Abstract
Main conclusion
Mechanosensitive channels are integral membrane proteins that rapidly translate extrinsic or intrinsic mechanical tensions into biological responses. They can serve as potential candidates for developing smart-resilient crops with efficient root systems.
Abstract
Mechanosensitive (MS) calcium channels are molecular switches for mechanoperception and signal transduction in all living organisms. Although tremendous progress has been made in understanding mechanoperception and signal transduction in bacteria and animals, this remains largely unknown in plants. However, identification and validation of MS channels such as Mid1-complementing activity channels (MCAs), mechanosensitive-like channels (MSLs), and Piezo channels (PIEZO) has been the most significant discovery in plant mechanobiology, providing novel insights into plant mechanoperception. This review summarizes recent advances in root mechanobiology, focusing on MS channels and their related signaling players, such as calcium ions (Ca2+), reactive oxygen species (ROS), and phytohormones. Despite significant advances in understanding the role of Ca2+ signaling in root biology, little is known about the involvement of MS channel-driven Ca2+ and ROS signaling. Additionally, the hotspots connecting the upstream and downstream signaling of MS channels remain unclear. In light of this, we discuss the present knowledge of MS channels in root biology and their role in root developmental and adaptive traits. We also provide a model highlighting upstream (cell wall sensors) and downstream signaling players, viz., Ca2+, ROS, and hormones, connected with MS channels. Furthermore, we highlighted the importance of emerging signaling molecules, such as nitric oxide (NO), hydrogen sulfide (H2S), and neurotransmitters (NTs), and their association with root mechanoperception. Finally, we conclude with future directions and knowledge gaps that warrant further research to decipher the complexity of root mechanosensing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
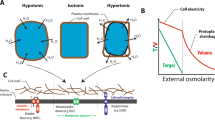
Plant growth and productivity are significantly influenced by root development and the overall health of the root system. Due to their dynamic phenotypic plasticity, roots offer a variety of benefits to plants, including anchoring, mineral, and water intake, as well as the ability to respond biotic and abiotic stresses (El Amrani 2023). The most striking feature of plant root system is their ability to communicate and influence beneficial microbiota which provides an array of beneficial traits to plants in terms of growth promotion and stress adaptability (Bao et al. 2014; Koevoets et al. 2016; Ryan et al. 2016; Anten and Chen 2021). Therefore, maintaining crop yields in the face of climate change and increased population growth necessitates the creation of robust root systems with flexible root system architecture (RSA). In nature, roots are very often exposed to a wide range of mechanical stressors, such as wind, rain, snow, sound, touch, insects, soil particles or stones, drought, salinity and flooding which have a significant impact on their growth and development traits (Moulia et al. 2011; Jin et al. 2013; Yuan et al. 2014; Hartmann et al. 2021; Mousavi et al. 2021; Yoshimura et al. 2021; Guichard et al. 2022). These mechanical stimuli causes short-term biochemical changes such as calcium burst, ROS wave formation, hormone synthesis, and pH modifications that regulates root architecture, developmental, and adaptive responses (Ghosh et al. 2016; Rodrigo-Moreno et al. 2017; Khait et al. 2023). For instance, root thigmomorphogenesis is the most visible morphological effect of mechanostimuli in roots (Chehab et al. 2009; Bidzinski et al. 2014). Similarly, positive gravitropism and touch stimuli can also influence a variety of root development patterns on the agar surface, including waving, skewing, helixing, and circumnutation (Monshausen and Gilroy 2009a; Silverberg et al. 2012; Zha et al. 2016). On the other hand, when root tips face soil obstructions, they avoid them by shifting the direction of their growth. Previous studies have shown that sound treatment significantly enhances root length, root vigor, and fresh weight in Dendranthema morifolium. According to Li et al. (2020), wind exposure increased the dry mass of tomato roots by about 34.63%. Interestingly, it was found the roots of Zea mays grow toward the vibrations caused by moving water (Gagliano et al. 2017). Therefore, these studies further support the notion that roots have evolved a complex sensing mechanism to perceive these mechanical cues and translate them into biochemical responses to drive changes in their growth direction (tropism) and RSA (Monshausen and Gilory 2009b). Another important biochemical modification triggered by mechanical stress is auxin repartition and cell development due to the auxin efflux carrier PINFORMED1 (PIN1), which can alter its abundance at the plasma membrane (PM) and intracellular localizations (Landrein et al. 2015). Previous studies have also shown that mechanical stimuli also affects the structure of microtubules (Liu et al. 2015; Ganguly et al. 2020). In addition, mechanical forces exerted above the ground can also be transmitted intrinsically to the soil, which in turn exerts reaction forces on the roots (Hartmann et al. 2021). Even without external mechanical factors, roots are also mechanically stressed by intrinsic turgor pressures and during cell division (Monshausen and Haswell 2013; Weber et al. 2015). These stress patterns can be predicted based on tissue stage, shape, and anisotropic growth: longitudinal versus transverse stress in different plant species. Nevertheless, many studies have reported that the reorientation of microtubules regulates mechanics-driven morphogenesis in plants in response to mechanical stress (Hejnowicz et al. 2000; Hamant et al. 2008; Robinson and Kuhlemeier 2018). These asymmetric stress patterns act as adaptive responses to control posture and mechanical disturbances in plants (Almeras and Fournier 2009). In these cases, MS ion channels serve as adaptable sensors that respond to intrinsic (osmotic pressure) or extrinsic mechanical stimuli (touch, sound, gravity, and water) that allow ions to flow from one side of the membrane to the other side along its electrochemical gradient (Martinac 2012; Peyronnet et al. 2014; Ranade et al. 2015). These mechanically gated ion channels are pore-forming transmembrane proteins that are ubiquitously distributed in both eukaryotic and prokaryotic cell membranes and open in response to the mechanical stage of the cell wall (Kloda and Martinac 2002; Pivetti et al. 2003; Haswell et al. 2011). In plants, during mechanical stress, such as osmotic pressure, touch, gravity, and pathogenic invasion, MS channels enable the transmembrane transfer of a monoatomic ion by altering the conformation of the protein and switching the multimeric complex from a non-conducting (closed) to a conducting (open) state (Basu and Haswell 2017; Guichard et al. 2022). However, the precise mechanism of gating MS channels remains unknown.
