Abstract
Pycnogonida (sea spiders) are bizarre marine arthropods that are nowadays most frequently considered as being the sister group to all other chelicerates. The majority of pycnogonid species develops via a protonymphon larva with only three pairs of limbs affiliated with the future head region. Deviating from this, the hatching stage of some representatives shows already an advanced degree of trunk differentiation. Using scanning electron microscopy, fluorescent nucleic staining, and bright-field stereomicroscopy, postembryonic development of Pseudopallene sp. (Callipallenidae), a pycnogonid with an advanced hatching stage, is described. Based on external morphology, six postembryonic stages plus a sub-adult stage are distinguished. The hatching larva is lecithotrophic and bears the chelifores as only functional appendage pair and unarticulated limb buds of walking leg pairs 1 and 2. Palpal and ovigeral larval limbs are absent. Differentiation of walking leg pairs 3 and 4 is sequential. Apart from the first pair, each walking leg goes through a characteristic sequence of three externally distinct stages with two intermittent molts (limb bud—seven podomeres—nine podomeres). First external signs of oviger development are detectable in postembryonic stage 3 bearing three articulated walking leg pairs. Following three more molts, the oviger has attained adult podomere composition. The advanced hatching stages of different callipallenids are compared and the inclusive term “walking leg-bearing larva” is suggested, as opposed to the behavior-based name “attaching larva”. Data on temporal and structural patterns of walking leg differentiation in other pycnogonids are reviewed and discussed. To facilitate comparisons of walking leg differentiation patterns across many species, we propose a concise notation in matrix fashion. Due to deviating structural patterns of oviger differentiation in another callipallenid species as well as within other pycnogonid taxa, evolutionary conservation of characteristic stages of oviger development is not apparent even in closely related species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pycnogonida are an exclusively marine arthropod group with a unique morphology, being characterized by a prominent anterior proboscis that bears the mouth distally, four pairs of long spindly walking legs (hence, their common name sea spiders) and a slender, often only tube-like trunk. The phylogenetic affinities of these bizarre arthropods have been a matter of continuous debate (see Dunlop and Arango 2005 for review), with the most recent molecular phylogenetic analyses tending to recover them within chelicerates, as sister group to all other subgroups, although with often only weak support (e.g. Bourlat et al. 2008; von Reumont et al. 2009; Koenemann et al. 2010; Meusemann et al. 2010; Regier et al. 2010; Rota-Stabelli et al. 2011). Alternatively, they are considered as sister group to all remaining euarthropods (Zrzavý et al. 1997; Edgecombe et al. 2000; Giribet et al. 2001; Scholtz and Edgecombe 2006; Bamber 2007; Rota-Stabelli et al. 2011 (morphological analysis)).
Relative to other arthropod taxa, pycnogonid development remains insufficiently documented and understood. Up to now, there are just few detailed embryonic studies, the majority of which dating back to the late nineteenth and early to mid-twentieth century (e.g. Morgan 1891; Meisenheimer 1902; Dogiel 1913; Sanchez 1959) and only a handful having been conducted recently (e.g. Brenneis et al. 2008, 2011; Ungerer and Scholtz 2009; Machner and Scholtz 2010). Compared to this, postembryonic development has received more attention, with several current contributions, mostly in form of scanning electron microscopic (SEM) studies (e.g. Behrens 1984; Bain 2003a; Vilpoux and Waloszek 2003; Bogomolova and Malakhov 2004; Lovely 2005; Maxmen 2006; Bogomolova 2007, 2010; Cano and López-González 2009; Cano Sánchez and López-González 2010). Consequently, there are by now detailed and well-documented descriptions of postembryonic external morphogenesis for some species, but total coverage of the various pycnogonid taxa is still far from complete.
Already early on, different modes of postembryonic development have been described for pycnogonids (e.g. Dohrn 1881; Dogiel 1913). However, in the last years, attempts have been made to systematically collect available data and to classify distinct developmental pathways. To date, five different pathways have been distinguished by different authors, with slight variations in the names proposed (e.g. Bain 2003b; Bogomolova and Malakhov 2006; Bogomolova 2007, 2010; Burris 2011). Four of them share a protonymphon larva as hatching stage, which is characterized by the possession of a larval proboscis, only three pairs of larval limbs (chelifores plus the palpal and ovigeral larval limbs) and an undifferentiated post-ovigeral trunk region. Yet, they differ in postembryonic life cycle features (parasitic on/in invertebrates versus remaining attached to the father’s oviger), mode of nutrition (actively feeding versus lecithotrophic) and the pattern of trunk and walking leg differentiation (strictly sequential versus synchronized). By contrast, the fifth pathway of postembryonic development begins with the hatching of a larva that bears already the anlagen of at least two walking legs and represents thus a further advanced stage relative to a protonymphon larva. This hatching larva is known in the literature as “attaching larva” (Nakamura 1981), since it remains attached to the father’s oviger for some subsequent molts.
In a previous paper (Brenneis et al. 2011), we have presented a description and staging of the embryonic morphogenesis in Pseudopallene sp. (Callipallenidae). Here, we describe the postembryonic development of the same species, which falls into the fifth developmental pathway. Applying SEM as standard method and supplementing it in some stages with light microscopy, fluorescent nucleic stains coupled to confocal laser scanning microscopy, and histology, we present the currently most complete description of callipallenid postembryonic development based on material collected in the field. We compare and discuss several features of callipallenid development as well as of pycnogonid development in general against an evolutionary–phylogenetic background. Furthermore, we suggest the renaming of the callipallenid (and probably in some cases nymphonid) hatching stage, due to lack of precision of the established term “attaching larva”.
Materials and methods
Specimen collection and fixation procedures
Adult specimens of Pseudopallene sp. were collected in coastal waters near Eaglehawk Neck, Tasmania. During SCUBA diving, individuals were taken by hand from their prey, the arborescent soft bryozoan Orthoscuticella sp. (Cheilostomata, Catenicellidae), put into small plastic jars, and transferred into buckets of fresh sea water upon return to the surface. Males carrying egg batches as well as postembryonic stages 1 and/or 2 (PS 1 and 2) were singled out, the latter being thereupon carefully removed from the ovigers with tweezers. Since postembryonic stage 3 (PS 3) has already left the male, this and all following stages had to be collected directly from the bryozoan colonies. For this purpose, pieces of the latter were brought to the field laboratory and checked using a stereomicroscope.
Postembryonic stages were either (1) fixed in 4% paraformaldehyde in sea water and kept in the fixative for several months, (2) fixed in Bouin’s fixative for 30–40 min, followed by several washes and final preservation in 70% ethanol, (3) directly transferred into 70% ethanol for SEM preparations (postembryonic stage 3 and later).
Scanning electron microscopy
Postembryonic specimens were checked for attaching dirt particles and carefully treated in a bathsonicator (Elma® Elmasonic One, several seconds at 35 kHz) for cleaning purposes. All stages were put through a graded ethanol series (each step lasting at least 1 h, sometimes up to 24 h), critically point-dried (BAL-TEC CPD 030) and sputter-coated with gold (BAL-TEC SCD 005). Images were taken with a Zeiss LEO 1430 scanning electron microscope. Global contrast and brightness values of some of the images were afterwards adjusted using ADOBE Photoshop CS3.
Nucleic staining
Only paraformaldehyde-fixed material of postembryonic stages 1 and 2 was used for these procedures. Specimens were transferred to TBS (TRIS–HCL buffered saline; 0.9% NaCl and 10 mM Tris–HCl; pH 7.5) and stained with the nucleic acid marker Sytox®Green (Invitrogen Molecular Probes®, 5 nmol/ml in TBS) overnight at 4°C. Following thorough washing in TBS, specimens were mounted in Vectashield® Mounting Medium (Vector Laboratories, Inc.). Tiny pieces of plasticine were fixed to the corners of cover slips, acting as spacers.
Stereomicroscope images
Bright-field images and fluorescence images of postembryonic stages were taken with a Zeiss Lumar V12, z-stacks being created with Zeiss AxioVision software (version 4.7.10) and subsequently merged to a single image using Heliconsoft Helicon Focus software (version 4.50). Color images of postembryonic stages were taken under a stereomicroscope coupled to a digital camera.
Confocal laser scanning microscopy
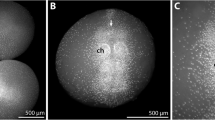
Image stacks were taken with a Leica DM IRE2 confocal laser-scanning microscope equipped with a Leica TCS SP2 AOBS laser-scanning unit, step sizes ranging from 1.0 to 1.5 μm between successive scanning planes. In postembryonic stage 2, advantage was taken of the cuticular autofluorescence under excitation with the UV laser (405 nm), as this enabled to visualize features of the external morphology as well as distinct borders between walking leg podomeres. Subsequent analyses were performed with the 3D reconstruction program “Imaris 7” (Bitplane AG, Switzerland). The “extended section mode” of this program allows generation of virtual sections with individually definable thickness by inclusion of a variable number of images. The “blend” option renders scanned structures non-transparent and thus facilitates evaluation of surface and external shape of an object.
Histology
Bouin-fixed specimens of postembryonic stage 1 were dehydrated in a graded ethanol series and embedded in the plastic resin Technovit 7100 (Kulzer Histo-Technik) following the proposed standard protocols. Semi-thin sections (1.5 μm) were cut with a microm HM 355 microtome, stretched at 60°C on a heating plate and stained in a methylenblue azure II basic fuchsin solution.
Terminology
We identified six stages during postembryonic development of Pseudopallene sp. plus a sub-adult stage. Postembryonic stages are numbered consecutively from PS 1 to PS 6. Morphological differences of consecutive stages point to intermittent molts. The actual molting, however, was observed only between PS 1 and PS 2. The term sub-adult is herein used for specimens with the overall external morphology of an adult, except for the gonopores and an apophysis on the oviger (in males only), which are lacking. Nonetheless, it cannot be excluded that these specimens undergo more than one molt until they reach maturity. A summary of postembryonic morphogenesis, including an overview of all identified stages and their respective degree of differentiation of various external structures and body regions is presented in Fig. 1. Throughout the study, we apply the traditional taxonomic classification proposed by Hedgpeth (1947) that is widely used in the pycnogonid literature (e.g. Child 1979, 1998; Stock 1994) and has been tested in recent phylogenetic analyses (Arango and Wheeler 2007; Nakamura et al. 2007). At species level, names were updated to the current suggestions by Bamber and El Nagar (2011).
