Abstract
The larval ascidian brain (sensory vesicle) is located on the dorsal side of the trunk region and forms part of the anterior central nervous system. Sensory organs such as the otolith, ocellus, and hydrostatic-pressure organ reside in the brain. The brain coordinates the core roles of the larval nervous system. The brain is derived from anterior animal a-line blastomeres. The default fate of these blastomeres is epidermis, and the inductive signals from anterior vegetal blastomeres convert the fate into brain. It remains unclear, however, when these inductive interactions take place. To determine when, we examined whether partial embryos derived from brain-lineage blastomeres isolated at various stages express neural and epidermal marker genes. Partial embryos derived from brain-lineage blastomeres isolated after the 32-cell stage expressed all the neural marker genes examined. The expression of the epidermal marker gene was first reduced in partial embryos when blastomeres were isolated at the 64-cell stage. Moreover, the process for brain specification seemed to continue after the 110-cell stage. We also investigated the function of HrEts, an ascidian homolog of Ets transcription factors, to elucidate the molecular mechanism of brain induction. HrEts functions were inhibited by the use of antisense morpholino oligonucleotides. Loss of Ets functions resulted in loss of the expression of some of the neural marker genes and the ectopic expression of the epidermal marker gene in brain precursor cells. These results suggest that HrEts is an essential transcription factor that mediates ascidian brain induction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
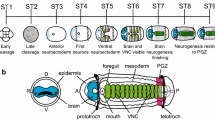
The central nervous system (CNS) of ascidian tadpole larvae comprises the anterior brain (sensory vesicle) in the cranial region and the posterior nerve cord in the trunk and tail regions (reviewed by Meinertzhagen and Okamura 2001). In the larval brain, sensory organs and neurons reside in the walls of the brain cavity. The brain is followed by the anterior part of the nerve cord, which is often called the posterior sensory vesicle. A visceral ganglion lies in the neck region. The nerve cord in the tail simply consists of ependymal glial cells. The cell lineage of the brain is illustrated in Fig. 1. The brain is derived from a4.2 (anterior animal) blastomeres of 8-cell-stage embryos, and the nerve cord (the rest of the CNS) is derived from A4.1 (anterior vegetal) and b4.2 (posterior animal) blastomeres (Figs. 1a, f; Nishida 1987). At the next cleavage, the a4.2 blastomere divides into a5.3 and a5.4 blastomeres. At the 32-cell stage, the a6.5 blastomere, daughter of a5.3, is restricted to give rise to neural tissues, brain, and palps. On the other hand, the a6.7 blastomere, daughter of a5.4, still has both neural and epidermal fates (Fig. 1g). The expression of a neural marker gene (Hroth) is initiated at this stage only in a6.5 blastomeres of the two brain lineage blastomeres (a6.5 and a6.7; Wada et al. 1996; Hudson and Lemaire 2001). Activation of mitogen-activated protein kinase (MAPK/ERK) is also seen only in a6.5 blastomeres (Nishida 2003). After two successive cleavages, a8.17, a8.19, and 8.25 blastomeres of 110-cell-stage embryos are restricted to form brain, and Hroth expression and activation of MAPK are observed in all of these blastomeres.
Cell lineages of ectodermal tissues, brain, palp, and epidermis in the animal hemisphere and a temporal sequence showing the initiation of the expression of neural and epidermal marker genes used in this study. a–f Schematic diagrams illustrating ascidian embryos. Anterior is to the left. a Eight-cell embryo (lateral view). b–e Sixteen-, 32-, 64-, and 110-cell-stage embryos in animal view. Red hatches represent brain-lineage blastomeres whose developmental fate is not restricted to the brain. Blastomeres colored red are restricted to form brain, and those colored orange and green are restricted to form palp and epidermis, respectively. Short bars between blastomeres indicate daughter cells generated by the previous cleavages. f Tailbud embryo (lateral view). g Temporal sequence of the expression of marker genes (upper), and lineage tree (lower) showing brain, palp, and epidermis lineages in the a-line of one lateral half. Black rectangles surrounding blastomere names indicate the blastomeres used in blastomere isolation experiments shown in Figs. 2 and 3 (NP neural-plate stage, N neurula stage)
Epidermal fate is specified autonomously by the maternally localized determinant in ascidian embryos (Nishida 1992). The formation of brain from the anterior edge of the animal hemisphere depends on inductive influence from the vegetal A-line blastomeres (Okado and Takahashi 1988; Nishida 1991; Okamura et al. 1994; Meinertzhagen and Okamura 2001; Lemaire et al. 2002). a-line blastomeres that have a default fate of epidermis acquire a brain fate by neural induction. As to the period required for brain induction, however, reports disagree (Okado and Takahashi 1993; Inazawa et al. 1998; Darras and Nishida 2001).