The first MS channel to be cloned was a bacterial MscL (MS channel large conductance) (Sukharev et al. 1994), followed by a mammalian MS channel (Patel et al. 1998). Since then, numerous MS channels have been identified in various taxa, owing to the availability of whole-genome sequencing and contemporary technologies. The patch-clamp electrophysiology technique was initially used to define the first MS channel activity in plant membranes shortly after being identified in animal cells (Haswell 2007). To date, five families of MS channels have been identified based on their subcellular localization and ionic preferences: MscS-like (MSL) proteins (Haswell et al. 2008; Maksaev and Haswell 2012); Mid1-complementing activity (MCA) proteins (Nakagawa et al. 2007; Furuichi et al. 2012); reduced hyperosmolality-induced [Ca2+]i increase (OSCA) channels (Yuan et al. 2014), Piezo channels (Mousavi et al. 2021), and Two-Pore Domain Potassium (TPK) channels (Maathuis 2011), respectively. They differ in their structure, which is mainly based on their transmembrane segment: MCA has a single transmembrane segment, MSL (heptamers), OSCA (pentamers), Piezo (trimers), and TPK (dimers) (Wilson et al. 2013; Saotome et al. 2018; Maity et al. 2019). However, to date, only three complex MS membrane stretch-activated channel families (MSL, MCA, and TPK1) have been fully characterized because of their overlapping localization (plasma membrane, tonoplast, mitochondrial matrix, and plastid stroma), structural diversity, ion selectivity, and biological functions in the plant system (Nakagawa et al. 2007; Haswell et al. 2008; Martinac et al. 2014; Ranade et al. 2015; Basu and Haswell 2017). A detailed list of MS ion channels that differ based on ion selectivity, biochemical properties, localization, and biological roles in plant systems is shown in Table 1.
Homologs of plant MS channels are also found in other organisms such as fungi, bacteria, archaea, protists, and animals, except for MCA, mainly found in plants. The roles of different MS channels are well-documented in animals, yeast, and bacteria, where they perform crucial functions in regulating different traits. In plants they are involved in various developmental processes, including pollen tube growth (MSL8), chloroplast organization (MSC1), cell wall damage (MCA), biotic/abiotic stresses (MSL1/4; MCA1/2), cell division (MSL2/3), reproductive stages (MSL1-6), and lateral root emergence (Haswell and Meyerowitz 2006; Nakayama et al. 2007; Hamilton et al. 2015; Saddhe and Kumar 2015; Zhang et al. 2017; Lee et al. 2019). However, few studies have demonstrated the role of MS ion channels during mechanical stress in different plant tissues (Hartmann et al. 2021). For example, MSL8 has a mechanosensory function in pollen (Hamilton et al. 2015), MSL10 functions as a sensor for cell swelling (Basu and Haswell 20172020), OSCA1 plays a role in osmosensation (Yuan et al. 2014), and OSCA1.3 controls stomatal closure during pathogen attacks (Thor et al. 2020). Denness et al. (2011) validated an MCA mutant in Arabidopsis and reported that MCA was required for root penetration on solid agar and responded to cellulose inhibition. The PIEZO ion channel has recently been identified as an important player in root mechanotransduction in A. thaliana (Mousavi et al. 2021). This study demonstrated that the main inhibition of root growth was observed in mutant PIEZO lines, as well as a reduction in their ability to penetrate hard agar. Additionally, they stated that PIEZO 1 was expressed in the root tip's columella and lateral root cap cells when exposed to significant mechanical stress during root growth or stress (Mousavi et al. 2021). Haswell et al. (2008) reported two MSL channels (MSL9 and MSL 10) in root cells required for mechanostimulation. Similarly, MSL9 and MSL10 show mechanosensitive activity in root protoplasts and cell signaling (Peyronnet et al. 2008; Basu et al. 2020). According to another study, OSCA1 plays a crucial role in calcium transients and is involved in root osmosensing (Yuan et al. 2014). These studies have provided novel insights into the role of MS channels in roots. However, despite the availability of high-throughput techniques, how they sense and become activated in response to different mechanical stimuli remains largely unknown (Bello-Bello et al. 2022).
Two models have been proposed and are widely recognized in animal systems: the force-from-lipid mechanism (Martinac et al. 1990; Teng et al. 2015), in which MS channels act as direct mechanosensors of tension in the lipid bilayer without requiring any external components, and the force-from-filament mechanism (Chalfie 2009; Katta et al. 2015), in which the extracellular matrix and/or cytoskeleton act as mediators of the force that pulls the MS channels open. However, in plants, two computational models were designed based on in vivo experiments to unravel the complexity of plant organ structures in response to mechanical and biochemical cues (Marconi and Wabnik 2021). Lockhart (1965) first described the mechanohydraulic cell growth equation or water uptake and cell wall mechanics, stating that the single-cell expansion rate is a function of the cell volume, cell wall extensibility, turgor pressure, and threshold. A mechanohydraulic model in a multicellular context was recently proposed to reconcile the previous assumptions (Long et al. 2020). The force-derived lipid mechanism of MS channels (Piezo1, OSCA1.2, MCA2, and MSLs) was initially studied in bacterial and mammalian systems (Martinac et al. 1990; Kung 2005; Teng et al. 2015; Cox et al. 2016; Syeda et al. 2016; Murthy et al. 2018; Yoshimura et al. 2021; Li et al. 2020). However, the future challenge is to map forces at the cellular level to understand which conditions and locations MS channels are activated. MS ion channels have been documented in all three domains (eukaryotes, eubacteria, and archaebacteria), suggesting they appeared early during evolution (Martinac and Kloda 2003). However, there is a significant knowledge gap regarding their function in plant growth and stress responses. There are few reports that have highlighted the role of different MS channels like MCA, MSL, OSCAs, Piezo in plants but there upstream and downstream signaling cascades remains largely unknown. Hence, integrating genetic, molecular, and electrophysiological techniques could provide fascinating insights into the role of MS channels, from mechanical perception to organismal behavior. In this review, we discuss the role of mechanopriming in root biology. Next, we focused on the adaptive role of root MS channels. Furthermore, we provide insights into their signal perception cascades, such as cell wall-mediated activation, and mechanotransduction players, such as Ca2+, ROS, hormones, NO, H2S, and NTs, highlighting knowledge gaps. Finally, we provide an update on root thigmomorphogenesis, cell wall compartments, and downstream signaling cascades in response to mechanical stimuli in root biology.
Mechano priming in plants opens new avenues for crop improvement
Mechano priming has become one of the most promising method for improving plant growth and stress adaptability in sustainable agriculture. In plants, mechanopriming induces transcriptional, translational and metabolic changes that improve their growth and stress adaptability. For instance, Ca2+ and ROS burst, activation of hormonal signaling pathways and antioxidant enzyme activity and cytoskeleton reorganization are some of the key events that occurs in plants after mechanopriming (Ghosh et al. 2021). Over the last decade, several studies have shown that regular mechanical priming can significantly improve plant stress resilience and fitness. For example, mechanical treatment of the aerial parts of sunflowers and Sitka spruce leads to modifications in their RSA and biomechanical properties, thus forming roots that are stronger and more resilient to bending (Stokes et al. 1997; Goodman and Ennos 1998). Iida (2014) reported that mechanical treatment of wheat and barley seedlings enhanced growth-related traits, such as root growth, longer spikes, improved tillers, reduced lodging, and higher yield. A previous study showed that touch stimulation in the root triggers directional growth responses essential for avoiding obstacles and navigating a stimulus-heavy soil environment (Weerasinghe et al. 2009). Similarly, wind stimulation affects the adaptive propagation of the root system and the restructuring of root growth patterns in various plant species (Coutand et al. 2008; Nam et al. 2020). Another study of mechanical perturbations in young Sitka spruce clones showed enhanced root growth traits and tolerance to bending (Stokes et al. 1997). Similarly, various studies have revealed biochemical and molecular changes in roots after mechanostimulation. For instance, touch treatment in Arabidopsis promoted extracellular ATP release in the root and shoot and identified the key regulator, namely the heterotrimeric G-protein complex (Weerasinghe et al. 2009). According to Scott and Allen (1999), mechanical stimulation of Arabidopsis roots results in a transient and local increase in intracellular Ca2+. On the other hand, it has been found that physical impedance in maize roots leads to a high accumulation of ethylene (ET) in roots, which is associated with aerenchyma formation (Sarquis et al. 1991; He et al. 1996). Another study showed that mechanopriming increases cold tolerance in tomato plants by modulating different biochemical and morphological traits (Keller and Steffen 1995). Intriguingly, it was observed that touch priming in Zea mays leaves produces volatile signals that triggers chemical defense in non-prime plants (Markovic et al. 2019). According to Chehab et al. (2012), bending of Arabidopsis plants exhibit higher jasmonic acid (JA)-dependent disease resistance against the fungus Botrytis cinerea and the herbivore Trichoplusia ni, respectively. On the other hand, touch stimuli in Arabidopsis plants improves resistance to fungal pathogens (Benikhlef et al. 2013).