Summary of postembryonic morphogenesis in Pseudopallene sp. Horizontal gray bars indicate specific features in relation to the described postembryonic stages of the present study. Brief descriptions of morphological characteristics are provided for each feature. Disruptions and switches of gray value along a horizontal bar indicate that observed changes in the morphological feature in question are coupled to intermediate molts. Gradual changes of gray values symbolize processes with no direct relation to molting. ff fixed chela finger, mf movable chela finger, tc terminal claw
Results
Habitat and coloration of the postembryonic stages
The body of a newly hatched PS 1 is egg-shaped and externally unsegmented (Fig. 2d, e). It bears the proboscis, chelifores, and distinct elongate anlagen of walking legs 1 and 2 (Fig. 2c–e). Hatching of PS 1 takes place simultaneously with the molting of an embryonic cuticle (see Brenneis et al. 2011). This stage is lecithotrophic, contains a copious amount of yellowish yolk and stays attached to the father’s ovigers (Fig. 2a, b). PS 2 also remains at first attached to the father’s ovigers (Fig. 4a), but in contrast to PS 1, the majority of the yellow yolk has been used up and PS 2 is thus more transparent. This allows discerning some internal structures through the cuticle (Fig. 4b, c), the most conspicuous being the whitish mass of the ventral nervous system comprising already the anlagen of all four walking leg ganglia. PS 2 is the oldest stage found on the ovigers and represents the stage leaving the father. All following stages, from PS 3 up to the sub-adults, are found clinging to the bryozoan Orthoscuticella colonies on which the adults prey. Most likely, these stages also feed on the bryozoans. From that point on, postembryonic specimens are less clear (Figs. 5a, d and 6a) and have a yellowish (sub)cuticular pigmentation similar to the sub-adults and adults (Fig. 8a). This coloration hampers observation of internal structures in vivo and in fixed material under the stereomicroscope. However, in living specimens, the slightly deeper colored orange midgut diverticula are frequently visible through the cuticle and extend into the articulated walking legs of the respective postembryonic stage (data not shown).
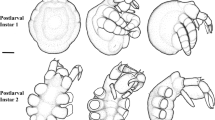
Postembryonic stage 1 of Pseudopallene sp. (“walking leg-bearing larva”). Stereomicroscope images, SEM micrographs, nucleic stainings, and histological sections. a Egg-bearing adult male of Pseudopallene sp., ventral view. The distalmost package (dashed outline) on the ovigers consists of hatched PS 1 that stay attached to it. b Removed package of hatched PS 1. The dorsal yellowish yolk is set of from the ventral whitish tissue of the proboscis and limbs. c Ventral overview. PS 1 bears proboscis, chelifores, and the unarticulated anlagen of walking legs 1 and 2. d Dorsal overview. No external segmentation of the trunk is detectable. e Lateral overview, older specimen. Note the bumpy appearance but not truly articulated condition of the walking leg anlagen. f Detail of the proboscis tip. The mouth opening is triangular. Posteriorly, a distinct constriction marks the borders of the ventrolateral proboscis antimeres (arrowhead). g Detail of the chela. The movable finger tapers into one terminal tip, the fixed finger exhibits an additional subterminal tip (arrow). h Detail of the spinning gland process. Hardened thread-like secretion (arrow) protrudes from the distal pore of the process. i Detail of the hind body region, dorsal towards the top. A shallow external groove (arrow) indicates the position of proctodeum formation. The primordia of walking leg 3 are only barely visible under the wrinkled cuticle. j Posterior view, Imaris volume (blend). Nuclei have immigrated interiorly where the proctodeum is formed. The primordia of walking leg 3 flank the anal tubercle laterally. k Histological section through hind body region. A connection is formed between the immigrating cells of the proctodeum and the yolk-containing midgut. ch chelifore, ff fixed chela finger, hb hind body region, mf movable chela finger, mo mouth opening, ov oviger, pr proboscis, proc proctodeum, sgp spinning gland process, wl walking leg
Morphogenesis during postembryonic development
External body segmentation
The body of PS 1 is egg-shaped and lacks external segmentation lines (Fig. 2d, e). Intersegmental constrictions are formed one at a time during each of the subsequent three molts. Accordingly, PS 2 shows only a single distinct constriction between the walking leg segments 1 and 2, which leads to the external delimitation of the anterior cephalosoma (Fig. 4e). In PS 3, walking leg segment 2 is distinctly demarcated from the more posterior trunk region, whereas in PS 4, the adult condition has been reached, being characterized by a distinct cephalosoma, the separate walking leg segments 2 and 3, and the posterior walking leg segment 4 from which the finger-like anal tubercle is projecting (Fig. 5c, d).
Ocular tubercle
PS 1 lacks eyes and shows no indication of the ocular tubercle at the anterodorsal body pole (Fig. 2d, e). The ocular tubercle is first visible as a slight elevation in PS 2 (Fig. 4e). Laterally, elevations of the lateral sensory organs start to protrude, but pigmented eye cups are still missing, at least in specimens still attaching to the father (Fig. 4c). In PS 3—the first completely free-living stage—the four pigmented eye cups are fully developed on a still rather shallow ocular tubercle with distinctly protruding lateral sensory organs (Fig. 5c, d). The ocular tubercle attains its more elevated shape in PS 4, being then positioned anterodorsally on the cephalosoma (Fig. 6c, d). From this stage on, no further significant structural changes occur.
Proboscis
Orientation
The orientation of the proximodistal axis (p-d axis) of the proboscis is shifting during postembryonic development. In PS 1, the proboscis is ventrally directed (Fig. 2c, e) and in PS 2 it bends slightly more posteroventrally (Fig. 4d). From PS 3 to 6, the long proboscis points almost directly posteriorly, running along the ventral side of the trunk (Figs. 5b, 6b and 7a, e). When the sub-adult stage is reached, it has shifted again slightly further anteriorly, resulting in the final posteroventrally directed p-d axis.
Shape
In PS 1, the proboscis is cylindrical and distally bears a triangular mouth with a pronounced notch on the posterior margin (Fig. 2f). Thus, the distalmost portion of the proboscis retains the vaguely horseshoe-like shape of late embryonic stages (see Brenneis et al. 2011). After the next molt (PS 2), the mouth opening is distinctly Y-shaped, it is surrounded by an elevated cuticular ridge with a very deep posterior constriction and two shallow anterolateral ones, lying in line with the extending arms of the Y-shaped mouth (Fig. 4f). In the following stages, the proboscis base grows increasingly wider in comparison to its distal tip. In PS 3, the tapering along the p-d axis remains almost imperceptible (Fig. 5e). In PS 4, however, the proboscis base has already reached twice the diameter of the tip (Fig. 6b, f) and in the following PS 5 and PS 6 it expands even further to almost threefold the tip’s diameter (Fig. 7f). The actual tapering does not extend along the entire p-d-axis, but is restricted to the proximal two thirds of the proboscis. As a consequence, the distal third of the proboscis resembles a slender cylindrical tube (Figs. 6b, f and 7f). The cuticular ridge surrounding the Y-shaped mouth is retained throughout these changes, but the previously pronounced indentations at the borders of the proboscis antimeres become increasingly less distinct from PS 3 onwards (Figs. 5e, 6f and 7g). In PS 6, we were able to observe a row of three inconspicuous pores on each of the three sides of the Y-shaped mouth (Fig. 7g).
Chelifore and its associated glands
In all postembryonic stages, the three-articled chelifores flank the proboscis laterally. In PS 1, it is the only functional appendage pair. The laterodistal margin of the scape bears a short process with a distal pore (Fig. 2h). It is associated with a spinning gland that is located in the chelifore scape (see Brenneis et al. 2011). Its thread-like secretions serve to attach the individual specimens to the egg matrix surrounding the father’s ovigers. The fixed finger of the chela bears a strongly sclerotized terminal tip and a second, outwards positioned subterminal tip. The movable finger narrows into just one sclerotized tip that grips in between the latter two when the chela is closed (Fig. 2g). In PS 2, the scape is elongated in relation to the palm and reaches twice the length of the latter; the spinning gland process is still retained (Fig. 4f). From PS 3 on, the scape has almost three times the length of the palm and the spinning gland process is reduced (Fig. 5b, e). The palm has attained a characteristic trapezoid shape, widening from proximal to distal, and the movable finger is articulating at the palm’s distal side. Presumable sensory setae on the palm become increasingly numerous with the number of postembryonic molts (Figs. 5b, e, 6b, e and 7b, f).
From PS 2 to 6, the movable finger terminates in a peculiar cork-screw-like tip (Figs. 4d, g, 5e, 6e and 7f). In these stages, a pore is found at the tip of each chela finger (Fig. 4g). The pore on the movable finger opens at the end of a tube-like extension projecting from its curved tip (Fig. 4g). In our recent embryonic study on Pseudopallene sp. (Brenneis et al. 2011), we have shown that a single copious chela gland develops during embryonic morphogenesis. It reaches distally into the movable finger and connects to its external pore. However, with the applied methods, we were unable to detect a comparably distinct connection between chela gland and pore of the fixed finger. In PS 2, the chela gland extends beyond the proximal border of the scape. The most proximal section of the gland runs laterally to the entodermal midgut diverticula reaching into the proximal portion of the chelifores (Fig. 4h). The gland’s extensions were not followed in later stages.