Recent work suggests that the FGF (fibroblast growth factor)/MAPK signaling pathway plays a pivotal role in brain induction in ascidians. Exogenous basic FGF induces the expression of a neuron-specific sodium channel gene (TuNa1; Inazawa et al. 1998), a neural-plate marker gene (HrETR-1), a neural-tissue-specific β-tubulin gene (HrTBB2; Darras and Nishida 2001), and Orthodenticle/Otx homologs (Darras and Nishida 2001; Hudson and Lemaire 2001) in isolated a-line blastomeres. The expression of epidermal markers is suppressed by basic FGF treatment (Darras and Nishida 2001). Thus, FGF induces the formation of brain and suppresses epidermal fate. Consistent with this, inhibition of FGF/MAPK signaling blocks the formation of brain. Treatment with FGF receptor inhibitor and injection of dominant-negative Ras protein lead to loss of the anterior nervous system and HrETR-1 expression in a-line-derived neural cells (Kim and Nishida 2001). Similarly, in embryos treated with inhibitors of MEK (ERK kinase/MAPKK), brain-lineage blastomeres lose the expression of neural marker genes and express an epidermal marker gene instead (Kim and Nishida 2001; Hudson et al. 2003).
Ets family proteins are target transcription factors of the Ras/MAPK signaling pathway (reviewed by Wasylyk et al. 1998; Yordy and Muise-Helmericks 2000). Recently, an Ets family gene of the ascidian Halocynthia roretzi, HrEts, was identified. It has been shown that HrEts is required for the expression of the Hroth gene, the earliest and essential neural marker gene for brain formation in ascidian embryos (Wada et al. 1996; Wada and Saiga 1999), as a downstream target transcription factor of FGF/MAPK signaling (Miya and Nishida 2003).
In this study, we aimed to elucidate the mechanism of brain induction in ascidian embryos at the cellular and molecular levels. First, we examined the period of brain induction. Next, we investigated the functions of the HrEts transcription factor in brain induction by using antisense morpholino oligonucleotides (MO) and examining the expression of various neural and epidermal marker genes. The results suggest that brain induction takes place progressively from the 32-cell stage to at least the 110-cell stage, and that HrEts is required for brain precursor cells to express some brain-specific genes and to suppress epidermis-specific gene expression.
Materials and methods
Animals and embryos
Adults of H. roretzi were collected or purchased from fishermen in the vicinity of Asamushi Marine Biological Station, Aomori, Japan, and Otsuchi Marine Research Center, Iwate, Japan. Naturally spawned eggs were artificially fertilized with a suspension of non-self sperm and raised in Millipore-filtered (pore size 0.45 µm) seawater containing 50 µg/ml streptomycin sulfate and 50 µg/ml kanamycin sulfate at 9–13°C.
Cloning of H. roretzi β-arrestin
In MAGEST, a database of maternal mRNA of Halocynthia eggs (Kawashima et al. 2000; Makabe et al. 2001), we found clone 009F24, which encodes a β-arrestin homolog that is zygotically expressed in the cranial region of tailbud embryos. To obtain the entire cDNA of β-arrestin, we carried out rapid amplification of cDNA ends (RACE) using a SMART RACE cDNA amplification kit (Clontech). The reverse primers for 5′ RACE and the forward primer for 3′ RACE were designed on the basis of the sequence of 009F24: primer for 5′ RACE, 5′-CCTTGCGAACCTCTGCACTCGGCT-3′; primer for 3′ RACE, 5′-ACCCTGCAACCAGCTAATGGTGACACGG-3′. The gene was named Hrarr (H. roretzi β-arrestin). The database accession number for the complete Hrarr cDNA sequence is AB109741.
Isolation of blastomeres
Embryos were manually devitellinized with tungsten needles and reared in agar-coated plastic dishes filled with seawater. Blastomeres were identified and isolated from embryos with a fine glass needle under a stereomicroscope. Isolated blastomeres were cultured separately as partial embryos.
Microinjection and treatment with cytochalasin B
To suppress the function of HrEts, we used an antisense morpholino oligonucleotide (MO; Gene Tools). The nucleotide sequence of the MO was 5′-TCATGGCGACCTTGGCCTGAAGATG-3′ (Miya and Nishida 2003). HrEts MO (1.5–2 ng) was injected into each fertilized egg. The injected embryos were allowed to develop up to the 110-cell stage and then transferred to seawater containing 2.5 µg/ml cytochalasin B (Sigma) to permanently arrest further cleavage. These embryos were fixed for in situ hybridization when unarrested control embryos developed to appropriate stages. To confirm the specificity of the effect of HrEts MO, we coinjected 150–200 pg synthetic HrEts mRNA that lacks the target sequence of HrEts MO (Miya and Nishida 2003) together with HrEts MO into each egg. HrEts mRNA was transcribed from pBluescriptHTB (Akanuma et al. 2002) containing HrEts cDNA, by using a T3 mMessage mMachine kit (Ambion) and a Poly(A) Tailing kit (Ambion).
Whole-mount in situ hybridization
Whole-mount in situ hybridization was performed by using digoxigenin (DIG)-labeled antisense probes, as described (Miya et al. 1994, 1997). For partial embryos, the protocol was modified slightly. Hybridization and washing were carried out in 96-well silent screen plates (Nalge Nunc), the bottoms of which were sealed with a nylon membrane with 3.0-µm pores (Minokawa et al. 2001; Ogasawara et al. 2001). Treatment with proteinase K and post-fixation before hybridization were omitted, since these damaged partial embryos. Plasmids containing cDNAs for Hroth (Wada et al. 1996), HrETR-1 (Yagi and Makabe 2001), HrTBB2 (Miya and Satoh 1997), HrEpiC (Ueki et al. 1991; Ueki and Satoh 1995) and Hrarr were used to prepare the DIG-labeled RNA probes. The expressions of Hroth and HrETR-1 were monitored at the neural plate stage, and those of HrTBB2 and Hrarr at the middle to late tailbud stage, when the expression of each gene is strongest and specific to neural tissues. For evaluation of background staining, the signal of samples was compared with that of partial embryos derived from non-brain-lineage blastomeres (B4.1 and b4.2) of 8-cell embryos (Fig. 1a). The expression of HrEpiC in partial embryos was monitored at the neural plate stage, and that in cleavage-arrested embryos at the middle to late tailbud stage.
