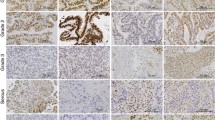
Expressions of estrogen receptors (ER) (A1), progesterone receptors (PR) (A2), and androgen receptors (AR) (A3) in non-neoplastic, normal endometrium (proliferative phase). B A low-grade endometrial stromal sarcoma (ESS) with typical infiltrative margins (B1), multiple lymphatic invasions (B2), and numerous thin-walled, small blood vessels (B3). C Expressions of ER (C1), PR (C2), and AR (C3) in a low-grade ESS. D, E A high-grade endometrial sarcoma (undifferentiated endometrial sarcoma) showing infiltrative pattern (D1), tumor cell necrosis (D2), and significant nuclear atypia (D3). Although the malignant stromal cells were completely negative for ER and PR (data not shown), a positive immunoreaction (1+ to 2+) for AR could be identified in many areas of this high-grade (undifferentiated) sarcoma (E1, E2, E3)
Results
Results are summarized in Table 2. Positive immunoreactions for ER and PR in benign and malignant stromal tumors were observed in 14 (70%) and 17 (85%) cases, respectively. Androgen receptor positivity was found in 9 (45%) cases. While all three ESN cases were PR positive, two of them were positive for ER and AR. In 17 sarcomas (14 ESS, low grade and 3 undifferentiated endometrial sarcoma), positive reactions for ER, PR, and AR were observed in 12 (75%), 14 (83%), and 7 (41%) cases, respectively. In low-grade ESS, positive immunoreactions for ER, PR, and AR were found in 11 (79%), 13 (93%), and 5 (36%) cases, respectively (Fig. 1B, C). Although two of the three undifferentiated endometrial sarcomas were negative for both ER and PR, a positive reaction of highly atypical tumor cells for AR was observed in one of the ER and PR negative cases (Fig. 1D, E) (Table 2).
Discussion
Previous studies demonstrated ER and PR in normal proliferative and secretory phase endometrium, both in endometrial glands and in stromal cells [19, 26]. A previous study also showed high ER and PR content in epithelium and stromal cells of simple and complex hyperplasia [2]. In atypical complex hyperplasia and endometrioid adenocarcinoma, however, the receptor content was significantly lower compared with that of normal proliferative or hyperplastic endometrium [2, 17, 19]. A few studies have demonstrated nuclear staining for AR in normal glands, endometrial stromal cells, and in endometrioid adenocarcinomas [3].
Several previous studies revealed expression of ER and PR in a high percentage of endometrial stromal sarcoma [13, 21, 22, 29]. However, to the best of our knowledge, the issue of AR expression in benign and malignant endometrial stromal neoplasms has not been examined previously.
According to our study, 45% of endometrial stromal neoplasms were AR positive. In sarcomas (low and high grades), a positive immunoreaction for AR was observed in 41% of examined cases. In all cases, the distribution of nuclear positivity for ER, PR, and AR among the tumor cells was quite heterogeneous (Table 2).
At least a partial response of low-grade ESS to hormonal therapy with progestins has been observed [8, 12, 18]. Recurrent or metastatic low-grade ESS have also been reported to be stabilized or suppressed with progestational agents in more than 50% of patients [5, 9, 20]. Tumors with a high level of progesterone receptors are most likely to respond to progestin therapy [20, 23, 28]. Treatment with gonadotropin-releasing hormone agonists has also been used in a few studies [14, 24]. However, although the vast majority of low-grade ESS have been shown to express ER and PR, some of the tumors, even with high ER and PR content, did not respond to adjuvant hormonal treatment [6, 25, 30]. This could be, at least in part, due to the heterogeneity of tumor cells in terms of expression of ER and PR and the presence or absence of other steroid receptors, such as AR. It is possible that the proportional distribution and concentration of each steroid receptor (ER, PR, and AR) among tumor cells influences the response to hormonal treatments. The biological significance of AR (stimulation versus inhibition of the growth of neoplastic endometrial stromal cells) and its interaction(s) with ER and PR in ESS requires further investigation.
In summary, our study shows for the first time that ARs are expressed in 45% of endometrial stromal neoplasms. Thus, in addition to ER and PR, the immunohistochemical examination of AR in endometrial stromal neoplasms, particularly in low-grade ESS, may have impact on the postoperative management of the patients.