Palp and oviger
In Pseudopallene sp., both sexes lack the palp and not even a primordial anlage of it is discernible during development. There is no indication of an oviger anlage during embryonic development (see Brenneis et al. 2011), first external signs of oviger development being detectable in PS 3. The primordium is a minute cuticular bulge at the ventral side of the trunk, just posterior to the insertion of the proboscis (Fig. 5f). In PS 4, this bulge has elongated into an unarticulated finger-like projection (Fig. 6a, f). After the next molt (PS 5), the oviger comprises six externally distinguishable podomeres and a poorly defined terminal claw (Fig. 7b, c). The uneven appearance of the distalmost sixth podomere is indicative of the ongoing internal development. This region was observed to bear distally one or few leaf-shaped cuticular outgrowths as first signs of the future strigilis. The terminal claw is distally equipped with a similar leaf-shaped cuticular outgrowth (Fig. 7c). In PS 6, the oviger is finally fully developed, comprising ten podomeres plus the terminal claw (Fig. 7h). Podomeres 6–10 arose most likely by subdivision of the sixth podomere of the preceding stage. The distalmost four podomeres form now the strigilis, each podomere being equipped with a row of spines resembling denticulate leafs. The margins of the terminal claw are distinctly denticulate, i.e. bearing a row of tiny cuticular pointed teeth (Fig. 7h).
Walking legs
In PS 1, the elongate anlagen of walking legs 1 and 2 show no external articulation (Fig. 2c, e). The cuticle fits the developing appendages just loosely, with locally more stretched and bumpy regions where underlying tissue of the developing walking legs presses against it. Distally, the limb anlagen grow more slender, but lack a sharp terminal claw. In SEM preparations of PS 1, the primordium of walking leg 3 is barely discernible as a slight elevation that is covered by wrinkled cuticle (Fig. 2i). However, in nucleic stains, it is recognizable as a small bud flanking the ventrally directed anal tubercle (Fig. 2j). Shortly before the molt from PS 1 to 2, the entire cuticle is more tautened, due to the pressure from the growing tissues underneath (Fig. 3a). In nucleic stains of such stages, an internal folding of the walking leg tissue due to spatial constraints is visible (Fig. 3f, g). After manual removal of the cuticle, the extreme compression is also visible in SEM preparations and it becomes clear that the differentiating proximal parts of the future walking legs (i.e. regions of coxae 1–3, femur and tibia 1) are interiorly curled into a compressed s-shape prior to the molt (Fig. 3h, i). With this compression, a direct correlation of regions along the externally visible p-d axis of PS 1 walking leg anlagen to future definite podomeres proves difficult. Based on a combination of the SEM data and nucleic stains, only the distalmost portion of premolting PS 1 walking leg anlagen, being demarcated by a distinct external cuticular fold (Fig. 3a–d, f, h), can be securely identified as giving rise to the tarsus, propodus, and terminal claw. The already sclerotized spines of the prospective propodus heel and terminal claw are pressed into the interior by the cuticle of PS 1 (Fig. 3h). During the molt from PS 1 to 2 the cuticle rips horizontally in anterior to posterior direction, usually starting at the base of the proboscis and the chelifore scapes (Fig. 3b–e). PS 2 emerges anterodorsally from the exuvia, which remains in one piece, except for the old chelifore cuticle that (at first) stays attached to the chelifores (Fig. 3e). During and after the molt, walking legs 1 and 2 stretch dramatically and are now truly articulated. In contrast to PS 1, the cuticle of PS 2 is covered with mainly bifurcating sensory setae (Fig. 3h). Walking leg 1 has already attained the adult number of nine podomeres (from proximal to distal: coxa1, coxa2, coxa3, femur, tibia1, tibia2, tarsus, propodus, and terminal claw lacking auxiliary claws; Fig. 4i; Supplementary Fig. 1a). Walking leg 2 has only seven podomeres (Fig. 4j; Supplementary Fig. 1b). These podomeres can be set into relation to the future adult ones, based on their shape and position along the p-d axis of the leg as well as some faint external lines that seem to correspond to internal subdivisions. They represent the three equally long coxae, a transient podomere that will subdivide into the future femur and tibia1, tibia 2, and a podomere with close resemblance to the future propodus, which will proximally most likely give rise to the tarsus, and the terminal claw. The anlage of walking leg 3 of PS 2 is just an unarticulated limb bud that extends posteriorly and flanks the anal tubercle (Fig. 4d, e; Supplementary Fig. 1b). From PS 3 to 5, this leg differentiation pattern is shifted posteriorly, in a stepwise fashion of one segment per molt (Figs. 5g, h, 6g, h and 7d). Accordingly, PS 3 has a nine-articled walking leg 2, a seven-articled walking leg 3, and an unarticulated limb bud of walking leg 4. PS 5 is the first stage with four articulated walking legs possessing adult composition.
Molting of postembryonic stage 1 of Pseudopallene sp. SEM micrographs and nucleic stainings. a Lateral overview of premolting PS 1. The cuticle is more tautened than in freshly hatched PS 1. The distalmost portion of the externally unarticulated walking leg 1 will give rise to the propodus and tarsus (+ terminal claw) in PS 2. b–d Sequence of molting specimens, lateral view. The cuticle rips from anterior to posterior, along the bases of proboscis and the limbs (dashed lines). e Lateral view of emerging PS 2. PS 2 leaves the old cuticle anterodorsally. f and g Detail of walking leg 1 of an old PS 1 specimen. Underneath the cuticle (arrowheads) the tissue of the growing walking leg is pressed into folds (arrows). The tissue folds extend in part far into the leg anlage. Proximally, correlation of folds to future leg articulation is ambiguous. Distally, the region giving rise to the prospective tarsus and propodus as well as the terminal claw can be identified. f Imaris volume (blend), anterior view. g Optical section, Imaris section mode. h Detail of walking leg 1 of molting PS 1, cuticle manually removed. Terminal claw and heel spines of propodus and tarsus are still pressed into the tissue. The leg is covered with setae (mostly bifurcating). i Overview of molting PS 1, ventral view, cuticle manually removed. The chelifores remain stuck in the cuticle of PS 1 (white arrows). Note the curved, vaguely s-shaped appearance of the still compressed walking legs (dashed white line). ch chelifore, cut old cuticle, pr proboscis, pro + ta precursor of tarsus and propodus, tc terminal claw, wl walking leg
Postembryonic stage 2 of Pseudopallene sp. Stereomicroscope images, SEM micrographs, and nucleic stainings. a Egg-bearing adult male of Pseudopallene sp., ventral view. PS 2 specimens stay attached to the ovigers and cover more proximally attached egg packages. b Ventral overview. Note the transparency of the cuticle. Ganglia of the ventral nervous system are visible as whitish mass in the trunk. Midgut diverticula extend into the articulated walking legs 1 and 2. c Detail of the cephalosoma, anterior view. The shallow ocular tubercle lacks pigmented eye cups. The whitish brain shimmers through the cuticle (dashed outline). d Ventral overview. The unarticulated limb bud of walking leg 3 is directed posteriorly, the anal tubercle (arrow) points ventrally. e Dorsal overview. A distinct intersegmental constriction between first and second walking leg segment delimits the cephalosoma. f Detail of the proboscis tip and cheliforal scape. The mouth opening is Y-shaped and surrounded by a cuticular ridge that has distinct indentations (arrowheads) at the borders of the proboscis antimeres. The spinning gland process with its thread-like secretions is not yet reduced. g Detail of the chela tip. The delicate curved tip of the movable finger and the roundish tip of the fixed finger each bear a pore of the chela gland (arrow and arrowhead, respectively). h Optical section through chelifores and proboscis, Imaris section mode. Autofluorescence of the cuticle gives the outlines of the structures. The chela gland extends through the entire chelifore into the cephalosoma. Its last third runs closely attached to the midgut diverticulum that reaches into the scape. Note the strong autofluorescent signal of the cuticularized pharynx with its oyster basket. i Detail of the completely articulated walking leg 1, posterior view, dorsal towards the right. No auxiliary claws are formed. j Detail of walking leg 2, posterior view, dorsal towards the bottom. Only seven podomeres (including terminal claw) are externally distinguishable. Note the ventrally pointing anal tubercle with the slit-like cuticular fold (arrow) of the anus at its tip. AF autofluorescence, cg chela gland, ch chelifore, cx coxa, fe femur, ff fixed chela finger, gd gut diverticula, mf movable chela finger, ns nervous system, ot ocular tubercle, ov oviger, pha pharynx, pr proboscis, pro propodus, sgp spinning gland process, ta tarsus, tb tibia, tc terminal claw, wl walking leg
Postembryonic stage 3 of Pseudopallene sp. Stereomicroscope images and SEM micrographs. a Ventral overview. Three pairs of articulated walking legs are formed. Note the non-transparency of this stage. b Ventral overview, same specimen as in a. The proboscis is directed posteriorly, running along the ventral side of the trunk. Note the ventrally pointing anal tubercle (arrow) and the flanking unarticulated limb buds of walking leg 4. Asterisk marks a damaged walking leg tip. c Detail of the cephalosoma, anterior view. The lateral sensory organs protrude from the ocular tubercle. Epizoans are attached to in various regions of the cuticle (arrowheads). d Detail of the cephalosoma, dorsal view. The ocular tubercle bears four pigmented eye cups. Arrowheads mark epizoans. e Detail of proboscis and chelifores, posteroventral view. At the slightly tapering tip of the proboscis, the Y-shaped mouth is surrounded by a cuticular ridge without distinct indentations. The movable finger of the chela possesses still the elongated curved tip (arrows). f Detail of the ventral side of the trunk, posterior view. The first elevation of the outgrowing oviger is detectable. Arrowheads mark epizoans. g Detail of the completely articulated walking leg 2, anterior view, dorsal towards the right. Femur and tibia 1 as well as tarsus and propodus are fully developed. h Detail of walking leg 3, posterior view, dorsal towards the right. Only seven podomeres (including terminal claw) are externally distinguishable. The future subdivision of the femur-tibia1 precursor podomere is indicated by framed arrows. Note the slit-like anal opening (white arrow). ch chelifore, cx coxa, fe femur, ls lateral sensory organ, ot ocular tubercle, ov oviger, pr proboscis, pro propodus, ta tarsus, tb tibia, tc terminal claw, wl walking leg