Results
Brain induction starts at the 32-cell stage and still progresses at the 110-cell stage
The ascidian larval brain originates from anterior animal cells, the a4.2 pair at the 8-cell stage (Fig. 1a, f, g). During cleavages, brain fate (red in Fig. 1) is restricted to the anterior-most descendants of the a4.2 pair, and the other descendants assume fates of epidermis (green) and palps (orange), which are part of the peripheral nervous system (Fig. 1b–g). In this study, we focused on the a5.3 brain lineage after the 16-cell stage. The a5.4 brain lineage may have different characteristics from the a5.3 lineage. The lineage trees of both show different configurations (Fig. 1g) and it has been reported that at the 64-cell stage, a7.13 brain-lineage blastomeres express an epidermal marker gene, HrEpiC, whereas a7.9 and a7.10 blastomeres do not (Fig. 1g; Ishida et al. 1996).
Molecular markers used in this study are listed in Fig. 1g, and shown in Fig. 2a, c, e, g, and i. Hroth, an ascidian homolog of the Orthodenticle/Otx gene, is expressed in brain/palp precursor cells at and after the 32-cell stage (Wada et al. 1996). At the neural-plate stage, its expression is seen in brain and palp progenitor cells (Fig. 2a). Hroth is also expressed in A9.14 and A9.16 blastomeres of nerve cord precursor cells, although relatively weakly (arrowheads in Fig. 2a). The expression of HrETR-1, which encodes an elav-type RNA-binding protein, starts at the 110-cell stage and continues in the cells that build up the neural plate, including brain precursor cells (Fig. 2c; Yagi and Makabe 2001). A nervous-system-specific β-tubulin gene, HrTBB2, is expressed in the presumptive brain region after the neural plate stage (Fig. 2e; Miya and Satoh 1997). Hrarr encodes a Halocynthia homolog of β-arrestin. β-Arrestins regulate the activity of G-protein-coupled receptors and are expressed mainly in the nervous systems in various animals (reviewed by Pierce and Lefkowitz 2001). In larvae of another ascidian, Ciona, β-arrestin is expressed in the brain (Nakagawa et al. 2002). We observed that β-arrestin was expressed in the cranial region during Halocynthia embryogenesis (Fig. 2g). The major domain of Hrarr expression, however, was more posterior to the brain, and the domain was mainly derived from A-line neural cells. In addition, some a-line brain cells also expressed Hrarr (K. Taniguchi and H. Nishida, unpublished). At the 64-cell stage, expression of an epidermis-specific gene, HrEpiC, is initiated in epidermis precursors (Ishida et al. 1996). No expression was observed in the neural plate cells (Fig. 2i).
Expression of neural and epidermal marker genes in control whole embryos and partial embryos derived from isolated blastomeres that are indicated on the top of each column (cell stages are indicated in parentheses). The gene examined is indicated on the left of each row. a, c, e, g, i Spatial expression patterns of neural marker genes in normal embryogenesis. Dorsal views. Embryos in a, c, and i are at the neural-plate stage. Embryos in e and g are at the middle-tailbud stage. Arrows indicate the brain precursor cells. Arrowheads in a indicate the expression in A-line neural plate cells. b, d, f, h, j Expression of the marker genes in partial embryos derived from brain-lineage blastomeres isolated at various cleavage stages. The position of each blastomere is indicated in Fig. 1a–e. Scale bars 100 µm
To uncover when brain fate was determined, we examined the abilities of isolated blastomeres to express neural marker genes autonomously and to suppress the default expression of an epidermal marker gene. Blastomeres were isolated at various stages between the 8-cell stage, when blastomeres were separated into animal and vegetal hemispheres, and the 110-cell stage, when presumptive brain blastomeres were restricted to form the brain and lost the ability to form epidermis in isolation (Nishida 1991). The isolated blastomeres were cultured separately as partial embryos until reaching appropriate stages for each molecular marker to be studied. The expressions of marker genes were examined by in situ hybridization.
The results obtained by a series of blastomere isolations are shown in Fig. 2 and summarized in Fig. 3. The earliest neural marker gene, Hroth, was expressed at a very low level, if any, in partial embryos derived from a4.2 blastomeres of 8-cell embryos and from a5.3 blastomeres of 16-cell embryos (Fig. 2b). By contrast, in partial embryos isolated at the 32-cell stage and later, the expression of Hroth was dramatically increased (a6.5, a7.9 and a7.10, a8.17 and a8.19 in Figs. 2, 3). This coincides with the fact that the expression of Hroth is initiated in a6.5 at the 32-cell stage in normal embryogenesis. This is also consistent with previous observation that ablation of A-line blastomeres before the 32-cell stage results in loss of Hroth expression in the a-line blastomeres, and ablation of A-line blastomeres after the 32-cell stage does not affect the expression of Hroth (Wada et al. 1999). Therefore, cell contact with A-line blastomeres until the 32-cell stage is sufficient for the a-line brain-lineage cells to express Hroth.
