Abstract
We describe the phylogenetic analysis and expression pattern of the Xenopus radial spoke protein 3 (RSP3) gene during early development. The Xenopus RSP3 protein presents characteristic features of the RSP3 family. It contains a radial spoke domain, which is 75 and 72 % identical to the corresponding region of human and Chlamydomonas RSP3 proteins, respectively. Examination of the phylogenetic relationship between the Xenopus RSP3 protein and its known homologues from different deuterostomes indicates that the RSP3 proteins are highly conserved among deuterostomes. Whole-mount in situ hybridization analyses show that Xenopus RSP3 is a maternal mRNA enriched in the animal hemisphere during cleavage stages. The expression is detected in the dorsal region of the embryo during gastrulation, then in the presumptive neuroectoderm at the end of gastrulation. During neurulation and at the subsequent stages, the expression of RSP3 mRNA is detected in the entire multiciliated cells of epidermis. At tail-bud stages, it is progressively expressed in the otic vesicles and sequentially expressed in the nephrostomes. Expression could be also detected in the floor plate of the neural tube. This expression pattern persists until at least late tail-bud stages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eucaryote cilia and flagella are highly ordered organelles that are involved in generating directional movement. The remarkably conserved architecture of these organelles is formed by a microtubule-based 9 + 2 scaffold called the axoneme (Barber et al. 2011; Pigino and Ishikawa 2012). Defects in the function of these organelles are linked to several human diseases called ciliopathies (Fliegauf et al. 2007). Cilia also play an important role in early development for the generation of directed fluid flow that is essential for a variety of developmental and physiological processes, including directed cell migration and the determination of the left–right asymmetry of organ development (Basu and Brueckner 2008; Blum et al. 2009; Werner and Mitchell 2012). Thus, understanding the generation and function of ciliated cells should help to elucidate the molecular basis of human diseases. The surface of Xenopus embryo develops a punctate pattern of ciliated cells and has been a suitable model for the analysis of the molecular determination of ciliated cell fate (Park et al. 2008; Wessely and Obara 2008).
The radial spoke is an ubiquitous component of motile cilia and flagella. It is formed by a complex of at least 23 distinct radial spoke proteins (RSPs) that have been identified in Chlamydomonas flagella and play an essential role in the regulation of cilia and flagellar motility (Yang et al. 2006; Mizuno et al. 2012). In mammals as in Chlamydomonas, radial spoke protein 3 (RSP3) is one of the 23 radial spoke structural components, which has been shown to function as a protein kinase A-anchoring protein required for the assembly of radial spoke and flagellar motility (Gaillard et al. 2001; Jivan et al. 2009). Although it is a component of the radial spoke, the expression pattern during early development is not clear. In this study, we report the phylogenetic analysis of RSP3 and its temporal–spatial expression pattern during Xenopus early development. We show that Xenopus RSP3 mRNA is localized to the multiciliated cells of epidermis, otic vesicles, and the nephrostomes.
Materials and methods
Sequence comparison and phylogenetic analysis
The deduced amino acid sequence of Xenopus RSP3 was aligned with related sequences from other species by the ClustalW program. Phylogenetic trees using the neighbor-joining and maximum likelihood methods with default settings and a Bayesian tree estimated by Bayesian inference were constructed as described previously (Kong et al. 2012).
Xenopus embryos and in situ hybridization
Xenopus embryo manipulation was described previously (Li et al. 2010). For whole-mount in situ hybridization, the RSP3 antisense probe in pBluescript was linearized with Not I, and the sense probe was linearized with EcoR I. They were labelled with digoxigenin-11-UTP using T7 and T3 RNA polymerase (Roche), respectively. Some embryos were sectioned to better detect hybridization signals inside the embryos. After whole-mount hybridization, some embryos were also made transparent in 2:1 benzyl benzoate/benzyl alcohol.
Results and discussion
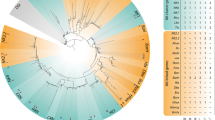
A Xenopus cDNA clone was initially identified by in situ hybridization screening of restricted gene expression during early development (Bourdelas et al. 2009). A BLAST search indicated that the nucleotide sequence of this cDNA was 100 % identical with the RSP3 cDNA sequence in the database (NM_001127814). This Xenopus RSP3 cDNA sequence encodes a protein with 383 amino acids, which shares high degree of conservation and characteristic features with respect to the RSP3 proteins from other species (Fig. 1). In particular, the radial spoke domain (amino acids 43–332) has 75 and 72 % identity with the corresponding region of human and Chlamydomonas RSP3 proteins, respectively. In addition, an A-kinase anchoring protein (AKAP) domain (amino acids 174–193) is also highly conserved (Fig. 1). Phylogenetic relationship between the Xenopus RSP3 protein and its known homologues from different deuterostomes including sea urchins, acorn worms, tunicates, and amphioxus was analyzed using the maximum likelihood (Fig. 2a), neighbor-joining (Fig. 2b) methods, and Bayesian inference (Fig. 2c). The analyses by different methods come to a similar conclusion that the Xenopus RSP3 groups into a vertebrate subgroup and the RSP3 proteins are highly conserved among deuterostomes.