Postembryonic stage 4 of Pseudopallene sp. Stereomicroscope images and SEM micrographs. a Ventral overview. Four pairs of articulated walking legs are formed. Buds of the ovigers are detectable on the ventral side of the trunk b Ventral overview. The anal tubercle (arrow) is pointing posteriorly, in line with the trunk. c Dorsal overview. The trunk is fully segmented (white arrowheads). The arrow indicates the posteriorly directed anal tubercle. d Lateral overview. The ocular tubercle is a prominent elevation on the cephalosoma. Arrowheads mark the external segment borders. The future subdivision of the femur-tibia1 precursor podomere in walking leg 4 is indicated by the framed arrows. e Detail of the chelae, anteroventral view. Arrows mark the delicate elongated curved tip of the movable chela finger. Note the trapezoid shape of the palm. f Detail of proboscis and the oviger anlagen. The proboscis tapers distinctly along the p-d axis. The oviger anlage is an elongate but unarticulated limb bud. g Detail of walking leg 3, posterior view. Femur and tibia 1 as well as tarsus and propodus are fully developed. h Detail of walking leg pair 4, ventrolateral view. Only seven podomeres (including terminal claw) are externally distinguishable. The future subdivision of the femur-tibia1 precursor podomere is indicated by a framed arrow. ch chelifore, cx coxa, fe femur, ff fixed chela finger, mf movable chela finger, ot ocular tubercle, ov oviger, pr proboscis, pro propodus, ta tarsus, tb tibia, tc terminal claw, wl walking leg
Postembryonic stages 5 and 6 of Pseudopallene sp. SEM micrographs. a–d PS 5. a Ventral overview. b Posteroventral view. The still incompletely articulated ovigers are ventrally covered by the proboscis and chelifores. c Detail of oviger tip. The long distalmost podomere bears single cuticular spines (arrows), reminiscent of the future leaf-shaped denticulate spines of the strigilis. d Detail of walking leg 4, posterior view. Femur and tibia 1 as well as tarsus and propodus are fully developed. e–h PS 6. Asterisks mark damaged regions. e Ventral overview. f Detail of the cephalosoma, anteroventral view. The base of the proboscis has 2.5–3 times the diameter of the tip. The delicate curved tip of the movable chela finger is still developed (arrows). The distal part of the completely articulated oviger protrudes from underneath the chelifores. g Detail of proboscis tip. The Y-shaped mouth opening is flanked on each side by three minute pores (arrowheads). h Detail of oviger tip. The distalmost four podomeres each bear a single row of leaf-shaped denticulate spines, forming together the strigilis. The terminal claw is equipped with two rows of denticulate outgrowths (arrowheads). ch chelifore, cx coxa, fe femur, ot ocular tubercle, ov oviger, pr proboscis, pro propodus, ta tarsus, tb tibia, tc terminal claw, wl walking leg
Anal tubercle (“abdomen”)
Orientation
From PS 1 to 3, the anal tubercle is directed ventrally and positioned in between the posteriormost walking leg anlagen of the respective stage (Figs. 2e, 4d, j and 5b, h). From PS 4 on, it projects horizontally, in line with the body axis (Figs. 6b, c and 7b).
Proctodeum and anal opening
PS 1 possesses still no anal opening, and histological sections show that the proctodeum is formed during this stage (Fig. 2k). At the distalmost point of the hind body region, cells immigrate and seem to establish contact with the still yolk-filled midgut. Nucleic stains show the basal displacement of nuclei at the point of the proctodeum formation quite distinctly (Fig. 2j). Externally, this region is only characterized by a shallow indentation of the cuticle (Fig. 2i). After the next molt (PS 2), the slit-like cuticular fold at the position of the future anus is visible (Fig. 4j; Supplementary Fig. 1b). However, in none of the investigated PS 2 specimens, the anal opening was observed to be distinctly open. Therefore, we cannot exclude that it opens only after the next molt to PS 3, which has definitely started active feeding.
The sub-adult
The ocular tubercle of the sub-adult of Pseudopallene sp. does not show any significant changes in comparison to PS 6 (Fig. 8e). The massive proboscis is cylindrical, the tip mamilliform without the slender tube-like appearance of the distal portion seen in the preceding stages. A conspicuous constriction is present around halfway of the proboscis’ length (Fig. 8a, b). The Y-shaped mouth at the tip of the proboscis lacks the surrounding unornamented cuticular ridge of the previous stages. Instead, each of its sides is flanked by three roundish papillae with a central pore. These papillae correspond in their position to the three minute pores observed on each side of the mouth in PS 6. They are placed within a field of small tube-like projections, some of which also bearing an opening at their distal pole (Fig. 8d). Apparently, these papillae and small tubes possess glandular function. The chelifore has undergone significant modifications. Its scape is sub-equal in length to the bulky palm and its distally projecting fixed finger (Fig. 8b). The movable finger has lost its slender corkscrew-like protrusion and is now more massive with a simple distal tip (Fig. 8g), making the chela more suitable for gripping and crushing compared to the preceding stages. No pores of chela glands could be found at the chela tips, which are directed medially and positioned directly in front of the mouth opening. The oviger has not changed significantly and is folded underneath the ventral side of the trunk (Fig. 8g). Each of the strigilis podomeres still bears a single row of leaf-like spines (Fig. 8h). The three coxae of the walking legs have further differentiated, coxa 2 being now distinctly longer than coxa 1 and coxa 3 (Fig. 8g). Indicative of the sub-adult stage, coxa 2 still bears as yet no gonopore on its ventral side (Fig. 8f).
Sub-adult of Pseudopallene sp. Stereomicroscope images and SEM micrographs. a Detail of the cephalosoma, anterior view. The eye cups on the ocular tubercle are of orange-brown color (arrows). Arrowheads indicate constriction of the proboscis. b Same specimen and view as in a. Note again the distinct proboscis constriction (arrowheads). c Detail of proboscis tip. The Y-shaped mouth is on each side surrounded by three papillae in a field of small tube-like projections. d Magnification of three papillae. Each papilla bears a single pore, some of the surrounding small tubes are also equipped with distal pores (arrows). e Detail of ocular tubercle, anterior view. Note the protruding lateral sensory organs. f Coxa 2 of walking leg 4, ventral view. The elongated coxa 2 lacks still gonopores. g Posterior view. The movable chela finger lacks the cork-screw-shaped delicate tip of the preceding stages. The ovigers are held ventral to the trunk. h Detail of the distal oviger portion. The four distalmost podomeres of the strigilis are each equipped with one row of leaf-shaped cuticular spines. The terminal claw bears two rows of denticulate cuticular outgrowths on its distal half. an Anus, ch chelifore, cx coxa, ff fixed chela finger, ls lateral sensory organ, mf movable chela finger, ot ocular tubercle, ov oviger, pr proboscis, wl walking leg
Discussion
The hatching larvae within Callipallenidae—a comparison
Nakamura (1981) was the first to describe the complete postembryonic development of a callipallenid, Propallene longiceps. Similar to Pseudopallene sp. (see Brenneis et al. 2011), this species develops an embryonic cuticle, which is simultaneously molted with the shedding of the egg membrane. Nakamura considers the late prehatching embryo with cuticle as a “first instar larva” and proposes its correspondence to the protonymphon larva of other pycnogonid subtaxa. As a consequence, he designates the actual hatching stage as “second instar larva”. This staging system was adopted in a later work on callipallenid development (Bain 2003a: Austropallene cornigera). However, as we have pointed out in a previous study (see Brenneis et al. 2011), callipallenid development does not exhibit any embryonized stage comparable to a hatched protonymphon larva. Furthermore, it appears in our opinion counterintuitive to consider a developmental period prior to hatching as “first instar larva” of postembryonic development. Hence, we suggest the shedding of the egg membrane as the unmistakable beginning of the postembryonic period in callipallenids, all prior stages being part of embryonic morphogenesis.
To date, data on postembryonic development are not yet available for all groups included in the Callipallenidae. Yet, a comparison of the hatching larvae in the different groups investigated so far shows already some notable differences (Fig. 9).
Comparison of callipallenid development (schematic). The various callipallenid groups show differing degrees of embryonization. At the time of hatching, the “walking leg-bearing larvae” exhibit deviating levels of differentiation of the post-ovigeral trunk. Accordingly, there is no morphologically uniform hatching stage of callipallenids. Representatives possessing hatching larvae with a comparatively low degree of trunk differentiation (e.g. Propallene) tend to leave the father’s oviger at an earlier stage than species with far advanced hatching larvae (e.g. Callipallene, Neopallene). Black arrows represent intermittent molts, the white arrow indicates further differentiation during embryonic development
Pseudopallene
A fragmentary description of the late embryonic development as well as the two first postembryonic stages of Pseudopallene spinipes (Meinert 1899; Bogomolova and Malakhov 2003, 2004) indicate a very similar course of development compared to Pseudopallene sp. investigated in the present study, including the characteristics of the hatching larva.
Propallene
Compared to Pseudopallene, the hatching larva of Propallene longiceps (“second instar larva” sensu Nakamura 1981) bears less prominent anlagen of walking leg pairs 1 and 2, which assume an elongated (but still unarticulated) shape only after the next molt (Nakamura 1981). Apparently, P. longiceps hatches with a slightly less developed trunk and undergoes an additional molt before reaching a stage comparable to the hatching PS 1 of Pseudopallene.
Stylopallene
Similar to Pseudopallene, newly hatched larvae of Stylopallene cheilorhynchus as well as S. longicauda possess two elongate unarticulated anlagen of walking leg pairs 1 and 2, and an internally but barely externally detectable primordium of walking leg 3 (Supplementary Fig. 2a–c).
Austropallene
In A. cornigera, the earliest described postembryonic stage closely resembles the PS 1 of Pseudopallene (Bain 2003a). The author, however, follows the staging scheme proposed by Nakamura (1981) and considers this stage as “third instar” (Bain 2003a). Unfortunately, embryonic development of A. cornigera was not investigated and the actual hatching never described. Hence, it remains somewhat ambiguous whether the first described stage does indeed represent the hatching larva.