Similarly, SV (sound vibration) priming has been reported to improve root growth in paddy rice and Actinidia chinensis (Bochu et al. 2003; Yang 2004). Previous research has demonstrated that during drought stress, SV priming shifts the direction of root development toward water (Gagliano et al. 2012, 2017). Furthermore, we have summarized the role of mechanopriming in stress resilience (Table 2 and Fig. 1). Overall, these studies provide concrete evidence of the impact of mechanopriming on root traits. However, how roots sense mechanical stimuli and translate mechanical signals into biochemical responses remains largely unknown. Therefore, it is necessary to conduct additional research to identify the molecular players that may function as sensors and transducers during root mechanosensing; this will help us better understand the molecular mechanism of mechanopriming and contribute to the development of future mechano-based smart, resilient crops that have significant agronomic value.
The application of mechanopriming in root biology. Thigmo-priming promotes several biochemical, physiological, and morphological characteristics, which improves the developmental and adaptive characteristics of roots in primed plants compared to naive plants. Aerenchyma formation, change in root navigation, root hair formation, vigor root are the major changes in primed plants. AUX auxin; BR brassinosteroid; ET ethylene; RSA root system architecture
Adaptive role of MS channels in root biology
Abiotic stressors such as drought, salinity, heavy metals, and flooding significantly affects root development and its functional attributes ultimately causing plant mortality (Ali et al. 2022; Aslam et al. 2022; Chen et al. 2022; Niu et al. 2022; Tyagi et al. 2023a, b). For instance, salt and drought impair root growth by causing nutrient and water imbalances, which ultimately result in osmotic and hydraulic failure (Van Zelm et al. 2020; Li et al. 2021). Similarly, flooding also affects roots by inhibiting gas diffusion which triggers hypoxia and alters metabolic functioning and energy production (Martínez-Alcántara et al. 2012). In plants, mechanosensitive ion channels are the primary candidates that can perceive diverse environmental cues and mediate growth and adaptive responses. For example, MCA1 and MCA2 can sense osmotic stress by regulating Ca2+ influx and exhibit sensory potentiation and involvement of plastidial potassium exchange antiporters (KEA) such as KEA1/2 and KEA3 in A. thaliana (Stephan et al. 2016). Previous studies have shown that overexpression of MCA1 increases Ca2+ accumulation and tolerance to hyperosmotic stress (Nakagawa et al. 2007). MCA1 is also known to play a significant role in the penetration of roots into tougher surfaces; as a result, it can shield roots from the mechanical pressure of hard soil particles. Similarly, MCA1 and MCA2 have been implicated in chilling and freezing tolerance and a transient increase in [Ca2+]cyt in Arabidopsis in response to cold shock. Similarly, the mca1/ mca2 double mutant was more sensitive to cold stress and showed a lower increase in [Ca2+]cyt when exposed to cold than the wild-type (Mori et al. 2018). In contrast, osca1 mutant lines display impaired Ca2+ levels and root growth under hyperosmotic stress, supporting the notion that OSCA1 is a critical osmosensor in Arabidopsis (Hou et al. 2014). OSCA1.1 is involved in sensing hydrotropism via Ca2+ influx, followed by the MIZU-KUSSEI 1 (MIZ1)-mediated signaling pathway (Akita and Miyazawa 2022). Similarly, MSL calcium channels have been reported to play key roles in plant adaptation to various stressors. For instance, MSL2 and MSL3 respond to osmotic stress (Wilson et al. 2011; Lee et al. 2019). The msl2 msl3 double mutant has short roots and a small number of lateral roots (Lee et al. 2019). Similarly, MSL8 is crucial for plant survival under osmotic stress (Hamilton et al. 2015). Recent research has demonstrated that the Arabidopsis PIEZO1 gene is necessary for root penetration and that pzo1 mutants have poor primary root growth under tougher conditions (Mousavi et al. 2021). Two pore channel 1 (TPC1), another important voltage- and ligand-gated cation channel, plays a significant role in calcium-mediated communication from the root to shoot, critical for plant development and adaptive responses (Ghosh et al. 2022). Furthermore, we have summarized the adaptive role of MS calcium channels in the roots (Table 3) and (Fig. 2). However, further research is needed to fully examine the potential of various MS channels in root adaptability, which will open new possibilities for creating robust smart crops based on root engineering.
Schematic illustration shows the adaptive role of MS channels in root stress biology. Overexpression and mutant studies showed the importance of different MS channels in plant (root) stress tolerance. This model shows how MS channel activation causes calcium burst, which are further decoded by different calcium sensors that modulate different adaptive and memory responses by evolving an array of dynamic signaling cascades, as highlighted in the figure. Many knowledge gaps regarding the participation of additional signaling players have been indicated with a question mark
Cell wall compartment—a new paradigm of MS channels activation in root biology
Plant cell walls offers a variety of functions in plants, including supporting their structural integrity, aiding in growth and development, and improving stress adaptability (Houston et al. 2016). It functions as a primary signaling hub that initiates a variety of signaling cascades linked to development and stress response, as well as also acts as first line of defense against various stressors (Rui and Dinneny 2020). Similarly, cell wall compartment is viewed as a key regulatory platform for mechanosignal transduction in response to different mechanical cues like touch, wind, bending and sound. However, unlike other stressors the role of cell wall in plant mechanosensing is not fully understood. Plant cells must constantly perceive and respond to changes in cell wall mechanics during stress and normal growth and development to survive (Rui and Dinneny 2020). One of the effects of losing the structural support of the cell wall is loss to plasma membrane integrity, which may lead to cell bursting and death (Feng et al. 2018). It is now well-documented that cells transmit mechanical stimulation into biochemical responses and use it as a driving force for activating different biological functions. This further supports the notion that plants like other organisms may have evolved unique mechano sensors to perceive different mechanostimuli. For instance, two group of sensors that are involved in mechanosensing in plants are MS channels and cell wall receptor-like kinases (Shi et al. 2014). So far, different MS ion channels (MCA1, MSL, PIEZO) have been identified in plants to serve as promising plant mechanosensors. However, the upstream elements involved in MS channel activation remain largely unknown. Second, the mechanism by which different mechanostimuli activate the MS channels is not fully understood. However, recent studies have highlighted the role of cell wall components (both chemicals and proteins), such as peptides, oligosaccharides (OGs), and receptor-like kinases (RLKs), and cellular conditions, such as apoplastic pH and ROS, in regulating the activity of MS channels (Bacete et al. 2022; Darwish et al. 2023). It is believed that plant cell wall integrity (CWI) sensors are essential for detecting mechanical signals in plants under stress and driving diverse cellular and adaptive responses through MS channel activation.