Sequence alignment between RSP3 proteins from different species, produced by ClustalW. The radial spoke domain (RSP3) is indicated and the AKAP domain is underlined. Dashes indicate absence of residues at the corresponding positions. Asterisks indicate identical residues, double dots indicate conserved substitutions, and dots indicate semi-conserved substitutions. Cr, Chlamydomonas reinhardtii; Mr, Megachile rotundata; Sp, Strongylocentrotus purpuratus; Sk, Saccoglossus kowalevskii; Ci, Ciona intestinalis; Bf, Branchiostoma floridae; Dr, Danio rerio; Xl, Xenopus laevis; Xt, Xenopus tropicalis; Gg, Gallus gallus; Mm, Mus musculus; Hs, Homo sapiens
Phylogenetic trees of RSP3 constructed using the maximum likelihood (a) and neighbor-joining (b) methods and the Bayesian inference (c). The values beside the branches represent the percentage of times that a node was supported in 1,000 bootstrap pseudoreplications (a and b) or the Bayesian posterior probability (c). The scale bars indicate an evolutionary distance of 0.1 substitutions per position. The Xenopus RSP3 is shown by a black dot and groups into a vertebrate subgroup. The identity of amino acid sequences between Xenopus RSP3 and its homologues in other species is indicated as percentage in the brackets following the species names. Sources of the sequences are from NCBI, except for the Branchiostoma floridae RSP3, which is from JGI (v1.0 Bf_V2_13: 1617944–1618190). Accession numbers for RSP3 from different species are listed as follows: Chlamydomonas reinhardtii (XP_001695406.1); Megachile rotundata (XP_003706939.1); Ciona intestinalis (NP_001027624.1); Saccoglossus kowalevskii (XP_002740588.1); Strongylocentrotus purpuratus (XP_783727.1); Xenopus laevis (NP_001121286.1); Xenopus tropicalis (NP_998863.1); Danio rerio (NP_001082864.1); Gallus gallus (XP_419702.11); Mus musculus (NP_080065.4 for RSP3a and NP_001077414.1 for RSP3b); and Homo sapiens (NP_114130.3)
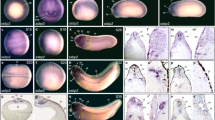
We then performed whole-mount in situ hybridization to examine the temporal and spatial expression of RSP3. The results indicated that Xenopus RSP3 is maternal mRNA strongly enriched in the animal hemisphere at the eight-cell stage (Fig. 3a). This expression pattern persists during cleavage stages, and at the late blastula stage, expression is still enriched in the animal hemisphere, although punctate expression pattern could be also detected in the vegetal hemisphere (Fig. 3b). At the early gastrula stage, the expression became weak and was more diffusely located in the dorsal half of the embryo (Fig. 3c). At the end of gastrulation, the expression level remains weak and was detected in the future neural plate; however, at this stage, new expression site was found in the dorsal and lateral blastopore (Fig. 3d). At the beginning of neurulation, RSP3 mRNA was first diffusely expressed in the neural plate and neural folds (not shown), but not in the gastrocoel roof plate (GRP), as revealed in sectioned embryos (Fig. 3e). The GRP consists of monociliated cells, and the cilia beating results in fluid flow towards the left side, which determines the left–right asymmetry of organ development (Blum et al. 2009). Thus, the absence of RSP3 expression in this tissue suggests that it may be not involved in this process. However, its expression in the multiciliated cells of epidermis became evident as neurulation proceeds (Fig. 3f). At the end of neurulation to the early tail-bud stage, RSP3 was predominantly expressed in the entire multiciliated epidermis (Fig. 3g). It could be observed that ciliated cells were more densely distributed in the anterior region than the trunk and posterior regions of the tail-bud stage embryos. Detailed examination indicated that, at the early tail-bud stage, some RSP3-expressing ciliated cells prefigure the otic vesicle (Fig. 3g). This becomes more evident as development proceeds. Thus, at the stage 30, in addition to the multiciliated cells of epidermis, RSP3 expression could be unambiguously detected in the otic vesicle, and also in the two anterior nephrostomes, but not, or very weakly, in the posterior one (Fig. 3h, j). We found that, from 3 independent experiments with 23 embryos at stages 29–30 hybridized with RSP3 probe, 20 embryos showed RSP3 expression in the two anterior nephrostomes. The region around the anus also exhibited dense ciliated cells (Fig. 3h). RSP3 expression was also enriched in the posterior tip of the neural tube (Fig. 3h). Sense probe did not show such labelling (Fig. 3i). In sectioned embryos, it could be observed that the neural tube expression was localized to the floor plate (Fig. 3k). At the stage 35, the same expression pattern persists, except that the third nephrostome, posterior to the two anterior ones, was also strongly marked by RSP3 expression (Fig. 3l, m). This observation suggests that RSP3 may be expressed sequentially in the nephrostomes during development. As at the stage 30, dense ciliated cells could be observed around the anus at the stage 35 (Fig. 3n). Interestingly, higher magnification of RSP3-stained cells indicates a heterogenous distribution of the mRNA within the multiciliated cells, no hybridization signal could be found at the sites corresponding likely to the multicilia (Fig. 3o). In embryos made transparent after whole-mount hybridization, multiciliated cells could be also easily observed in the dorsal and ventral fins (Fig. 3p). These three major expression sites including ciliated epidermis, otic vesicles, and nephrostomes persist until at least the stage 40 late tail-bud embryo (not shown).