Parapallene
A casual observation of the development of Parapallene avida (Hooper 1980) proves vague in its description of the hatching larva. The earliest depicted postembryonic stage possesses two elongated but apparently unarticulated walking leg anlagen (with terminal claw?) plus a prominent limb bud of walking leg 3 that appears further developed than the corresponding primordium in Pseudopallene, Austropallene, and Stylopallene.
Callipallene
In the genus Callipallene, detailed embryonic studies (Morgan 1891: C. brevirostris; Dohrn 1881, Sanchez 1959, Winter 1980: C. emaciata) have demonstrated that walking leg pairs 1–3 and the primordium of the walking leg pair 4 are already developed prior to hatching. In the hatching larva, walking legs 1–3 are already relatively long and folded on the ventral side, their distal tips being directed anteriorly. Externally, they are still unarticulated and covered by a loosely fitting cuticle, although, especially in the distal portions, demarcations of future podomeres begin to show through the latter (Supplementary Fig. 2d–f).
Neopallene
Dohrn (1881) reports for Neopallene campanellae a development comparable to Callipallene emaciata, including the morphology of the hatching larva.
These examples illustrate that the degree of differentiation of the post-ovigeral trunk and hence the degree of embryonization of development varies significantly between different callipallenid taxa (Fig. 9). On one end, Propallene longiceps hatches from the egg at a comparatively early stage with only primordial anlagen of walking legs 1 and 2, whereas hatching larvae of Callipallene species and Neopallene almost resemble adult morphology. However, even the relatively “early” hatching larva of Propallene still differs from the widespread protonymphon larva of many other pycnogonid groups by a more significantly advanced level of trunk development in combination with the absence of palpal and ovigeral larval limbs. Judging from available data, different degrees of embryonized development represent a general callipallenid feature, being to our knowledge found in all studied representatives regardless of geographic distribution or depth in which they are found. Accordingly, this developmental phenomenon does not appear to be as strongly influenced by environmental factors as has been suspected for other pycnogonid taxa (e.g. Bain 2003b; Bamber 2007). If true, the observed differences between the callipallenid hatching larvae might even be indicative of a gradual embryonization of development during the evolution of this pycnogonid lineage. Unfortunately, reliable hypotheses on the interrelationships of callipallenid taxa are still lacking. In older classifications (e.g. Munilla 1999) and recent phylogenetic analyses incorporating morphological characters (Arango 2002; Arango and Wheeler 2007), development has been largely neglected as source for phylogenetic information. Interestingly, the most recent studies (Arango and Wheeler 2007; Nakamura et al. 2007; Arabi et al. 2010) indicate a close relationship of Callipallenidae and Nymphonidae. In contrast to callipallenids, nymphonids show different developmental pathways (see, e.g. Brenneis et al. 2011 for discussion). For these two groups (if not even on larger scale), the different developmental pathways and the differing larval types can provide a number of characters for phylogenetic studies. Combined with characters of adult morphology as well as sequence data, analyses including developmental data would help assess the amount of phylogenetic information contained therein and contribute to our understanding of evolutionary transformations in pycnogonid development.
The callipallenid hatching stage: “attaching larva” versus “walking leg-bearing larva”
Since the complete description of development of Propallene longiceps (Sekiguchi et al. 1971; Nakamura 1981), the advanced hatching larva of callipallenids with the anlagen of at least two walking legs is known as “attaching larva”. This name relates to the behavior of the newly hatched offspring, remaining attached to the father’s oviger for one or more molts and abandoning it only after the first articulated and functional walking legs have developed. However, already older investigations (e.g. Dogiel 1913) and more recent studies (Bogomolova and Malakhov 2006; Bogomolova 2007, 2010; Cano and López-González 2009; Cano Sánchez and López-González 2010) have highlighted the existence of pycnogonid species in which big, lecithotrophic protonymphon larvae hatch from the egg membrane and subsequently remain attached to the father’s oviger for the major part of the postembryonic development. This illustrates that attachment of offspring is not exclusive for hatching callipallenid larvae with an advanced level of differentiation of the post-ovigeral trunk (see also discussions in Bogomolova and Malakhov (2006), Bogomolova (2007), and Burris (2011)). Accordingly, Nakamura’s behavior-based term “attaching larva” proves not precise enough. Therefore, we propose to substitute this term into the more morphology-based name “walking leg-bearing larva”. The possession of anlagen of at least two walking legs is a distinctive morphological feature of hatching stages of callipallenids and probably also some nymphonids (see Hoek 1881). This stands in clear contrast to all types of hatching protonymphon larvae (free-living and actively feeding as well as attaching and lecithotrophic) with their fairly undifferentiated post-ovigeral region. Therefore, we consider “walking leg-bearing larva” as representing a less confusing and more precise designation. However, contrary to earlier accounts on the postembryonic development of pycnogonids (e.g. Bain 2003b; Burris 2011) it has to be emphasized that the callipallenid “walking leg-bearing larvae” do not represent a morphologically uniform and definable stage as the protonymphon larva (see above). Instead, this term unifies hatching larvae that share an advanced but often differing degree of post-ovigeral trunk development and the lack of palpal and ovigeral larval limbs (see Fig. 9, Supplementary Fig. 2).
Walking leg development in Pycnogonida
The composition of the adult walking legs is one of the most conserved features of extant pycnogonids (Arnaud and Bamber 1987). With the exception of the first pair in Nymphonella (see Ohshima 1933), a walking leg consists of nine podomeres (from proximal to distal: coxa 1, coxa 2, and coxa 3, femur, tibia 1, tibia 2, tarsus, propodus, and terminal claw). Frequently, walking leg differentiation encompasses three distinct stages that are separated by two intermittent molts. First, an unarticulated limb bud is transformed via one molt into a long and articulated leg that still comprises an incomplete number of podomeres. Only after the next molt, the adult composition is attained.
-
1.
From limb bud to articulated walking leg
The internal articulation processes underlying the dramatic metamorphosis of a small and externally unarticulated limb bud into a significantly longer and many-articled leg remain poorly understood. Sanchez (1959) describes four distinguishable folded tissue regions underneath the cuticle of a limb bud in Nymphon gracile, which are referred to as proto-podomeres. The author even included details on the further subdivision of these putative proto-podomeres, but documentation of this developmental process remains unsatisfactory. As shown in our study on Pseudopallene sp., spatial constraints within the limb bud cuticle force the underlying walking leg tissue into numerous folds that cannot be unambiguously related to future leg podomeres, especially in more proximal regions. Hence, tissue folds alone do not permit infallible identification of future podomere regions. The constraints of the surrounding cuticle even lead to a compressed s-shape of the p-d axis of the developing walking legs 1 and 2 in externally linear-looking limb anlagen. A similar observation has already been made in Pycnogonum litorale (Dogiel 1913, p. 651, fig. 72) and in Propallene longiceps (Nakamura 1981). Due to this, also direct correlation of external cuticle folds along a limb bud’s p-d axis with future podomere borders has to be carried out with caution, especially when only external morphology has been investigated. For instance, descriptions of intermediate two- or three-articled legs in early postembryonic stages of callipallenids (e.g. Meinert 1899: Pseudopallene circularis, “Pallene brevirostris”, Callipallene hastata; Bogomolova and Malakhov 2003, 2004: Pseudopallene spinipes) certainly correspond to cuticle folds of externally unarticulated limb buds with underlying compressed tissue as seen in PS 1 of Pseudopallene sp. in this study. Reports of three-articled walking leg stages in some Nymphonidae (Bogomolova and Malakhov 2006: Nymphon grossipes; Bogomolova 2010: Nymphon macronyx), have been recently questioned in a study of the postembryonic development of Nymphon unguiculatum (Cano Sánchez and López-González 2010). We agree with the latter authors that the true articulation of these early leg stages is insufficiently proven. And even if the two constrictions along the elongate limb buds in nymphonids should indeed demarcate an early internal regionalization into three parts, the relationships between these early regions and the future leg podomeres remain to be established yet.
To gain a better understanding of initial regionalization events and podomere specification, investigation of pycnogonid species with a more accessible and (externally) less drastic walking leg development seems crucial. Promising candidates for such studies may be found again within Callipallenidae. During embryonic and postembryonic development of Pseudopallene sp., the anlagen of walking leg pairs 1 and 2 are too compressed to reveal more insights in this regard (see also Brenneis et al. 2011). However, within the genera Callipallene and Neopallene, embryonic development terminates in a well-advanced walking leg-bearing larva with long and anteriorly extended walking legs that appear to be internally far—perhaps even completely—differentiated (see Supplementary Fig. 2d–f, Dohrn 1881; Morgan 1891; Winter 1980). Therefore, investigation of embryonic morphogenesis in these groups would enable to follow walking leg development into late stages without the intermittent molts occurring in most other pycnogonid groups. Coupled with studies on the expression patterns of genes known to be involved in arthropod leg segmentation (see, e.g. Prpic and Damen 2009), this would not only contribute to a better understanding of the developmental processes within pycnogonids, but also enable comparisons to other arthropod taxa.
-
2.
Femur-tibia 1 and tarsus-propodus precursor podomeres as plesiomorphic feature of pycnogonid development?