In plants, CWI sensors continuously monitor the mechanical integrity of walls and have become one of the most interesting fields in plant mechanobiology. In plants, the most studied CWI sensors that have been well belong to the RLK family. Numerous CWI sensors have been identified in plants, including wall-associated kinases (WAKs), leucine-rich repeat receptor-like kinases (LRR-RLKs), Catharanthus roseus receptor-like kinase1-like (CrRLK1L) subfamily like FERONIA, THESEUS, leucine-rich repeat extensins (LRXs), and glycosylphosphatidylinositol-anchored (GPI)-anchored proteins, which play key roles in sensing abiotic, biotic, and mechanical stressors. The roles of various CWI sensors in response to different stressors or root traits have been studied in root biology. For instance, RALF34/THESEUS1 plays an important role in fine-tuning lateral root initiation (Gonneau et al. 2018). Similarly, RALF1-FERONIA activation distinctly regulated root developmental traits. It negatively regulates primary root growth and promotes root hair growth. Under high-sugar conditions, cellulose synthesis and anisotropic cell growth in the roots depend on two LRR-RLKs, FEI1 and FEI2 (Xu et al. 2008). Another important CWI sensor is arabinogalactan proteins (AGPs), such as salt overly sensitive 5 (SOS5), which play an important role in root development during salinity stress (Shi et al. 2003). Among the LRX-type CWI sensors, LRX1 and LRX2 are mainly associated with root hair growth and play a vital role in vacuolar mechanosensing (Dünser et al. 2019). However, little is known about how CWI sensors affect MS channels and various downstream signaling cascades that affect both root development and adaptation traits. Recent research on THESEUS1 suggests that it may regulate MCA1 channel activity, which is downstream of THESEUS1 and is implicated in lignification and hormone synthesis in response to isoxaben treatment, which prevents cellulose synthesis (Engelsdorf et al. 2018). Similarly, another study showed that MSL6 is phosphorylated by wall-associated kinase 1 (WAK1, another important CWI sensor) when it binds to oligogalacturonides (Kohorn et al. 2016). In root mechanosensing, FERONIA has been reported as a key regulator of calcium signaling during touch response (Shi et al. 2014). These studies highlight the role of CWI sensors or RLKs in the mechanosensing and regulation of the MS channel. Therefore, future research should focus on deciphering how cell wall reprogramming can activate CWI sensors (RLKs) and different MS channels, such as MCA, MSL, OSCA, and PIEZO, in roots under different environmental and mechanical stressors, which could provide novel insights into root mechanobiology. MS channels play an important roles in adaptive root responses to various abiotic and mechanical stressors. Therefore, identifying the upstream players that modulate or regulate MS channels and their downstream signaling cascades (calcium, ROS, and hormones) will open up new possibilities for crop improvement by employing new root traits. Furthermore, we propose a model that shows the importance of cell wall sensors and their signaling events in modulating MS channels (Fig. 3).
A model showing cell wall compartment as a new paradigm of MS channels activation in root biology. It describes how the upstream components such as peptides, oligosaccharides (OGs), receptor-like kinases (RLKs), apoplastic pH, and ROS act as important upstream players in regulating or activating MS channels in roots. This model highlights many missing links that warrant future investigations to decipher how the cell wall triggers MS channel activation and its allies. CNGC cyclic nucleotide-gated channel; FER FERONIA; GPIglycosylphosphatidylinositol-anchored protein; LRX leucine-rich repeat extensin; THE THESEUS and WAK wall-associated kinase
Downstream signaling cascades linked to MS channels in roots
Although the molecular mechanism underlying the perception of a mechanical stimulus remains unknown, the rapid calcium burst during mechanostimulation (touch and wind) has emphasized the importance of Ca2+ MS channels in plant mechanobiology. MS channels are predicted to be the most adaptable plasma membrane-based sensors that enable plants to detect various mechanical stimuli (Ridone et al. 2019). However, the upstream and downstream signaling pathways associated with MS channel activation remain largely unknown. Some important findings have emerged in the last decade on how MS channels transport calcium and other signaling molecules (ROS and anions), which are known to regulate diverse signaling cascades. The structural, metabolic, and signaling features of plants depends on the calcium as a macronutrient and secondary messenger (Marschner 2012). For nutritional traits (structural and metabolic) plants involve passive Ca2+ route via ion channels and relies on the operation of constitutive Ca2+ influx channels. In contrast, plants evolve rapid and transient ion channel-mediated calcium flow via cytosolic Ca2+ [Ca2+]cyt., referred to as a Ca2+ signal. Ca2+ is transported orchestratively across cell membranes by Ca2+-permeable ion channels, Ca2+-ATPases, and Ca2+/H+ exchangers, enabling Ca2+ physiological functions. This signal is crucial for translating internal and external inputs into physiological and gene expression responses by decoding them (Demidchik and Shabala 2018; Aslam et al. 2022). For instance, in different plants, MS channels such as MCA1, MCA2 (Nakagawa et al. 2007; Kurusu et al. 2012; Mori et al. 2018), OSCA (Yuan et al. 2014; Thor et al. 2020), PIEZO (Mousavi et al. 2021; Radin et al. 2021), and MSL10 (Basu and Haswell 2020) are known to stimulate Ca2+ transients, whereas MSL10 and MCA1 promote ROS production as a subsequent outcome of the activation of Ca2+ signaling (Kurusu et al. 2012; Basu and Haswell 2020). In roots, calcium burst regulate an array of signaling cascades driven by ROS, apoplastic and cytoplasmic pH, hormones, and other emerging signaling molecules such as NO. For example, calcium transients lead to the activation of the auxin and ethylene pathways, which are involved in the modulation of the root response to mechanical impedance (Masle 2002; Braam 2005; Okamoto et al. 2008; Yamamoto et al. 2008; Lee et al. 2020). The activation of calcium signaling also plays an important role in auxin-triggered primary root development. Recent studies have shown the involvement of Ca2+ signaling in auxin transport via PIN2 to mediate root bending (Lee et al. 2020). Previous studies have reported that when roots are mechanically restricted, their morphology frequently resembles that of roots exposed to ethylene, with the inhibition of root growth and an increase in the number of root hairs (Masle 2002; Buer et al. 2003). Another study revealed that mechanically induced root bending could promote the growth of lateral roots, accompanied by a local increase in Ca2+ levels and an asymmetric redistribution of auxin to the site of emergence. Ethylene and auxin signaling may serve as a significant downstream targets after Ca2+ signaling activation by MS channels in roots during mechanical stimuli which could regulate diverse traits of RSA. Hence, deciphering the relationship between MS channel-regulating ethylene and auxin-mediated signaling would offer new perspectives on root mechanism perception. Many studies have identified various genes crucial for decoding calcium-mediated downstream signaling in plants. For example, touch-responsive genes (TCH) are downstream components of the touch response that are tightly