A neural-plate-specific gene, HrETR-1, was not expressed in partial embryos derived from a4.2 and a5.3 blastomeres. However, 80% of embryos derived from a6.5 at the 32-cell stage exhibited a weak signal of HrETR-1 (Figs. 2d, 3). Since partial embryos derived from a6.6 epidermal precursors, sister cells of a6.5, exhibited no staining, the weak signal seen in the a6.5 partial embryos was unlikely to be background staining from in situ hybridization. The strength of the signal scarcely increased to a level comparable to that in intact embryos (Fig. 2c) in partial embryos, even if blastomeres were isolated at the 110-cell stage (Figs. 2d, 3).
The response of the neural tubulin gene, HrTBB2, was similar to that of Hroth (Figs. 2f , 3). HrTBB2 expression was first observed in 75% of partial embryos derived from a6.5 of 32-cell embryos. The expression was frequently seen only in some cells in each partial embryo. By contrast, in the partial embryos derived from a7.9 and a7.10 blastomeres at the 64-cell stage, HrTBB2 was expressed in entire partial embryos in all cases. Unexpectedly, the proportion of embryos expressing HrTBB2 at a high level decreased when the blastomeres were isolated from the 110-cell embryos (light blue bar in Fig. 3). This was observed in two independent experiments.
Slight staining for Hrarr was observed in a4.2- and a5.3-derived partial embryos (Fig. 2h). However, partial embryos derived from non-CNS-lineage cells (e.g. B4.1 blastomeres) of 8-cell embryos whose descendants never expressed Hrarr in normal development were also stained slightly. Therefore, this slight staining was regarded as non-specific background staining. A signal little stronger than background was seen in 57% of a6.5-derived partial embryos (Figs. 2h, 3). The proportion of embryos expressing Hrarr and the expression level in each partial embryo gradually increased as blastomeres were isolated at later stages. Thus, the profiles of gene expression were a bit different from gene to gene. However, all of them commonly showed qualitative contrast between the consequences of isolation at the 16- and 32-cell stages.
We also examined expression of the epidermal marker gene, HrEpiC, in partial embryos (Figs. 2j, 3). It was fully expressed in partial embryos that originated from isolated a4.2, a5.3, and a6.5 blastomeres. In the partial embryos derived from a7.9 and a7.10 of 64-cell embryos, the staining became weaker in all cases, although the proportion of stained embryos was almost the same (light green bars in Fig. 3). The proportion of weakly stained embryos decreased to 30% of partial embryos when blastomeres were isolated at the 110-cell stage. This is consistent with the previous observation that the presumptive brain blastomeres of the 110-cell embryos do not sustain the ability to form epidermis in isolation (Nishida 1991)
All these results suggest that brain precursor cells require cell contact with other lineage cells, at least until the 32-cell stage, for induction of the expression of most neural marker genes, and until the 64-cell stage for suppression of the epidermal marker gene, HrEpiC. In addition, continuous cell contact from the 32- to 110-cell stages would have to be maintained for completion of promotion of Hrarr and suppression of HrEpiC.
Inhibition of the function of HrEts disturbs the brain induction
Recent work demonstrates that FGF/MAPK signaling is involved in neural induction in ascidian embryos (Inazawa et al. 1998; Darras and Nishida 2001; Kim and Nishida 2001; Hudson and Lemaire 2001; Hudson et al. 2003; Nishida 2003). It has been suggested that HrEts, a Halocynthia homolog of vertebrate Ets1 and Ets2, encodes the target transcription factor of FGF signaling and functions as a regulator of Hroth expression in brain lineage blastomeres (Miya and Nishida 2003). Therefore, we examined the functions of HrEts in brain induction in more detail by using an antisense morpholino oligonucleotide (MO; Fig. 4). Injection of HrEts MO results in the loss of brain, palp, mesenchyme, and notochord, whose formation depends on FGF/MAPK signaling (Miya and Nishida 2003). We reconfirmed that the injection of HrEts MO results in the same aberrant morphology reported. It was, however, hard to evaluate the expression of neural marker genes in brain precursor cells, since morphology of such embryos was severely disturbed. Therefore in this study, we permanently arrested the cleavage at the 110-cell stage so that we are able to identify blastomeres. In cleavage-arrested ascidian embryos, blastomeres can undergo some processes of cell differentiation and express cell-type-specific markers according to the fate that they adopt during embryogenesis (e.g. Hudson et al. 2003). To confirm the specificity of HrEts MO, we carried out rescue experiments by coinjecting synthetic HrEts mRNA from which the target sequence of MO was mostly removed (Miya and Nishida 2003).