Expression pattern of Xenopus RSP3 during early development analyzed by whole-mount in situ hybridization. a An eight-cell stage embryo showing the expression in the four animal blastomeres, which are oriented on the top. b Localization of RSP3 transcripts in the animal hemisphere (oriented on the top) at the late blastula stage. Punctate expression can be observed in the vegetal hemisphere. c Dorso-vegetal view of a stage 11 early gastrula with low level of RSP3 expression in the dorsal half of the embryo. d Dorsal view of a stage 13 late gastrula with diffuse RSP3 expression in the future neural plate. Other expression sites include the dorsal and lateral regions of the blastopore. e Transverse section at the trunk region of a stage 15 early neurula shows the expression of RSP3 mRNA in the neural plate. f Dorsal view of a stage 20 late neurula shows RSP3 expression in the entire multiciliated cells of epidermis. Anterior is to the left. g In an early tail-bud embryo at the stage 24, the RSP3 expression site also prefigures the otic vesicle (arrowhead). Anterior is to the left. h At the stage 30, additional RSP3 expression sites are detected in the two anterior nephrostomes (arrows) and in the posterior neural tube. i A stage 30 embryo hybridized with sense probe. j Higher magnification corresponds to the rectangle in h. Note that RSP3 expression is not detected in the third nephrostome (dashed arrow). k Transverse section cut at the trunk region as indicated by the vertical line in h shows RSP3 expression in the floor plate of the neural tube (n). l At the stage 35, RSP3 expression appears in the third nephrostome (arrow). m, n Higher magnifications correspond to the boxes in l. o Higher magnification of multiciliated cells of the epidermis, showing heterogenous distribution of RSP3 mRNA. p A stage 35 embryo made transparent to show RSP3 expression in the dorsal and ventral fins
In summary, this analysis indicates the Xenopus RSP3 is a maternal mRNA, which may play an early role during development. However, the expression level decreases at the onset of gastrulation and then gradually increases only during neurulation. This suggests that the zygotic transcription of RSP3 probably starts only at the onset of neurulation. In addition, the localization of RSP3 transcripts from neurulation onward is strongly correlated to the differentiation of multiciliated cells, making it a new marker of ciliated epidermis in the Xenopus embryo. Furthermore, our analysis also indicates that the expression pattern of RSP3 prefigures the formation of otic vesicles. Interestingly, RSP3 is expressed sequentially in the nephrostomes. It is unlikely that this correlates with a sequential formation of the three nephrostomes since at least Wnt4 is expressed simultaneously in the three nephrostomes at stages 29–30 (Saulnier et al. 2002). Several other motile cilia-expressed genes, such as dnah9, are also localized to the nephrostomes (Vick et al. 2009); however, whether they are expressed sequentially in the nephrostomes is not clear. It will be thus of interest to perform a detailed comparison of the temporal expression pattern between RSP3 and these genes. Nevertheless, the expression of RSP3 in multiciliated cells, otic vesicles, and the nephrostomes suggests that these tissues may share common structures and/or function, at least in RSP3-expressing cells. It is also consistent with the characteristic feature that the nephrostomes are ciliated peritoneal funnels, which may be actively involved in various transport activities (Raciti et al. 2008). In addition to multiciliated cells, RSP3 may also have function in neural epithelia because its expression could be detected in the floor plate in Xenopus and in the developing neocortex, hippocampus, and cerebellum in the mouse embryo (Koukoulas et al. 2004). Since RSP3 contains an AKAP domain associated with signal transduction, this suggests that the RSP3-expressing cells may share common features as both scaffolds and signal transducers.