related to the initial Ca2+ signal (Braam et al. 1997). Previous studies have shown that mechanoperception in roots results in transcriptional reprogramming, which is primarily related to the initial Ca2+ induction, further highlighting the role of calcium in mechanoperception (Kimbrough et al. 2004; Lee et al. 2005; Monshausen and Gilroy 2009b; Zhang et al. 2020). ROS, a universal secondary messenger that regulates various cellular, developmental, and adaptive responses (Mohammadi et al. 2021; Ali et al. 2023) is another significant downstream signaling component of MS calcium channels. It is well-documented that plants under stress triggers the formation of ROS wave which leads to the activation of systemic signaling, however, its role in mechano systemic signaling remains enigmatic. Therefore, it is important to point out how mechanical signals triggers ROS wave in roots and how it activates systemic signaling in above ground organs. Recent study using various molecular and biochemical analyses have demonstrated the importance of ROS-mediated root development in response to mechanical impedance (Jacobsen et al. 2021). This study further showed that integration of ROS, ethylene, and auxin signaling cascades are crucial to mechano triggered root growth modifications. Ca2+ signaling is necessary for ROS generation in response to mechanical stimuli (Monshausen et al. 2009). The complexity of calcium- and ROS-driven signaling in root mechanosensing calls for further research and the determination of the MS channel that drives positive calcium and ROS interactions. It will be interesting to explore whether MS channels have distinctive calcium and ROS signaling patterns in response to various root mechanostimuli and how they regulate different root hormonal signatures, such as auxin and ethylene. Recently, the discovery of the H2O2 receptor (HPCA1) in plants has shown that ROS can activate calcium channels in guard cells, opening new directions for calcium and ROS signal transduction (Wu et al. 2020). It would be interesting to explore the role of H2O2, NO, and other emerging signaling pathways in root mechanosensing and how MS calcium channels modulate them during mechanostimulation or vice versa. Furthermore, we summarized the downstream signaling cascades that are directly or indirectly linked to MS channels in root biology (Fig. 4).
Signal perception and transduction in plant roots through mechanosensitive ion channels (MCA, MSL, OSCA, Piezo, TPK) triggered by different mechanical stimuli. After signal perception numerous downstream signaling cascades like calcium, ROS, and other anionic signaling molecules will be activated which in turn regulates diverse signaling cascades in roots. In roots, calcium burst is known to regulate an array of signaling cascades that are regulated by ROS, apoplastic and cytoplasmic pH, hormones (auxin, ethylene), and other emerging signaling molecules like NO and neurotransmitters which in turn regulates different RSA and adaptive responses. This model also highlights numerous knowledge gaps (such as calcium sensors, hormonal crosstalk, NO and H2S crosstalk, and NTs) that await discovery. SAC stretch-activated ion channel
Emerging signaling molecules in root mechanosignal transduction.
Stress driven signals in roots cause modifications in xylem hydraulics, mobile peptides, ROS, Ca2+ and hormones which causes change in their structure as well as in areal parts. Similarly, root mechanosensing also involves an array of signaling players and molecules such as cell wall sensors, MS channels, calcium, ROS, and hormones which determines the overall fate of mechanosignal transduction. However, in addition to calcium, ROS, and hormones, other signaling molecules like NO, H2S, and neurotransmitters have emerged crucial regulators of root mechanosignal transduction. For instance, NO is widely considered an important signaling molecule that regulates different root developmental and adaptive traits. Previous studies have shown that NO concentration and the nature of the stress either promote or inhibit root growth (Correa-Aragunde et al. 2004; Manoli et al. 2014). Similarly, NO has been reported to play important role in Arabidopsis following mechanostimulation. However, the mechanisms by which MS channel activation regulates NO synthesis and feedback regulation in plants remain unclear. In animals, NO biosynthesis is regulated by Ca2+ and its sensors, such as calmodulin (CaM). Therefore, it would be interesting to explore how mechanostimulation triggers calcium waves and how their sensors can regulate NO homeostasis, which can modulate different root and shoot development and plant stress responses. On the other hand, the role of ROS and NO cross talk in regulating root mechanosignal transduction warrants future investigation. As, ROS is key for the formation and function of NO in plants. Numerous studies have shown how NO and ROS can control a variety of plant responses, including leaf senescence, seed dormancy, nutrition homeostasis, hypersensitive responses (PCD), plant immunity, and stress responses (Kapoor et al. 2023; Kaya et al. 2023). Therefore, it will be interesting to find out how mechanostimuli triggered ROS influence NO production and how it regulates root traits as this would provide new light on root mechanobiology. In root developmental biology, ROS, NO and hormones (like auxin, ethylene) are crucial for the development of primary, lateral, adventitious root (AR), and root hair (Chen et al. 2012; Lombardo and Lamattina 2012). Future research on root mechanobiology will, therefore, also need to consider how mechanical stimuli alter these signaling signatures and their effect on above root traits.
Similar to NO, H2S is another emerging signaling molecule in root mechanobiology as it regulates an array of plant developmental and adaptive responses, either individually or in combination with other signaling molecules. Recent studies have shown that H2S promotes lateral and adventitious root formation but inhibits root hair (Li et al. 2022). As mechanical stress also regulates root developmental traits, it would be interesting to examine the role of H2S in root mechanosensing and signal transduction. Further studies should focus on the crosstalk between NO and H2S during root mechanical stress and how it affects root traits. Plant neurotransmitters such as melatonin, serotonin, acetylcholine, dopamine, and GABA have also become important signaling players in plant biology owing to their multifaceted functions in plant development and stress resilience (Akula and Mukherjee 2020; Arnao and Hernández-Ruiz 2021). NTs have emerged as new players in root biology that control various morphological, developmental, and adaptive root features (Guidotti et al. 2013; Ramesh et al. 2015; Duan et al. 2022). Numerous studies have shown that NTs control a range of root signaling molecules, including calcium, reactive oxygen species (ROS), nitric oxide (NO), and hormones. However, its role in the root mechanobiology remains unclear. Therefore, exploring how MS channel-triggered signaling can activate or regulate NTs will be interesting, providing new directions for the connection between neurotransmitters and plant mechanobiology. In addition, how these emerging signaling molecules interact with root signature hormones, such as auxin (AUX), ethylene, ABA, and brassinosteroid (BR), during mechanostimulation needs further attention. Furthermore, we summarized the overall events of MS-mediated signal perception and transduction in plant roots (Fig. 4).