Expression of neural and epidermal marker genes in cleavage-arrested embryos injected with HrEts antisense morpholino oligonucleotide (MO). a, e, i, m, q Schematic representation of 110-cell-stage embryo (left column animal view; right column vegetal view). Fates of each blastomere are shown by color (red brain, orange palp, purple posterior nerve cord). Black dots indicate the expression of Hroth (a), HrETR-1 (e), HrTBB2 (i), HrEpiC (m), and Hrarr (q). b–d, f–h, j–l, n–p, r–t Expressions of marker genes in 110-cell arrested embryos without injection, those injected with HrEts MO, and those coinjected with HrEts MO and HrEts mRNA. All embryos are viewed from the animal pole. Colored dots represent the blastomere identities as judged by the their sizes and the locations shown in the illustrations on the left. In the embryo shown in c, nerve cord blastomeres (with purple dots) lie below the focal plane and brain blastomeres (with red dots). Scale bar 100 µm
First, we examined the effect on Hroth. Blastomeres were identified on the basis of their positions and sizes, and are indicated by color codes in Fig. 4. In uninjected control embryos, the expression was observed in six brain precursor cells (red in Fig. 4) and four palp precursor cells (orange) of the a-line. Among nerve-cord-lineage blastomeres (purple), A8.7 and A8.8 blastomeres expressed Hroth. Endoderm precursors also expressed the gene. In embryos injected with HrEts MO, on average, only three a-line blastomeres expressed Hroth at the same level as in control embryos (n =52; Fig. 4c). The number of Hroth-positive a-line cells recovered to 7.5 cells in the embryos coinjected with MO and mRNA (n =45; Fig. 4d). The expression in the A-line and endoderm was not affected during this experiment.
In most control-arrested embryos, HrETR-1 was expressed in six brain lineage cells and eight A-line cells that gave rise to nerve cord (Fig. 4e, f). In the case of HrETR-1, the effect of HrEts MO was not obvious. The average numbers of positive cells were 5.3 in a-line cells and 7.6 in A-line cells in embryos injected with HrEts MO (n =58; Fig. 4g), and 5.6 in a-line cells and 7.5 in A-line cells in embryos coinjected with MO and mRNA (n =47; Fig. 4h).
Another neural marker gene, HrTBB2, was expressed in 6 brain progenitors and 12 nerve cord lineage cells, including 8 A-line cells and 4 b-line cells (Fig. 4i, j). In embryos injected with HrEts MO, the expression in brain and b-line-derived nerve cord lineage cells was almost completely extinguished (Fig. 4k). The average numbers of the expressing cells were 0.6 in a-line brain precursor cells, 0.1 in b-line, and 8.0 in A-line nerve cord precursor cells (n =32). In the rescued embryos (n =20; Fig. 4l), the expression in brain lineage cells recovered (4.0 cells on average), although the expression in b-line nerve cord was not much rescued (0.7 cells on average). The average number of A-line cells expressing HrTBB2 was 7.3 in those embryos.
Next we examined the expression of the epidermal marker gene, HrEpiC. In the control-arrested embryos, HrEpiC was expressed in all a-line blastomeres except six brain progenitor cells that were present at the anterior edge (Fig. 4m, n). In all 32 embryos injected with HrEts MO, the expression was also seen in brain precursor cells (Fig. 4o). This ectopic HrEpiC expression was restored in embryos coinjected with HrEts MO and HrEts mRNA (n =35; Fig. 4p). These results suggest that the function of HrEts is required for presumptive brain precursor cells to express some of the neural marker genes and to suppress HrEpiC expression.
HrEts may mediate part of the posteriorizing signal in A-line-derived neural cells
Within 12 nerve-cord lineage cells at the 110-cell stage, A8.7 and A8.8 pairs contribute to the posterior region flanking the brain in the trunk, which is called the posterior sensory vesicle (Nishida 1987; K. Taniguchi and H. Nishida, unpublished). Four cells of A8.7 and A8.8 pairs expressed the anterior neural marker gene, Hroth, in cleavage-arrested embryos (Fig. 4b). It has been shown in Ciona that inhibition of MEK signaling causes anteriorization of the A8.15 and A8.16 nerve cord precursor cells, which are fated to give rise to the nerve cord of the tail, and that all the A-line nerve cord cells express otx (Hudson et al. 2003). Therefore, we expected that the number of A-line cells expressing Hroth would increase when the function of HrEts, a candidate downstream component of MEK signaling, was inhibited. In embryos injected with HrEts MO, however, no effect was observed on the Hroth expression in the A-line cells (3.8 cells on average; Fig. 4c). By contrast, in the case of Hrarr, which is expressed mainly in the posterior sensory vesicle in addition to some a-line brain cells, the result was similar to that observed in Ciona. In control-arrested embryos, the expression of Hrarr was not seen in the a-line brain precursor cells, probably owing to the effect of cleavage arrest, and it was expressed only in A8.7 and A8.8 pairs, which are fated to become the posterior sensory vesicle (Fig. 4q, r). In 58% of the embryos injected with HrEts MO (n =32), the Hrarr-expression site expanded to the lateral region, which encompasses A8.15 and A8.16 (5.0 cells on average; Fig. 4s). The ectopic expression of Hrarr was negated by coinjection of HrEts mRNA (3.4 cells on average; n =20; Fig. 4t). Thus, the HrEts seems to be involved in the distinction between two types of nerve cord precursor by posteriorizing the A8.15 and A8.16 cells, although its requirement would be different between Hroth and Hrarr.