References
Aaro LA, Symmonds RE, Dockerty MB (1966) Sarcoma of the uterus. A clinical and pathologic study of 177 cases. Am J Obstet Gynecol 94:101–109
Bergeron C, Ferenczy A, Shyamala G (1988) Distribution of estrogen receptors in various cell types of normal, hyperplastic, and neoplastic human endometrial tissues. Lab Invest 58:338–345
Brys M, Semczuk A, Baranowski W, Jakowicki J, Krajewska WM (2002) Androgen receptor (AR) expression in normal and cancerous human endometrial tissues detected by RT-PCR and immunohistochemistry. Anticancer Res 22:1025–1031
Chang KL, Crabtree GS, Lim-Tan SK, Kempson RL, Hendrickson MR (1990) Primary uterine endometrial stromal neoplasms. A clinicopathologic study of 117 cases. Am J Surg Pathol 14:415–438
Evans HL (1982) Endometrial stromal sarcoma and poorly differentiated endometrial sarcoma. Cancer 50:2170–2182
Gadducci A, Sartori E, Landoni F, Zola P, Maggino T, Urgesi A, Lissoni A, Losa G, Fanucchi A (1996) Endometrial stromal sarcoma: analysis of treatment failures and survival. Gynecol Oncol 63:247–253
Gloor E, Schnyder P, Cikes M, Hofstetter J, Cordey R, Burnier F, Knobel P (1982) Endolymphatic stromal myosis. Surgical and hormonal treatment of extensive abdominal recurrence 20 years after hysterectomy. Cancer 50:1888–1893
Horowitz K, Rutherford T, Schwarz PE (1996) Hormone replacement therapy in women with sarcomas. CME J Gynecol Oncol 1:23–29
Katz L, Merino MJ, Sakamoto H, Schwartz PE (1987) Endometrial stromal sarcoma: a clinicopathologic study of 11 cases with determination of estrogen and progestin receptor levels in three tumors. Gynecol Oncol 26:87–97
Keen CE, Philip G (1989) Progestogen-induced regression in low-grade endometrial stromal sarcoma. Case report and literature review. Br J Obstet Gynaecol 96:1435–1439
Koss LG, Spiro RH, Brunschwig A (1965) Endometrial stromal sarcoma. Surg Gynecol Obstet 121:531–537
Krumholz BA, Lobovsky FY, Halitsky V (1973) Endolymphatic stromal myosis with pulmonary metastases. Remission with progestin therapy: report of a case. J Reprod Med 10:85–89
Lantta M, Kahanpaa K, Karkkainen J, Lehtovirta P, Wahlstrom T, Widholm O (1984) Estradiol and progesterone receptors in two cases of endometrial stromal sarcoma. Gynecol Oncol 18:233–239
Mesia AF, Demopoulos RI (2000) Effects of leuprolide acetate on low-grade endometrial stromal sarcoma. Am J Obstet Gynecol 182:1140–1141
Navarro D, Cabrera JJ, Leon L, Chirino R, Fernandez L, Lopez A, Rivero JF, Fernandez P, Falcon O, Jimenez P, et al (1992) Endometrial stromal sarcoma expression of estrogen receptors, progesterone receptors and estrogen-induced srp27 (24 K) suggests hormone responsiveness. J Steroid Biochem Mol Biol 41:589–596
Norris HJ, Taylor HB (1966) Mesenchymal tumors of the uterus. I. A clinical and pathological study of 53 endometrial stromal tumors. Cancer 19:755–766
Nyholm HC, Nielsen AL, Lyndrup J, Norup P, Thorpe SM (1992) Biochemical and immunohistochemical estrogen and progesterone receptors in adenomatous hyperplasia and endometrial carcinoma: correlations with stage and other clinicopathologic features. Am J Obstet Gynecol 167:1334–1342
Pellillo D (1968) Proliferative stromatosis of the uterus with pulmonary metastases. Remission following treatment with a long acting synthetic progestin: a case report. Obstet Gynecol 31:33–39
Pickartz H, Beckmann R, Fleige B, Due W, Gerdes J, Stein H (1990) Steroid receptors and proliferative activity in non-neoplastic and neoplastic endometria. Virchows Arch 417:163–171
Piver MS, Rutledge FN, Copeland L, Webster K, Blumenson L, Suh O (1984) Uterine endolymphatic stromal myosis: a collaborative study. Obstet Gynecol 64:173–178
Reich O, Regauer S, Urdl W, Lahousen M, Winter R (2000) Expression of oestrogen and progesterone receptors in low-grade endometrial stromal sarcomas. Br J Cancer 82:1030–1034
Sabini G, Chumas JC, Mann WJ (1992) Steroid hormone receptors in endometrial stromal sarcomas. A biochemical and immunohistochemical study. Am J Clin Pathol 97:381–386
Schilder JM, Hurd WW, Roth LM, Sutton GP (1999) Hormonal treatment of an endometrial stromal nodule followed by local excision. Obstet Gynecol 93:805–807
Scribner DR Jr, Walker JL (1998) Low-grade endometrial stromal sarcoma preoperative treatment with Depo-Lupron and Megace. Gynecol Oncol 71:458–460
Soper JT, McCarty KS Jr, Hinshaw W, Creasman WT, McCarty KS Sr, Clarke-Pearson DL (1984) Cytoplasmic estrogen and progesterone receptor content of uterine sarcomas. Am J Obstet Gynecol 150:342–348
Tamaya T, Arabori K, Okada H (1988) Relation between steroid receptor levels and prolactin level in the endometrial stromal cells. Acta Obstet Gynecol Scand 67:265–269
Tavassoli FA, Devilee P (2003) World Health Organization classification of tumors: pathology and genetics of tumors of the breast and female genital organs, IARC Press, Lyon
Thatcher SS, Woodruff JD (1982) Uterine stromatosis: a report of 33 cases. Obstet Gynecol 59:428–434
Tosi P, Sforza V, Santopietro R (1989) Estrogen receptor content, immunohistochemically determined by monoclonal antibodies, in endometrial stromal sarcoma. Obstet Gynecol 73:75–78
Wade K, Quinn MA, Hammond I, Williams K, Cauchi M (1990) Uterine sarcoma: steroid receptors and response to hormonal therapy. Gynecol Oncol 39:364–367
Acknowledgement
This study was supported by the Lore-Saldow-Reasearch-Fund. The authors thank Mrs. Margit Gogg-Kamerer and Ms. Andrea Sommersacher for their excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moinfar, F., Regitnig, P., Tabrizi, A.D. et al. Expression of androgen receptors in benign and malignant endometrial stromal neoplasms. Virchows Arch 444, 410–414 (2004). https://doi.org/10.1007/s00428-004-0981-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-004-0981-9