Filling the gap between MS channel perception and signal transduction
Despite significant structural and mechanical differences, MS channels are conserved in animal and plant cells with similar properties (Peyronnet et al. 2014). Over the past 10 years, significant advancements have been made in our understanding of membrane calcium transport. Most Ca2+-permeable channels have been studied electrophysiologically and cloned into heterologous expression platforms in animal systems. However, despite the availability of high-throughput tools, the functions of MS channels in plants remain largely unknown and have not been structurally confirmed. For example, (1) how are MS channels activated in the roots after perceiving mechanical cues? (2) How do MS channels selectively perceive individual or multi-root mechanostimuli? (3) How do they trigger downstream signaling cascades that result in root morphological, developmental, and adaptive responses? (4) How do MS channels sense abiotic stressors triggered by mechanostimuli, and do they differ from other mechanostimuli? (5) How do MS channels affect RSA in different crop systems? Do they have similar effects, or may they vary at the individual level? (6) How do MS channels affect the root architecture under different stresses? (7) Do root MS channels play a role in long-distance signaling? The scientific community can fill the knowledge gap between MS channel signal perception and transduction by addressing these important questions. In this regard, future studies should focus on some of the important themes that are linked to root MS channels involving various signaling events, such as cell wall sensors, NADPH oxidase, H+-ATPase, hormones, NO, H2S, NTs, and intracellular organelle signaling. These players play crucial role in regulating different plant developmental and adaptive responses against biotic and abiotic stresses (Ashraf and Aslam 2022). Recent studies have reported that mechanostimulation triggers the expression of cell wall-related genes, transcription factors, kinases, and phytohormones which are associated with calcium signaling activation (Darwish et al. 2023). On the other hand, the expression of NTs such as acetylserotonin methyltransferase (ASMT), G-protein-coupled receptor 1 (GCR1), acetylcholine (ACT), and dopamine beta-monooxygenase (DOMON) was found to be induced after exposure to 500 Hz SV frequency at 30 min time interval in Arabidopsis (Tyagi et al. 2023b). Therefore, more in depth studies are required to further underpin the role of hidden signaling players that are regulated by MS channels in plants. Furthermore, how MS channels participate in intracellular organelle root signaling, triggering epigenetic modifications, needs future investigation. In this regard, various molecular, biochemical, and physical tools, such as multi-omics, genetic studies using knockout mutants and genome editing, whole-cell and excised patch-clamp electrophysiology, live-cell imaging, indirect analysis, and mathematical modeling, are required to study the role of these players in MS channel perception and signal transduction (Fig. 5). Nevertheless, these tools have been instrumental in underpinning the intricacy of MS channel-triggered signaling in animals hence translating them into plant root mechanobiology will open new directions in how roots sense and translate mechanical cues into biochemical responses. Recent advances in plant MS channel research have revealed that MS channels regulate different root phenotypic traits. In this regard, a high-throughput phenotyping platform, deep learning, and computer vision will help thoroughly study root growth or RSA related to MS channel activity in different crops.
Application of high-throughput technologies to study the functional role of MS channels and the complexity of root mechanobiology. A Multi-omics can provide information on gene networks, differentially expressed genes, proteins, and metabolites which are regulated by MS channels and their role in root mechanosignal perception and transduction. B In-silico or bioinformatics analysis will help to provide structural and other features of MS channels. C Biochemical live-cell imaging for quantitative data analysis will provide real-time screening of MS channel linked molecules such as calcium, ROS, using FRET: Fluorescence resonance energy transfer; FRAP: Fluorescence recovery after photobleaching. D High throughput root phenotyping to capture root system architecture (RSA). E Artificial intelligence (AI)/machine learning (ML) will provide the phenotypic information on RSA regulated by MS channels. Overall, these tools can provide new insights into root mechanobiology
Root thigmomorphogenesis in response to mechanical stress
Plant morphogenesis is a complex and ubiquitous response comprising many interacting elements, including mechanical stress, biochemical signaling, and genetic conditions (Marconi and Wabnik 2021). In addition to the internal mechanical forces driven by organogenesis, external stresses are unpredictable and dangerous sources of permutation. These various manifestations of growth acclimation to mechanical permutations are called thigmomorphogenesis (Jaffe 1973). Among these, root thigmomorphogenesis is the most visible morphological effect of mechanostimuli in roots. Various studies have reported that mechanism-driven morphogenesis involves microtubule reorientation in response to mechanical stress (Hejnowicz et al. 2000; Hamant et al. 2008; Robinson and Kuhlemeier 2018). For example, during transient bending, wind, mechanical obstacles, and severe water stress, formation of the lateral root, increase in root biomass, change in root architecture (waving), reduced height growth, and increased diameter growth were reported to improve anchorage in Arabidopsis, Plantago major, Ulmus americana, and Prunus avium (Stokes et al. 1997; Telewski and Pruyn 1998; Coutand et al. 2008; Ditengou et al. 2008; Richter et al. 2009; Anten et al. 2010; Paul-Victor and Rowe 2011; Schoelynck et al. 2015; Zhdanov et al. 2021; Zhang et al. 2022). However, complex signaling cascades are known to play important roles in modulating the mechanical properties of cell walls during root thigmomorphogenesis, including Ca2+ signaling, pH modulation, polar auxin transport, microtubular function, and gravitropism (Monshausen et al. 2009; Bidzinski et al. 2014; Nam et al. 2020). Sometimes, responses can also occur far away from stimuli that are mechanically coupled (by either hydraulic or electrical signals), known as long-distance signaling, mainly due to hormone transport (Moulia et al. 2011; Lopez et al. 2014; Louf et al. 2017). For example, long-distance signaling has been observed in Arabidopsis after mechanical stress by rapidly synthesizing jasmonates that enhance plant growth and pest resistance (Chauvin et al. 2013). Similarly, tomato plants show reduced height when manually bent at the basal part of the stem (Coutand et al. 2000). Expression of the JAZ5 gene, encoding the jasmonate ZIM domain protein, was observed as a long-distance signal after exposing poplar plants to local flame wounding on the stem, which alters stem elongation and stimulates apices (Tixier et al. 2014). Therefore, plants respond to mechanical stress in many different ways, depending on the stress’s type, intensity, and duration.
Mechanosensing and mechanotransduction pathways have previously been described as an important driver for the root and shoot growth. The perception of mechanical stress by plants is a rapid process that triggers the quick conversion of mechanical signals into biological signals with the aid of two major candidates/sensors: (i) RLKs and (ii) MS ion channels (Bacete and Hamann 2020; Frachisse et al. 2020). Previously, only one Ca2+-permeable MS channel, MCA1, was reportedly involved in root thigmomorphogenesis (Nakagawa et al. 2007). Other channels like, AtOSCA1.1, attenuates water transpiration and root growth in response to osmotic stress, whereas maize ZmOSCA2.4 is also involved in drought tolerance in transgenic Arabidopsis (Yuan et al. 2014; Cao et al. 2020). Recently, MILDEW LOCUS O (MLO) genes were shown to play important roles in Arabidopsis root thigmomorphogenesis and encode Ca2+ channels (Bidzinski et al. 2014; Gao et al. 2023). Similarly, Procko et al. (2021) reported the involvement of MSL and OSCA in triggering touch-sensitive hair in the Venus flytrap (Dionaea muscipula). In addition, OsHOS1, an E3-ubiquitin ligase, has been reported to be an important mechanosensing regulator of root thigmomorphogenesis (root-curling phenotype) in rice (Lourenço et al. 2015). Several recent studies have reported the potential role of AtPIEZO (mainly present in the root cap) in Arabidopsis mechanosensing root growth in response to substrate impedance (Fang et al. 2021; Mousavi et al. 2021). However, Radin et al. (2021) reported that PpPIEZO 1 and 2 are localized in the tonoplast of the moss (Physcomitrium patens). Nevertheless, these downstream signaling players stretch the plasma membrane; hence, further research is required to understand the initial signaling cascades that perceive and translate mechanical signals into biochemical responses, which will offer new insights into the mechanisms underlying root thigmomorphogenesis. Among the RLK1 family members, FERONIA is known to play a role in developmental responses and hormonal signaling when exposed to mechanical stimuli, such as root nutation, root gravitropism, and roots encountering an impenetrable barrier (glass or hard agar medium) (Shih et al. 2014; Dong et al. 2019; Li et al. 2020). Functional validation using fer mutants has also shown impaired ion signaling, reduced MS gene expression, and altered root growth when exposed to touch or bending (Shih et al. 2014). However, despite the diversity of mechanically induced channels and cell wall receptors, their functions and distinct signaling pathways potentially activated by specific mechanical stresses have not yet been explored.