Discussion
Brain specification is initiated at the 32-cell stage and progresses during cleavage stages
In this study, we focused on the specification of brain fate during ascidian embryogenesis. While the fate of nerve cord in the posterior CNS is specified in a cell-autonomous manner, brain is induced by signals from vegetal blastomeres (Okada et al. 1997; Minokawa et al. 2001; reviewed by Meinertzhagen and Okamura 2001; Lemaire et al. 2002). It was still unclear, however, when brain induction starts and finishes. We tried to approach the issue by isolating brain-lineage blastomeres and then examined the expression of neural marker genes. Except for Hrarr, the neural marker genes used in this study are expressed in both brain and palps. Hrarr is expressed in anterior CNS including brain, but not in palps. Therefore, we can distinguish the formation of palp from that of brain by monitoring the expression of Hrarr. Our results showed that a6.5 blastomeres isolated at the 32-cell stage expressed all the neural marker genes, including Hrarr. At this stage, the expression of the earliest neural marker gene, otx, starts in a6.5 blastomeres in intact embryos (Wada et al. 1996; Hudson and Lemaire 2001). Moreover, MAP kinase is first activated in brain-lineage cells at this stage (Nishida 2003; Hudson et al. 2003). Concomitantly with MAP kinase activation, the sensitivity of otx expression to MEK inhibitors is reduced after the 32-cell stage (Hudson et al. 2003). All these data are consistent with the idea that brain specification starts at the 32-cell stage, when a6.5 brain-lineage blastomeres are restricted to neural fates, namely brain in the CNS and palp in the peripheral nervous system (Fig. 1g), both of which express otx. Partial embryos derived from 64-cell-stage embryos showed a higher proportion of expression of the neural marker genes except for HrETR-1. Moreover, the expression of Hrarr, which was specific to the anterior CNS but not to palps, further increased when blastomeres were isolated at the 110-cell stage. These data suggest that cell interactions start at or before the 32-cell stage, and continue during the 64- to 110-cell stages, when fate restriction to brain still continues (Fig. 1). On the other hand, HrETR-1 expression was considerably lower than the normal level even when blastomeres were isolated at the 110-cell stages, suggesting that cell interactions for brain specification are not complete even at this stage.
Every partial embryo derived from a6.5 fully expressed the epidermal marker gene simultaneously with neural marker genes (Fig. 3). Thus, induction of the expression of neural marker genes can be uncoupled from the inhibition of epidermal fate. The expression of HrEpiC was gradually reduced as isolations were carried out at later stages. Therefore, to suppress the epidermal fate, continuous cell contact is also required at least up to the 110-cell stage. In whole embryos at the 64-cell stage, HrEpiC expression had already started exclusively in epidermal precursors, and brain-lineage a7.9 and a7.10 blastomeres never expressed HrEpiC. By contrast, 98% of a7.9 and a7.10 blastomeres eventually expressed HrEpiC in isolation. Therefore, the state of brain-lineage cells at the 64-cell stage is still reversible in certain aspects.
Figure 5a represents a temporal sequence of brain induction. There are two processes. First, the presumptive brain precursor cells start to acquire the ability to autonomously express the neural genes through the cell-cell interactions that take place at or before the 32-cell stage. Next, suppression of epidermal genes is initiated at the 64-cell stage. But these two processes do not complete in a single cell cycle. This contrasts to the induction of notochord and mesenchyme, which is initiated and completed during the 32-cell stage (Nakatani and Nishida 1994; Kim and Nishida 1999). Continuous signaling over two or three cell cycles is required for completion of brain induction and for keeping the expression of epidermal genes silent. The 32- to 110-cell stage coincides well with the period of sensitivity to MEK inhibitor for otx and ETR-1 expression in Ciona (Hudson et al. 2003).
Temporal sequence of brain induction and HrEts-mediated signaling in ascidian embryos. a Acquisition of the ability to autonomously express neural marker genes starts at the 32-cell stage. It continues at least up to the 110-cell stage. Suppression of the epidermal marker gene’s expression is initiated at the 64-cell stage and is complete at the 110-cell stage. b Presumptive brain blastomeres receive the FGF signal. This signal activates the Ras/MAPK pathway, which then phosphorylates the HrEts protein. Activated HrEts turns on the transcription of the Hroth gene. Hroth would play core roles to suppress the expression of epidermis-specific genes at the 64-cell stage and to induce the expression of the late neural marker genes (e.g. HrTBB2) in brain lineage cells. An early neural marker gene, HrETR-1, is controlled by the FGF/MAPK pathway but not by HrEts. Another factor (X) would act downstream of the FGF/MAPK pathway to activate HrETR-1 at the 110-cell stage
HrEts mediates brain induction in ascidian embryos
Various lines of evidence suggest that the FGF/MAPK pathway initially triggers the specification of ascidian brain at cleavage stages (Inazawa et al. 1998; Darras and Nishida 2001; Hudson et al. 2003; Nishida 2003). HrEts acts downstream of the FGF/MAPK pathway and regulates the expression of Hroth (Miya and Nishida 2003). We investigated the functions of HrEts in more detail. Inhibition of function by HrEts MO disturbed the expression of an early neural marker gene, Hroth, and a later one, HrTBB2, in the brain-lineage cells. HrEpiC, an epidermal marker gene, was expressed in brain precursor cells instead. Orthodenticle/Otx genes are known to play important roles in brain development in Drosophila and vertebrates (Simeone 1998). Overexpression of Hroth leads to cell-autonomous expression of HrTBB2 and suppression of the expression of an epidermal marker gene in isolated a-line blastomeres (Wada and Saiga 1999). Therefore, it is plausible that reduction of Hroth expression by HrEts MO consequently causes loss of HrTBB2 expression and ectopic expression of the epidermal gene.