The first truly articulated stage during pycnogonid walking leg differentiation shows in the majority of cases still an incomplete number of podomeres. In Pseudopallene sp., the anlagen of walking legs 1 and 2 are already developed during embryonic development, while the remaining two walking leg pairs arise sequentially during postembryonic development. Apart from walking leg pair 1 (see below), the external differentiation of the walking legs follows a three-stage-sequence with two intermittent molts. The intermediate articulated leg is already functional and comprises only seven podomeres, two of them representing transient precursor podomeres. In line with our study, a precursor podomere giving rise to the adult femur and tibia 1 has been explicitly reported in several instances (e.g. Dohrn 1881: Neopallene campanellae; Morgan 1891: Tanystylum orbiculare; Sanchez 1959: Nymphon gracile; Nakamura 1981: Propallene longiceps; Bain 2003a: Austropallene cornigera; Bogomolova 2007: Nymphon grossipes; Bogomolova 2010: N. macronyx) and the term femur-tibia 1 has recently been introduced for this transient podomere (Cano Sánchez and López-González 2010: N. unguiculatum). More distally, incompletely articulated walking leg stages often lack a podomere resembling the adult tarsus. Our study does not provide evidence for the origin of this podomere in Pseudopallene sp. However, in other species, the split of the future tarsus from the proximal region of a propodus-like precursor podomere has been repeatedly described (Dohrn 1881: N. campanellae; Sanchez 1959: N. gracile; Bogomolova 2007: N. grossipes; Bogomolova 2010: N. macronyx), and the term tarsus-propodus has been proposed for the corresponding precursor podomere (Cano Sánchez and López-González 2010: N. unguiculatum). Apparently, femur-tibia 1 and tarsus-propodus are widespread precursor podomeres (or at least specific regions) occurring in the developing walking legs of different pycnogonid groups. Hence, they are here tentatively suggested as characteristic and plesiomorphic feature of pycnogonid walking leg development. However, as there is still a lack of developmental data on potentially basally branching taxa such as Austrodecidae and Colossendeidae (see, e.g. Arango and Wheeler 2007; Arabi et al. 2010) this evaluation has to remain preliminary.
-
3.
Variations in the pattern of pycnogonid walking leg development
Comparison across different taxa shows that a three-stage-sequence of walking leg differentiation as described for walking leg pairs 2–4 of Pseudopallene sp. (unarticulated limb bud—incompletely articulated leg—nine-articled leg) represents a recurrent motif of pycnogonid development. However, there are also several deviations from this pattern. For instance, in line with our results on Pseudopallene, the unarticulated limb bud of walking leg pair 1 in all other investigated callipallenids attains adult articulation through one molt only, thus skipping the incompletely articulated leg stage (Nakamura 1981: P. longiceps; Bain 2003a: A. cornigera; Bogomolova and Malakhov 2003, 2004: P. spinipes; Brenneis, pers. obs.: Stylopallene sp.). Alternatively, sometimes a distinct elevation of the cuticle is seen to precede the unarticulated limb bud of all or only some walking legs, leading in this case to four externally discernible stages of walking leg development (e.g. Okuda 1940: Achelia alaskensis; Dearborn 2003: Achelia gracilipes; Bogomolova 2007: N. grossipes; Bogomolova 2010: N. macronyx; Cano Sánchez and López-González 2010: N. unguiculatum).
In addition, the composition of the still incompletely articulated legs can vary. The seven-articled stage described for Pseudopallene is a widespread feature (see above). But in some other callipallenids (e.g. Nakamura 1981: P. longiceps; Brenneis, pers. obs.: Stylopallene sp.) and also other pycnogonid groups (e.g. Dearborn 2003: A. gracilipes), the first articulated leg stages comprise already eight podomeres. In these cases, the tarsus and propodus are already fully separated, but never femur and tibia 1. An interesting differentiation pattern is found in Pycnogonum litorale, whose postembryonic development has been documented no less than three times (Dogiel 1913; Behrens 1984; Vilpoux and Waloszek 2003). Surprisingly, the descriptions of walking leg differentiation differ between these studies, but due to the thorough documentation with original SEM images, Vilpoux and Waloszek (2003) are clearly the most reliable source. Walking leg pair 1 of P. litorale passes not only through a seven-articled but also an additional eight-articled stage (with separated tarsus and propodus) before attaining the adult composition. On the other hand, walking leg pairs 3 and 4 do not possess any incompletely articulated stage and transform via a single molt from limb bud to adult composition (similar to walking leg pair 1 in callipallenids). Hence, walking leg development in P. litorale shows a decrease of intermediate stages along the anterior-posterior axis. Finally, studies on Phoxichilidiidae with their endoparasitic postembryonic development (e.g. Dogiel 1913; Lebour 1916, 1945) and on species with ectoparasitic development characterized by synchronized differentiation of all four walking legs (e.g. Ohshima 1933: Nymphonella tapetis; Ohshima 1937: Ammothea sp.; Salazar-Vallejo and Stock 1987: Ammothella spinifera) have described a more gradual elongation of the walking leg anlagen, with no clear mention of podomere numbers in the different stages. However, in the first SEM study on the postembryonic development of a phoxichilidiid (Lovely 2005: Phoxichilidium tubulariae), walking leg differentiation is reported as adhering to the widespread three-stage-sequence, the incompletely articulated leg stages being already formed during the endoparasitic phase and at that point not yet functional. Similarly, Maxmen (2006) briefly mentions an intermediate seven-articled walking leg stage preceding the completely articulated adult leg in the phoxichilidiid Anoplodactylus eroticus. Hence, recent evidence suggests that even in the clearly derived endoparasitic developmental pathway of Phoxichilidiidae, a similar pattern of leg differentiation is found.
-
4.
Comparison of walking leg differentiation across pycnogonids
Cross-species comparisons of structural and temporal patterns of walking leg differentiation have been conducted previously for restricted numbers of pycnogonid species, often in form of space-consuming tables (e.g. Bain 2003a, b; Gillespie and Bain 2006; Cano Sánchez and López-González 2010). In an attempt to depict the documented patterns in a concise form that facilitates comparison across more species, we introduce an abbreviated notation in matrix fashion, as exemplified for Pseudopallene sp. in Fig. 10. The rows of the matrix represent the four walking legs, whereas the columns depict the sequence of postembryonic stages. Each matrix entry represents the number of externally differentiated podomeres (“+” standing for a first externally visible primordium, “0” for the unarticulated limb bud) of a given walking leg in a specific postembryonic stage. This short form of notation visualizes (1) the sequence of the anlage of the walking legs during postembryonic development based on the shift of the entries in the different rows, (2) the different stages of the external articulation process of each walking leg in its respective row, and finally (3) the number of developed walking legs and their structural composition in specific postembryonic stages within single columns. For cross-species comparisons, the matrices of different representatives can be easily aligned one below the other. Due to the differing degree of trunk and walking leg differentiation in hatching protonymphon larvae (with no walking leg anlagen at all) and walking leg-bearing larvae, the alignment of the columns (= postembryonic stages) has to be based on a corresponding degree of development in the post-ovigeral region (= number of walking leg anlagen), as opposed to the species-specific numbers of developmental stages. An overview of aligned matrices as extracted from the literature is given in Fig. 11. Here, similarities and differences in the developmental patterns are easily detectable. Within Callipallenidae, for instance, identical patterns are found in Pseudopallene sp. and A. cornigera. Propallene longiceps, on the other hand, differs in the structure of the incompletely articulated legs (tarsus already differentiated) and the fragmentary data on Stylopallene sp. indicate a pattern corresponding to this. With a better coverage of pycnogonid taxa and the filling of gaps in so far fragmentary descriptions (gaps in matrices indicate missing stages), the inclusion of such developmental patterns into future phylogenetic studies may indeed contribute to the resolution of pycnogonid interrelationships.
Fig. 10 Fig. 11 Walking leg differentiation patters within different pycnogonid taxa. For explanation of notation see Fig. 10 and text. Criterion for the alignment of matrices is solely the number and composition of the walking legs, because the species-specific numbers of the respective postembryonic stagings do often not refer to morphologically similar developmental stages. The entry value “8” indicates that tarsus and propodus are already separated, but femur and tibia 1 not yet. The value “3” relates to late limb bud stages where an apparent underlying regionalization of the tissue is detectable. This is not considered as true articulation. Since transformation from “0” to “3” is most likely not separated by a molt, both states are summarized in one entry
Oviger differentiation
Most callipallenids lack palps, whereas they do always bear ten-articled ovigers. In Pseudopallene sp., oviger outgrowth starts in PS 3, when three pairs of articulated walking legs are differentiated and the unarticulated limb bud of walking leg 4 is already visible. From that point on, it takes at least three more molts for the complete number of articles to develop.
When set in relation to trunk development and walking leg differentiation, this timing of the initial oviger outgrowth is found to be similar not only in other callipallenid representatives, but also in pycnogonid species developing via a protonymphon larva.
Regardless of the degree of embryonization encountered in callipallenids, first (external) signs of oviger development have been reported for postembryonic stages with three articulated walking legs and the unarticulated bud of walking leg 4 (Sanchez 1959: Callipallene emaciata; Dohrn 1881; Morgan 1891: C. brevirostris; Nakamura 1981: Propallene longiceps; Bain 2003a: Austropallene cornigera). Deviating observations indicating an earlier onset of oviger development (Meinert 1899: Pseudopallene circularis, “Pallene brevirostris”; Hooper 1980: Parapallene avida) are only poorly documented and find no support in recent SEM studies on callipallenids (Bain 2003a; Bogomolova and Malakhov 2004, present study). In pycnogonid taxa with protonymphon larvae (either free-living and ectoparasitic or attaching and lecithotrophic), the three-articled palpal and ovigeral larval limbs are largely reduced during postembryonic development before re-outgrowth and differentiation of the adult oviger sets in. Prior to this, however, the ovigeral larval limb is reduced to an unarticulated bud (e.g. Dohrn 1881: Endeis spinosa (re-outgrowth in males only); Morgan 1891: T. orbiculare; Dogiel 1913: Achelia laevis, Nymphon spinosum; Okuda 1940: A. alaskensis; Sanchez 1959: Achelia echinata, N. gracile; Dearborn 2003: A. gracilipes; Vilpoux and Waloszek 2003: P. litorale (re-outgrowth in males only); Bogomolova and Malakhov 2006: N. grossipes; Gillespie and Bain 2006: Tanystylum “bealensis”; Bogomolova 2010: N. macronyx), similar to the first oviger outgrowth in Pseudopallene and the other callipallenids. Those studies providing overall descriptions of the corresponding postembryonic stages show that the vestigial unarticulated ovigeral bud is typically reached when three articulated walking leg pairs plus the limb buds of walking leg pair 4 are developed. Even in some Phoxichilidiidae with their derived endoparasitic postembryonic development and the related early reduction of the palpal and ovigeral larval limbs after the first postembryonic molt, the stage emerging from the hydroids with three articulated walking legs and a bud of walking leg 4 still has just a tiny unarticulated bud in the place of the future adult oviger (Dogiel 1913: Anoplodactylus petiolatus, A. pygmaeus; Maxmen 2006: A. eroticus). Hence, we conclude that the onset of adult oviger differentiation represents a widely conserved feature of pycnogonid development, when seen in relation to the degree of differentiation of the post-ovigeral trunk. Depending on the type of the preceding postembryonic development, the first elevation of the future adult oviger represents either a vestige of the reduced ovigeral larval appendage or is developed anew in species without this larval appendage (i.e. in Callipallenidae).