References
Barber CF, Heuser T, Carbajal-González BI, Botchkarev VV Jr, Nicastro D (2011) Three-dimensional structure of the radial spokes reveals heterogeneity and interactions with dyneins in Chlamydomonas flagella. Mol Biol Cell 23:111–120
Basu B, Brueckner M (2008) Cilia multifunctional organelles at the center of vertebrate left-right asymmetry. Curr Top Dev Biol 85:151–174
Bourdelas A, Li HY, Carron C, Shi DL (2009) Dynamic expression pattern of distinct genes in the presomitic and somitic mesoderm during Xenopus development. Int J Dev Biol 53:1075–1079
Blum M, Beyer T, Weber T, Vick P, Andre P, Bitzer E, Schweickert A (2009) Xenopus, an ideal model system to study vertebrate left–right asymmetry. Dev Dyn 238:1215–1225
Fliegauf M, Benzing T, Omran H (2007) When cilia go bad: cilia defects and ciliopathies. Mol Cell Biol 8:880–893
Gaillard AR, Diener DR, Rosenbaum JL, Sale WS (2001) Flagellar radial spoke protein 3 is an A-kinase anchoring protein (AKAP). J Cell Biol 153:443–448
Jivan A, Earnest S, Juang YC, Cobb MH (2009) Radial spoke protein 3 is a mammalian protein kinase A-anchoring protein that binds ERK1/2. J Biol Chem 284:29437–29445
Kong WH, Yang YJ, Zhang TX, Shi DL, Zhang YJ (2012) Characterization of sFRP2-like in amphioxus: insights into the evolutionary conservation of Wnt antagonizing function. Evol Dev 14:168–177
Koukoulas I, Augustine C, Silkenbeumer N, Gunnersen JM, Scott HS, Tan SS (2004) Genomic organisation and nervous system expression of radial spoke protein 3. Gene 336:15–23
Li HY, Bourdelas A, Carron C, Shi DL (2010) The RNA-binding protein Seb4 is a target of MyoD and is required for myogenesis during Xenopus early development. Mech Dev 127:281–291
Mizuno N, Taschner M, Engel BD, Lorentzen E (2012) Structural studies of ciliary components. J Mol Biol 422:163–180
Park TJ, Mitchell BJ, Abitua PB, Kintner C, Wallingford JB (2008) Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat Genet 40:871–879
Pigino G, Ishikawa T (2012) Axonemal radial spokes: 3D structure, function and assembly. Bioarchitecture 2:50–58
Raciti D, Reggiani L, Geffers L, Jiang Q, Bacchion F, Subrizi AE, Clements D, Tindal C, Davidson DR, Kaissling B, Brändli AW (2008) Organization of the pronephric kidney revealed by large-scale gene expression mapping. Genome Biol 9:R84
Saulnier DM, Ghanbari H, Brändli AW (2002) Essential function of Wnt-4 for tubulogenesis in the Xenopus pronephric kidney. Dev Biol 248:13–28
Vick P, Schweickert A, Weber T, Eberhardt M, Mencl S, Shcherbakov D, Beyer T, Blum M (2009) Flow on the right side of the gastrocoel roof plate is dispensable for symmetry breakage in the frog Xenopus laevis. Dev Biol 331:281–291
Werner ME, Mitchell BJ (2012) Understanding ciliated epithelia: the power of Xenopus. Genesis 50:176–185
Wessely O, Obara T (2008) Fish and frogs: models for vertebrate cilia signaling. Front Biosci 13:1866–1880
Yang P, Diener DR, Yang C, Kohno T, Pazour GJ, Dienes JM, Agrin NS, King SM, Sale WS, Kamiya R, Rosenbaum JL, Witman GB (2006) Radial spoke proteins of Chlamydomonas flagella. J Cell Sci 119:1165–1174
Acknowledgments
This work was supported by grants from the Association Française contre les Myopathies and the Agence Nationale de la Recherche (ANR-09-BLAN-0262-03) to D.L.S. and the National Natural Science Foundation of China (30470902) and the Natural Science Foundation of Shandong (Y2007D13) to Y.J.Z. We thank X. Guo and X. Song for illustrations.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Hollemann
Rights and permissions
About this article
Cite this article
Zhang, YJ., Zhao, L., Meng, YP. et al. Xenopus radial spoke protein 3 gene is expressed in the multiciliated cells of epidermis and otic vesicles and sequentially in the nephrostomes. Dev Genes Evol 223, 183–188 (2013). https://doi.org/10.1007/s00427-012-0433-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-012-0433-5