HrEts may not be a sole target transcription factor of the FGF/MAPK pathway during ascidian embryogenesis
Figure 5b summarizes the signaling cascades that are involved in brain induction. Previous studies have demonstrated that components of the FGF/MAPK signaling pathway regulate the expression of several neural marker genes (Inazawa et al. 1998; Darras and Nishida 2001; Kim and Nishida 2001; Hudson and Lemaire 2001; Hudson et al. 2003). The results of Miya and Nishida (2003) and the present study indicate that Hroth, HrTBB2, and HrEpiC are expressed directly or indirectly downstream of HrEts. It has been shown that HrETR-1 expression is similarly induced by FGF treatment (Darras and Nishida 2001) and suppressed by MEK inhibitors (Kim and Nishida 2001; Hudson et al. 2003). However, the results of the present study suggest that HrETR-1 does not lie downstream of HrEts. Therefore, there might be another factor (X in Fig. 5b) downstream of MAP kinase that acts in parallel in order to activate the transcription of HrETR-1. Another possibility is that injected HrEts MO is not efficient at completely suppressing HrEts translation.
Recent work demonstrates that A-line-notochord blastomeres adopt nerve cord fate and express HrETR-1 and HrTBB2 when notochord induction is blocked by blastomere isolation or MEK inhibitor treatment (Kim and Nishida 2001; Minokawa et al. 2001; Hudson et al. 2003). Since inhibition of HrEts functions results in loss of expression of notochord markers, HrBra and notochord-specific antigen, HrEts is thought to be a component of the signaling cascade by which notochord formation is induced (Miya and Nishida 2003). In embryos injected with HrEts MO, however, no ectopic expression of neural marker genes was observed in notochord lineage cells. Therefore, HrEts is required for the expression of HrBra and its downstream notochord-specific products, but again unknown factors downstream of MAPK could be involved in suppression of nerve cord fate in notochord precursor cells.
References
Akanuma T, Hori S, Darras S, Nishida H (2002) Notch signaling is involved in nervous system formation in ascidian embryos. Dev Genes Evol 212:459–472
Darras S, Nishida H (2001) The BMP/CHORDIN antagonism controls sensory pigment cell specification and differentiation in the ascidian embryo. Dev Biol 236:271–288
Hudson C, Lemaire P (2001) Induction of anterior neural fates in the ascidian Ciona intestinalis. Mech Dev 100:189–203
Hudson C, Darras S, Caillol D, Yasuo H, Lemaire P (2003) A conserved role for the MEK signalling pathway in neural tissue specification and posteriorisation in the invertebrate chordate, the ascidian Ciona intestinalis. Development 130:147–159
Inazawa T, Okamura Y, Takahashi K (1998) Basic fibroblast growth factor induction of neuronal ion channel expression in ascidian ectodermal blastomeres. J Physiol 511:347–359
Ishida K, Ueki T, Satoh N (1996) Spatio-temporal expression patterns of eight epidermis-specific genes in the ascidian embryo. Zool Sci 13:699–709
Kawashima T, Kawashima S, Kanehisa M, Nishida H, Makabe KW (2000) MAGEST: MAboya gene expression patterns and sequence tags. Nucleic Acids Res 28:133–135
Kim GJ, Nishida H (1999) Suppression of muscle fate by cellular interaction is required for mesenchyme formation during ascidian embryogenesis. Dev Biol 214:9–22
Kim GJ, Nishida H (2001) Role of the FGF and MEK signaling pathway in the ascidian embryo. Dev Growth Differ 43:521–533
Lemaire P, Bertrand V, Hudson C (2002) Early steps in the formation of neural tissue in ascidian embryos. Dev Biol 252:151–169
Makabe KW, Kawashima T, Kawashima S, Minokawa T, Adachi A, Kawamura H, Ishikawa H, Yasuda R, Yamamoto H, Kondoh K, Arioka S, Sasakura Y, Kobayashi A, Yagi K, Shojima K, Kondoh Y, Kido S, Tsujinami M, Nishimura N, Takahashi M, Nakamura T, Kanehisa M, Ogasawara M, Nishikata T, Nishida H (2001) Large-scale cDNA analysis of the maternal genetic information in the egg of Halocynthia roretzi for a gene expression catalog of ascidian development. Development 128:2555–2567
Meinertzhagen IA, Okamura Y (2001) The larval ascidian nervous system: the chordate brain from its small beginnings. Trends Neurosci 24:401–410
Minokawa T, Yagi K, Makabe KW, Nishida H (2001) Binary specification of nerve cord and notochord cell fates in ascidian embryos. Development 128:2007–2017
Miya T, Nishida H (2003) An Ets transcription factor, HrEts, is target of FGF signaling and involved in induction of notochord, mesenchyme, and brain in ascidian embryos. Dev Biol 261:25–38
Miya T, Satoh N (1997) Isolation and characterization of cDNA clones for β-tubulin genes as a molecular marker for neural cell differentiation in the ascidian embryo. Int J Dev Biol 41:551–557