In addition to external mechanical stimuli, roots face mechanical strain during cell division and other growth traits that must be sensed to survive. For example, lateral roots emerge mainly due to the mechanical stress exerted from the inside. Nevertheless, roots respond to mechanical pressure in a complicated manner involving biological and physical mechanisms. The physical body deforms under mechanical stress. For instance, when an axially oriented force is applied, an elastic rod may buckle or thicken in response to compression while becoming thinner in stretching (Potocka and Szymanowska-Pulka 2018; Smithers et al., 2019). In mechanics, a physical body subjected to mechanical stress can be either elastic (reversible to its original form) or plastic (nonreversible) (Meyers and Chawla 2008). According to Niklas (1992), plants and their tissues exhibit characteristics of all three types of materials but are not entirely plastic, elastic, or viscous. Despite their physical structure, roots operate as living plant organs because they can perceive environmental changes and initiate various adaptive processes. However, the mechanism by which they sense and respond to mechanical stressors is not fully understood. Generally, growing root tips in their natural habitat, soil, come into contact with an array of impediments and soil particles that cause mechanical strain in some situations, may experience displacement and morphological changes, and in others, may inhibit further growth. The root cap is the primary part exposed to environmental stressors and can sense these cues to maintain root growth and adaptive responses.
Interestingly, the root cap plays an important role in root biology by acting as a barrier between the soil and growing root, directing root growth, and minimizing friction between the soil and root (secreting mucilage) (Bengough and McKenzie 1997). Previous studies have also demonstrated that mechanical stress in roots has a significant impact on aboveground plant parts, such as retarded shoot growth (Potocka and Szymanowska-Pulka 2018), altered leaf growth and morphology, decreased leaf number (Grzesiak 2009), area (Kobaissi et al. 2013), and elongation rates (Young et al. 1997), as well as stomatal closure (Roberts et al. 2002). However, the question remains about how the root caps sense mechanical cues and translate them into biochemical responses that modulate different root traits.
Many studies have highlighted how roots undergo developmental changes under abiotic stress, and various key elements that regulate root developmental and adaptive responses have been identified. In other words, the roots frequently adapt to their surroundings and exhibit significant developmental plasticity. For example, under drought stress, roots show a parsimonious root architecture (Lynch 2013, 2018) with fewer lateral/axial roots that grow deeper and move toward greater water availability (Zhan et al. 2015; Dinneny 2019; Gandullo et al. 2021). In contrast, roots show positive hydrotropism, suggesting they bend toward water-rich areas that greatly influence drought tolerance and RSA (Dietrich et al. 2017; Dinneny 2019). During positive root hydrotropism, abscisic acid (ABA) was found to be a key player in regulating an array of signaling events in roots toward hydrotropism (Dinneny 2019). Another drought avoidance strategy is controlling lateral root emergence under drought conditions using hydro patterning and xerobranching. Many important downstream signaling players such as ABA, auxin, MIZU KUSSEI1 (MIZ1), subclass III Snf1-related kinases (SnRK2s), AUXIN RESPONSE FACTOR 7 (ARF7), IAA3 (indole-3-acetic acid), DEEPER ROOTING 1 (DRO1), and the quantitative trait locus for SOIL SURFACE ROOTING 1 have been identified in regulate different root traits during drought stress.
In contrast, halotropism is a noticeable response of the primary root, affecting the root architecture in saline soils. A previous study has shown that negative halotropism (root bending away from salty environments) is the most adaptable strategy for avoiding salinity stress (van Zelm et al. 2020). Auxin and its regulatory signaling cascades are the main players driving halotropism responses in roots during salinity stress (van den Berg et al. 2016). Recent research has highlighted the significance of root hair growth and development under drought and salt stress, in addition to the adjustment of primary and lateral roots. These include EXPB7 (He et al. 2015), WOX11 (Cheng et al. 2016), GLABRA2 (Wang et al. 2020), Triptychon (TRY) (Leng et al. 2021) and salt-overly sensitive (SOS) TFs (Wang et al. 2008) have been shown to positively or negatively regulate root hair growth during drought and salinity stress. Plant roots undergo dramatic morphological changes in response to flooding to survive. Recently, the Hydraulic Conductivity of Root 1 (HCR1), a Raf-like MAPKKK pathway, has been identified as a major player in improving crop flood resilience (Shahzad et al. 2016). These studies provide novel insights into root biology and the signaling cascades that drive growth and adaptation responses to abiotic stressors.
Similarly, mechanical stimuli, such as bending and barrier contact, showed significant changes in ROS, ethylene, and auxin signaling pathways, which were functionally validated using ethylene-insensitive mutants, PIN mutants, pharmacological assays, and image analysis (Lee et al. 2020; Jacobsen et al. 2021). However, recent studies have shown that plant roots also experience mechanical strain during abiotic stressors, including drought, salinity, and flooding, which have a significant impact on its development and functions. Therefore, it is necessary to study the combined effect and tissue-specific expression patterns of mechanical and abiotic stressors on roots using single-cell RNA-seq methods and to develop a model that can shed light on how these two external stressors govern root traits, which will offer new possibilities for crop development in sustainable agriculture.