Once adult oviger development has set in, the developmental sequence of intermediate morphological stages and the number of intermittent molts varies between different pycnogonid species. This ontogenetic variation is less surprising between different lineages, given the diversity of podomere numbers in adult ovigers (nine or ten in Colossendeidae, Nymphonidae, Callipallenidae, Ammotheidae, and Pycnogonidae, and four to eight in Rhynchothoracidae, Phoxichiliididae, Endeididae, and Austrodecidae); yet, even between closely related groups, deviations in the developmental sequence can be observed. For instance, in the investigated callipallenid species, the next stage of oviger development is an unarticulated but distinctly elongated limb bud (Nakamura 1981: P. longiceps; Bain 2003a: A. cornigera; present study). However, in P. longiceps (Nakamura 1981), the next molt results in a drastic metamorphosis from the unarticulated limb bud into the complete ten-articled oviger, whereas oviger differentiation in Pseudopallene sp. is more gradual, with an intermediate six-articled stage in PS 5 prior to the completed oviger of PS 6. Similar differences in the developmental sequence of oviger differentiation have been noted in closely related ammotheid species (e.g. Dearborn 2003). These observations indicate that even in species with a comparable structure of the adult ovigers, the sequence of morphologically distinct stages during development does not seem to be strictly conserved.
In the traditional view (e.g. Munilla 1999), the diversity of oviger composition in extant pycnogonids has been considered as representing the result of a gradual reduction trend during pycnogonid evolution. However, phylogenetic analyses (e.g. Arango 2002; Arango and Wheeler 2007; Nakamura et al. 2007; Arabi et al. 2010) clearly show a more scattered distribution of the taxa with high and low podomere numbers of the oviger. This calls for an explanation that might account for multiple and probably even bidirectional evolutionary events. Since the oviger represents a specialized appendage that may have evolved from a “normal” trunk appendage, the same mechanisms as seen in walking leg development could have contributed to its morphological diversification in the various pycnogonid taxa. During walking leg development, some specific precursor podomeres of incompletely articulated walking legs subdivide into adult podomeres (see above). Therefore, it appears feasible that an oviger with a higher number of podomeres (e.g. in Callipallenidae) might have evolved by an additional subdivision event of an already existing oviger podomere. Conversely, evolution of an oviger with fewer podomeres might be the result of the omission of such developmental subdivisions. Interestingly enough, our SEM study of the postembryonic development of Pseudopallene does indicate subdivision events of transient podomeres during oviger development: The distalmost four podomeres forming the strigilis of the complete oviger in PS 6 seem to arise from the long, undivided distal podomere in the oviger of PS 5. Comparative studies combining adult morphology, developmental sequences and leg-segmentation gene expressions of pycnogonid species with a more gradual and therefore morphologically accessible oviger differentiation (as seen, e.g. in Pseudopallene) may eventually lead to the identification of corresponding regions along the oviger p-d axis and thus reveal new insights into the development and evolution of oviger diversity.
Late postembryonic and sub-adult stages
From PS 6 on, we were unable to ascertain the number of molts before sub-adult morphology is reached in Pseudopallene sp. Size differences between PS 6 specimens could be indicative of the existence of several, externally hard to distinguish stages. Similar to the sub-adult, PS 6 as described herein may thus potentially represent a grouping of different molting stages. Notably, we found no stage representing an intermediate condition between PS 6 morphology and the sub-adult appearance, although the latter differs significantly from the former in the shape of the proboscis and the chelifores. This suggests that the observed changes do indeed occur during a single molt. In order to resolve the remaining uncertainties of late postembryonic development, a more rigorous sampling over an extended time period and especially the establishment of a successful laboratory culture would prove invaluable. In addition, investigations of internal organogenesis promise to be useful, since the degree of gonad differentiation has been already shown to exhibit significant differences in externally similar looking late stages of Propallene longiceps (Nakamura 1981). Nonetheless, apart from the laboratory culture-based study on this species (Nakamura 1981), we present here the currently most complete description of postembryonic development in a callipallenid.
References
Arabi J, Cruaud C, Couloux A, Hassanin A (2010) Studying sources of incongruence in arthropod molecular phylogenies: sea spiders (Pycnogonida) as a case study. C R Biol 333:438–453
Arango CP (2002) Morphological phylogenetics of the sea spiders (Arthropoda: Pycnogonida). Org Divers Evol 2:107–125
Arango CP, Wheeler WC (2007) Phylogeny of the sea spiders (Arthropoda, Pycnogonida) based on direct optimization of six loci and morphology. Cladistics 23:1–39
Arnaud F, Bamber RN (1987) The biology of Pycnogonida. Adv Mar Biol 24:1–96
Bain BA (2003a) Postembryonic development in the pycnogonid Austropallene cornigera (Family Callipallenidae). Invertebr Reprod Dev 43:181–192
Bain BA (2003b) Larval types and a summary of postembryonic development within the pycnogonids. Invertebr Reprod Dev 43:193–222
Bamber RN (2007) A holistic re-interpretation of the phylogeny of the Pycnogonida Latreille, 1810 (Arthropoda). Zootaxa 1668:295–312
Bamber RN, El Nagar A (2011) Pycnobase: World Pycnogonida Database. http://www.marinespecies.org/pycnobase
Behrens W (1984) Larvenentwicklung und Metamorphose von Pycnogonum litorale (Chelicerata, Pantopoda). Zoomorphology 104:266–279
Bogomolova EV (2007) Larvae of three sea spider species of the genus Nymphon (Arthropoda: Pycnogonida) from the White Sea. Russian J Mar Biol 33:145–160
Bogomolova EV (2010) Nymphon macronyx (Arthropoda, Pycnogonida), another pycnogonid species with “lecytotrophic protonymphon” development. Zool Zhurnal 89:528–544
Bogomolova EV, Malakhov VV (2003) Larvae of sea spiders (Arthropoda, Pycnogonida) from the White Sea. Entomol Rev 83:222–236
Bogomolova EV, Malakhov VV (2004) Fine morphology of larvae of sea spiders (Arthropoda: Pycnogonida) from the White Sea. Zool Bespozvon 1:3–28
Bogomolova EV, Malakhov VV (2006) Lecithotrophic protonymphon is a special type of postembryonic development of sea spiders (Arthropoda, Pycnogonida). Dokl Biol Sci 409:328–331
Bourlat SJ, Nielsen C, Economou AD, Telford MJ (2008) Testing the new animal phylogeny: a phylum level molecular analysis of the animal kingdom. Mol Phylogenet Evol 49:23–31
Brenneis G, Ungerer P, Scholtz G (2008) The chelifores of sea spiders (Arthropoda, Pycnogonida) are the appendages of the deutocerebral segment. Evol Dev 10:717–724
Brenneis G, Arango CP, Scholtz G (2011) Morphogenesis of Pseudopallene sp. (Pycnogonida, Callipallenidae). I: embryonic development. Dev Genes Evol. doi:10.1007/s00427-011-0382-4
Burris ZP (2011) Larval morphologies and potential developmental modes of eight sea spider species (Arthropoda: Pycnogonida) from the southern Oregon coast. J Mar Biol Assoc UK 91:845–855
Cano Sánchez E, López-González PJ (2010) Postembryonic development of Nymphon unguiculatum Hodgson 1915 (Pycnogonida, Nymphonidae) from the South Shetland Islands (Antarctica). Polar Biol 33:1205–1214
Cano E, López-González PJ (2009) Novel mode of postembryonic development in Ammothea genus (Pycnogonida: Ammotheidae) from Antarctic waters. Sci Mar 73:541–550
Child CA (1979) Shallow water Pycnogonida of the Isthmus of Panama and the coasts of Middle America. Smithson Contrib Zool 23:1–86
Child CA (1998) The marine fauna of New Zealand: Pycnogonida (sea spiders). NIWA Biodivers Mem 109:1–71
Dearborn GK (2003) Post-embryonic development of the sea spider Achelia gracilipes (Chelicerata: Pycnogonida). Dep Biol Sci. Univ Alberta. Master Thesis. pp 1–86
Dogiel V (1913) Embryologische Studien an Pantopoden. Z Wiss Zool 107:575–741
Dohrn A (1881) Die Pantopoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. Fauna und Flora des Golfes von Neapel. Wilhelm Engelmann, Leipzig
Dunlop JA, Arango CP (2005) Pycnogonid affinities: a review. J Zool Syst Evol Res 43:8–21
Edgecombe GD, Wilson GDF, Colgan DJ, Gray MR, Cassis G (2000) Arthropod cladistics: combined analysis of histone H3 and U2 snRNA sequences and morphology. Cladistics 16:155–203
Gillespie JM, Bain BA (2006) Postembryonic development of Tanystylum bealensis (Pycnogonida, Ammotheidae) from Barkley Sound, British Columbia, Canada. J Morphol 267:308–317
Giribet G, Edgecombe GD, Wheeler WC (2001) Arthropod phylogeny based on eight molecular loci and morphology. Nature 413:157–161
Hedgpeth JW (1947) On the evolutionary significance of the Pycnogonida. Smithson Misc Coll 106:1–53
Hoek PPC (1881) Report on the Pycnogonida, dredged by H.M.S. Challenger during the years 1873–76. Zool Chall Exp 10:1–167