Miya T, Makabe KW, Satoh N (1994) Expression of a gene for major mitochondrial protein, ADP/ATP translocase, during embryogenesis in the ascidian Halocynthia roretzi. Dev Growth Differ 36:39–48
Miya T, Morita K, Suzuki A, Ueno N, Satoh N (1997) Functional analysis of an ascidian homologue of vertebrate Bmp-2/Bmp-4 suggests its role in the inhibition of neural fate specification. Development 124:5149–5159
Nakagawa M, Orii H, Yoshida N, Jojima E, Horie T, Yoshida R, Haga T, Tsuda M (2002) Ascidian arrestin (Ci-arr), the origin of the visual and nonvisual arrestins of vertebrate. Eur J Biochem 269:5112–5118
Nakatani Y, Nishida H (1994) Induction of notochord during ascidian embryogenesis. Dev Biol 166:289–299
Nishida H (1987) Cell lineage analysis in ascidian embryos by intracellular injection of a tracer enzyme. III. Up to the tissue restricted stage. Dev Biol 121:526–541
Nishida H (1991) Induction of brain and sensory pigment cells in the ascidian embryo analyzed by experiments with isolated blastomeres. Development 112:389–395
Nishida H (1992) Developmental potential for tissue differentiation of fully dissociated cells of the ascidian embryo. Roux’s Arch Dev Biol 201:81–87
Nishida H (2003) Spatio-temporal pattern of MAP kinase activation in embryos of the ascidian Halocynthia roretzi. Dev Growth Differ 45:27–37
Ogasawara M, Minokawa T, Sasakura Y, Nishida H, Makabe KW (2001) A large-scale whole-mount in situ hybridization system: Rapid one-tube preparation of DIG-labeled RNA probes and high throughput hybridization using 96-well silent screen plates. Zool Sci 18:187–193
Okada T, Hirano H, Takahashi K, Okamura Y (1997) Distinct neuronal lineage of the ascidian embryo revealed by expression of a sodium channel gene. Dev Biol 190:257–272
Okado H, Takahashi K (1988) A simple ‘neural induction’ model with two interacting cleavage-arrested ascidian blastomeres. Proc Natl Acad Sci USA 85:6197–6201
Okado H, Takahashi K (1993) Neural differentiation in cleavage-arrested ascidian blastomeres induced by a proteolytic enzyme. J Physiol 463:269–290
Okamura Y, Ono F, Okagaki R, Chong JA, Mandel G (1994) Neural expression of a sodium channel gene requires cell-specific interaction. Neuron 13:937–948
Pierce KL, Lefkowitz RJ (2001) Classical and new roles of β-arrestins in the regulation of G-protein-coupled receptors. Nat Rev Neurosci 2:727–733
Simeone A (1998) Otx1 and Otx2 in the development and evolution of the mammalian brain. EMBO J 17:6790–6798
Ueki T, Satoh N (1995) Sequence motifs shared by the 5′ flanking regions of two epidermis-specific genes in the ascidian embryo. Dev Growth Differ 37:597–604
Ueki T, Makabe KW, Satoh N (1991) Isolation of cDNA clones for epidermis-specific genes of the ascidian embryo. Dev Growth Differ 33:579–586
Wada S, Saiga H (1999) Vegetal cell fate specification and anterior neuroectoderm formation by Hroth, the ascidian homologue of orthodenticle/otx. Mech Dev 82:67–77
Wada S, Katsuyama Y, Sato Y, Itoh C, Saiga H (1996) Hroth, an orthodenticle-related homeobox gene of the ascidian, Halocynthia roretzi: its expression and putative roles in the axis formation during embryogenesis. Mech Dev 60:59–71
Wada S, Katsuyama Y, Saiga H (1999) Anteroposterior patterning of the epidermis by inductive influences from the vegetal hemisphere cells in the ascidian embryo. Development 126:4955–4963
Wasylyk B, Hagman J, Gutierrez-Hartmann A (1998) Ets transcription factors: nuclear effectors of the Ras-MAP-kinase signaling pathway. Trends Biochem Sci 23:213–216
Yagi K, Makabe KW (2001) Isolation of an early neural maker gene abundantly expressed in the nervous system of the ascidian, Halocynthia roretzi. Dev Genes Evol 211:49–53
Yordy JS, Muise-Helmericks RC (2000) Signal transduction and the Ets family of transcription factors. Oncogene 19:6503–6513
Acknowledgements
The authors thank members of the Asamushi Marine Biological Station and the Otsuchi Marine Research Center for help in collecting live ascidian adults, and members of the Misaki Marine Biological Laboratory for help in maintaining them. The authors are also grateful to Drs. S. Wada and H. Saiga for Hroth cDNA; Drs. K. Ishida, T. Miya and N. Satoh for HrEpiC and HrTBB2 cDNAs; and Ms. K. Yagi and Dr. K. W. Makabe for HrETR-1 cDNA. This work was supported by Grants-in-Aid from the JSPS and MEXT, Japan (13480245 and 13044003).
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by N. Satoh
Rights and permissions
About this article
Cite this article
Akanuma, T., Nishida, H. Ets-mediated brain induction in embryos of the ascidian Halocynthia roretzi . Dev Genes Evol 214, 1–9 (2004). https://doi.org/10.1007/s00427-003-0368-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-003-0368-y