Hooper J (1980) Some aspects of the reproductive biology of Parapallene avida Stock (Pycnogonida: Callipallenidae) from Northern New South Wales. Aust Zool 30:473–483
Koenemann S, Jenner RA, Hoenemann M, Stemme T, von Reumont BM (2010) Arthropod phylogeny revisited, with a focus on crustacean relationships. Arthropod Struct Dev 39:88–110
Lebour MV (1916) Notes on the life history of Anaphia petiolata (Kröyer). J Mar Biol Assoc UK 11:51–56
Lebour MV (1945) Notes on the Pycnogonida of Plymouth. J Mar Biol Assoc UK 26:139–165
Lovely EC (2005) The life history of Phoxichilidium tubulariae (Pycnogonida: Phoxichilidiidae). Northeast Nat 12:77–92
Machner J, Scholtz G (2010) A scanning electron microscopy study of the embryonic development of Pycnogonum litorale (Arthropoda, Pycnogonida). J Morphol 271:1306–1318
Maxmen A (2006) Pycnogonid development and the evolution of the arthropod body plan. Harvard Univ, Cambridge, Massachusetts. PhD Thesis. pp 1–177
Meinert F (1899) Pycnogonida. The Danish Ingolf-Expedition. Bianco Luno (F. Dreyer), Copenhagen
Meisenheimer J (1902) Beiträge zur Entwicklungsgeschichte der Pantopoden. I. Die Entwicklung von Ammothea echinata Hodge bis zur Ausbildung der Larvenform. Z Wiss Zool 72:191–248
Meusemann K, von Reumont BM, Simon S, Roeding F, Strauss S, Kück P, Ebersberger I, Walzl M, Pass G, Breuers S, Achter V, von Haeseler A, Burmester T, Hadrys H, Wägele JW, Misof B (2010) A phylogenomic approach to resolve the arthropod tree of life. Mol Biol Evol 27:2451–2464
Morgan TH (1891) A contribution to the embryology and phylogeny of the pycnogonids. Stud Biol Lab Johns Hopkins Univ 5:1–76
Munilla T (1999) Evolución y filogenia de los picnogónidos. In: Melic A, de Haro JJ, Mendez M, Ribera I (eds) Evolución y filogenia de Arthropoda. Sociedad Entomológica Aragonesa, Zaragoza, pp 273–279
Nakamura K (1981) Post-embryonic development of a pycnogonid, Propallene longiceps. J Nat Hist 15:49–62
Nakamura K, Kano Y, Suzuki N, Namatame T, Kosaku A (2007) 18S rRNA phylogeny of sea spiders with emphasis on the position of Rhynchothoracidae. Mar Biol 153:213–223
Ohshima H (1933) Young pycnogonids found parasitic on nudibranchs. Annot Zool Jpn 14:61–66
Ohshima H (1937) The life-history of “Nymphonella tapetis” Ohshima (“Pantopoda, Eurycydidae”). Extr C R XII Congr Int Zool Lisb 1935:1616–1627
Okuda S (1940) Metamorphosis of a pycnogonid parasitic in a hydromedusa. J Fac Sci, Hokkaido Imperial Univ 7:73–86, Ser 6
Prpic NM, Damen WGM (2009) Notch-mediated segmentation of the appendages is a molecular phylotypic trait of the arthropods. Dev Biol 326:262–271
Regier JC, Shultz JW, Zwick A, Hussey A, Ball B, Wetzer R, Martin JW, Cunningham CW (2010) Arthropod relationships revealed by phylogenomic analysis of nuclear protein-coding sequences. Nature 463:1079–1083
Rota-Stabelli O, Campbell L, Brinkmann H, Edgecombe GD, Longhorn SJ, Peterson KJ, Pisani D, Philippe H, Telford MJ (2011) A congruent solution to arthropod phylogeny: phylogenomics, microRNAs and morphology support monophyletic Mandibulata. Proc R Soc B 278:298–306
Salazar-Vallejo S, Stock JH (1987) Apparent parasitism of Sabella melanostigma (Polychaeta) by Ammothella spinifera (Pycnogonida) from the Gulf of California. Rev Biol Trop 35:269–275
Sanchez S (1959) Le développement des Pycnogonides et leurs affinités avec les Arachnides. Arch Zool Exp Gén 98:1–102
Scholtz G, Edgecombe GD (2006) The evolution of arthropod heads: reconciling morphological, developmental and palaeontological evidence. Dev Genes Evol 216:395–415
Sekiguchi K, Nakamura K, Onuma S (1971) Egg-carrying habit and embryonic development in a pycnogonid, Propallene longiceps. Zool Mag 80:137–139
Stock JH (1994) Indo-west pacific Pycnogonida collected by some major oceanographic expeditions. Beaufortia 44:17–77
Ungerer P, Scholtz G (2009) Cleavage and gastrulation in Pycnogonum litorale (Arthropoda, Pycnogonida): Morphological support for the Ecdysozoa? Zoomorphology 128:263–274
Vilpoux K, Waloszek D (2003) Larval development and morphogenesis of the sea spider Pycnogonum litorale (Ström, 1762) and the tagmosis of the body of Pantopoda. Arthropod Struct Dev 32:349–383
von Reumont BM, Meusemann K, Szucsich NU, Dell’Ampio E, Gowri-Shankar V, Bartel D, Simon S, Letsch HO, Stocsits RR, Luan Y, Wägele JW, Pass G, Hadrys H, Misof B (2009) Can comprehensive background knowledge be incorporated into substitution models to improve phylogenetic analyses? A case study on major arthropod relationships. BMC Evol Biol 9:119
Winter G (1980) Beiträge zur Morphologie und Embryologie des vorderen Körperabschnitts (Cephalosoma) der Pantopoda Gerstaecker, 1863. I. Entstehung und Struktur des Zentralnervensystems. Z Zool Syst Evol-Forsch 18:27–61
Zrzavý J, Hypsa V, Vlásková M (1997) Arthropod phylogeny: taxonomic congruence, total evidence and conditional combination approaches to morphological and molecular data sets. In: Fortey RA, Thomas RH (eds) Arthropod relationships. Chapman and Hall, London, pp 97–107
Acknowledgments
Karen Gowlett-Holmes and Mick Baron are thanked for sharing their knowledge of Tasmanian dive sites and their invaluable help in collecting Pseudopallene sp. The help of Paul Whitington, Roy Swain, Glenn Johnstone, and Jonny Stark with the logistics of organizing the essential chemicals for processing the developmental stages is greatly appreciated. David Staples kindly provided hatching larvae of Stylopallene cheilorhynchus and S. longicauda. We are grateful to Wilfried Bleiss and Gabriele Drescher for assistance with the scanning electron microscope. Ekaterina Ponomarenko is thanked for the translation of Russian literature.
Collection of animals and their offspring was made possible by permits of the Tasmanian Department of Primary Industries, Parks, Water and Environment (permit nos. 6039 and 9255). Export of collected material was permitted by the Australian Department of the Environment, Water, Heritage and the Arts (permit nos. WT2008-4394 and WT2009-4260). GB was in part supported by the Studienstiftung des deutschen Volkes and CPA was supported by Australian Biological Resources Study (ABRS, grant 204-61). The project was funded by the Deutsche Forschungsgemeinschaft (Scho 442/13-1).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by S. Roth
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Walking leg articulation in PS 2 of Pseudopallene sp. Imaris volume, autofluorescent signal. a Walking leg 1, anterior view. The leg possesses the adult number of podomeres, the podomere borders being discernible as regions with higher signal intensity. b Walking leg 2, anterior view. The leg possesses only seven podomeres (including terminal claw), regions of future subdivisions in precursor podomeres are not yet assessable. Note high signal intensity in the cuticular fold relating to the anal opening (arrowhead). cx Coxa, fe femur, pro propodus, ta tarsus, tb tibia, tc terminal claw, wl walking leg (JPEG 57 kb)
Supplementary Fig. 2
Walking leg-bearing larvae of Stylopallene cheilorhynchus and Callipallene sp. SEM micrographs and nucleic stainings. a–c S. cheilorhynchus. a Freshly hatched PS 1, anterolateral view. Proboscis and chelifores of the specimen are still inserted in the ripped egg membrane (arrow) and probably an embryonic cuticle. The egg membrane is covered by a hardened sticky matrix via whose stalk-like projection (directed to the left) the egg remains attached to the father’s oviger. The unarticulated limb buds of walking leg 1 and 2 protrude freely from the egg membrane. b PS 1, ventral view. Note the folds of the walking leg tissue beneath the cuticle. c PS 1, posterior view. A tiny interior primordium of walking leg 3 is detectable in the hind body region. The proctodeum/anus is forming beneath the cuticle. The distalmost portion of walking leg 2 will most likely give rise to tarsus and propodus as well as the terminal claw. Proximal to this region, the differentiating tibia 2 may be discernible. d–f Callipallene sp. d Hatched and still attaching PS1, anterolateral view. Note the elongate anlagen of walking leg pairs 1–3. The arrow indicates a piece of ripped egg membrane (plus part of embryonic cuticle?) that still covers the proboscis and is still attached to the spinning gland of the chelifore. Presumptive podomere regions are labeled along walking leg 1. e PS 1, lateral view, Imaris volume (blend). The spinning gland process is distinctly visible due to its autofluorescent signal. A shallow elevation of the developing ocular tubercle lies slightly anterior to walking leg 1. Note the primordium of walking leg 4 as well as the interior tissue folds of walking legs 1–3 that may partially directly relate to the borders of future leg podomeres. f PS 1, ventral view. Walking leg pair 3 is bent in an anterior direction, being wedged between walking leg pairs 1 and 2. The primordium of walking leg pair 4 is already externally detectable, flanking the hind body region that still lacks an anus. ch chelifore, cx coxa, fe femur, ot ocular tubercle, pr proboscis, pro propodus, sgp spinning gland process, ta tarsus, tb tibia, tc terminal claw, wl walking leg (JPEG 124 kb)
Rights and permissions
About this article
Cite this article
Brenneis, G., Arango, C.P. & Scholtz, G. Morphogenesis of Pseudopallene sp. (Pycnogonida, Callipallenidae) II: postembryonic development. Dev Genes Evol 221, 329–350 (2011). https://doi.org/10.1007/s00427-011-0381-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-011-0381-5