Conclusion and future perspectives
Plants have evolved a sophisticated mechanism to perceive environmental cues to maximize their growth and survival. Like other biotic and abiotic stressors, mechanical forces generated by turgor pressure, cellular growth and environmental factors such as touch, wind, rain, and sound also influence plant development and their adaptive responses. Despite the existence of high-throughput techniques, the molecular basis of plant mechanoperception and signal transduction remains largely unknown. Interestingly, root mechanobiology has become one of the most important areas for plant scientists to unravel the molecular complexity of plant mechanoperception and signal transduction. So far, two major classes of molecular players like RLKs and MS channels have been identified in root mechanoperception. However, their precise role in response to different root mechano stimuli has not been fully understood. The MS calcium channels are molecular switches used to perceive and transmit mechanical signals. Although MS calcium channels are extensively studied in other organisms, such as bacteria, yeast, and animals, their functions in plant mechanosensing are not entirely understood. Over the last 10 years, plant biologists have shown a real renaissance of interest in unraveling how inner and outer mechanical strains affect the RSA. Numerous studies have shown that mechanopriming and mechanical stress modify root development and adaptive traits. However, the molecular mechanisms underlying the effect of mechanical strain on the RSA remain largely unknown. Additionally, root thigmomorphogenesis is the most obvious morphological result of mechanostimuli (Bello-Bello et al. 2022); however, little is known about the role MS channels, and the other signaling cascades involved in it. Additionally, several questions remain unanswered and await discovery regarding how these channels are involved in root mechanosignal transduction, root development, systemic signaling (from root to shoot), root–microbe interaction, and root–biotic and abiotic–stress interactions. Therefore, future multidisciplinary research including plant physiology, functional genomics, live-cell imaging, bioinformatic analysis, and other high-throughput tools are required to decipher the role of MS channels in root mechanobiology which will pave the way for developing future smart crops. This review provides a perspective on MS channels and their important roles in root mechanosensing. However, to better understand the complex nature of mechanosignal perception and transduction, it is necessary to determine the primary targets (upstream and downstream) of MS channels as well as their molecular functional dynamics. In conclusion, owing to their multifaceted roles, MS channels such as MCA, MSL, OSCA, and PIEZO can be prime candidates for the development of future smart resilient crops with improved root systems.
Data availability
Not applicable.
Abbreviations
- CWI:
-
Cell wall integrity
- LRX:
-
Leucine-rich repeat extensin
- MCAs:
-
Mid1-complementing activity channels
- MS:
-
Mechanosensitive
- MSLs:
-
Mechanosensitive-like channels
- NO:
-
Nitric oxide
- NTs:
-
Neurotransmitters
- OSCA:
-
Reduced hyperosmolality-induced Ca2+ increase
- PIEZO:
-
Piezo channels
- RLK:
-
Receptor-like kinase
- ROS:
-
Reactive oxygen species
- RSA:
-
Root system architecture
- TPK:
-
Two-pore domain potassium
References
Akita K, Miyazawa Y (2022) The mechanosensitive Ca2+ channel, OSCA11, modulates root hydrotropic bending in Arabidopsis thaliana. Environ Exp Bot 197:104825. https://doi.org/10.1016/j.envexpbot.2022.104825
Akula R, Mukherjee S (2020) New insights on neurotransmitters signaling mechanisms in plants. Plant Signal Behav 15:1737450. https://doi.org/10.1080/15592324.2020.1737450
Ali S, Tyagi A, Park S, Mir RA, Mushtaq M, Bhat B, Mahmoudi H, Bae H (2022) Deciphering the plant microbiome to improve drought tolerance: mechanisms and perspectives. Environ Exp Bot 201:104933
Ali S, Tyagi A, Bae H (2023) ROS interplay between plant growth and stress biology: challenges and future perspectives. Plant Physiol Biochem 203:108032
Alméras T, Fournier M (2009) Biomechanical design and long-term stability of trees: morphological and wood traits involved in the balance between weight increase and the gravitropic reaction. J Theor Biol 256(3):370–381
Anten NPR, Chen BJW (2021) Detect thy family: Mechanisms, ecology and agricultural aspects of kin recognition in plants. Plant Cell Environ 44:1059–1071. https://doi.org/10.1111/pce.14011
Anten NP, Alcalá-Herrera R, Schieving F, Onoda Y (2010) Wind and mechanical stimuli differentially affect leaf traits in Plantago major. New Phytol 188(2):554–564
Arnao MB, Hernández-Ruiz J (2021) Melatonin as a regulatory hub of plant hormone levels and action in stress situations. Plant Biol 23:7–19. https://doi.org/10.1111/plb.13202
Ashraf MA, Aslam M (2022) pH sensing in plants. Mol Plant 15(10):1510. https://doi.org/10.1016/j.molp.2022.09.019
Aslam M, Fakher B, Greaves JG, Jakada BH, Qin R, Qin Y (2022) A CBL-interacting protein kinase, AcCIPK18, from Ananas comosus regulates tolerance to salt, drought, heat stress and Sclerotinia sclerotiorum infection in Arabidopsis. Environ Exp Bot 194:104728
Bacete L, Hamann T (2020) The role of mechanoperception in plant cell wall integrity maintenance. Plants 9(5):574. https://doi.org/10.3390/plants9050574
Bacete L, Schulz J, Timo Engelsdorf T et al (2022) THESEUS1 modulates cell wall stiffness and abscisic acid production in Arabidopsis thaliana. Proc Natl Acad Sci USA 119(1):e2119258119
Bao Y, Aggarwal P, Robbins NE et al (2014) Plant roots use a patterning mechanism to position lateral root branches toward available water. Proc Natl Acad Sci USA 111:9319–9324. https://doi.org/10.1073/pnas.1400966111
Basu D, Haswell ES (2017) Plant mechanosensitive ion channels: an ocean of possibilities. Curr Opin Plant Biol 40:43–48. https://doi.org/10.1016/j.pbi.2017.07.002
Basu D, Haswell ES (2020) The mechanosensitive ion channel MSL10 potentiates responses to cell swelling in Arabidopsis seedlings. Curr Biol 30:2716-2728.e6. https://doi.org/10.1016/j.cub.2020.05.015
Basu D, Shoots JM, Harkess A, Veley KM, Haswell ES (2020) Interactions between the N- and C-termini of mechanosensitive ion channel AtMSL10 support a three-step mechanism for activating its signaling function. bioRxiv. https://doi.org/10.1101/726521
Becker D, Geiger D, Dunkel M, Roller A, Bertl A, Latz A et al (2004) AtTPK4, an Arabidopsis tandem-pore K+ channel, poised to control the pollen membrane voltage in a pH-and Ca2+ -dependent manner. Proc Natl Acad Sci USA 101(44):15621–15626
Beier MP, Tsugawa S, Demura T, Fujiwara T (2020) Root shape adaptation to mechanical stress derived from unidirectional vibrations in Populus nigra. Plant Biotechnol 37:423–428. https://doi.org/10.5511/plantbiotechnology.20.0813a
Bello-Bello E, López-Arredondo D, Rico-Chambrón TY, Herrera-Estrella L (2022) Conquering compacted soils: uncovering the molecular components of root soil penetration. Trends Plant Sci 8:814–827
Bengough AG, McKenzie BM (1997) Sloughing of root cap cells decreases the frictional resistance to maize (Zea mays L.) root growth. J Exp Bot 48:885–893. https://doi.org/10.1093/jxb/48.4.885
Benikhlef L, L’Haridon F, Abou-Mansour E, Serrano M, Binda M, Costa A, Lehmann S, Métraux JP (2013) Perception of soft mechanical stress in Arabidopsis leaves activates disease resistance. BMC Plant Biol 13:133
Bidzinski P, Noir S, Shahi S et al (2014) Physiological characterization and genetic modifiers of aberrant root thigmomorphogenesis in mutants of Arabidopsis thaliana MILDEW LOCUS O genes. Plant Cell Environ 37:2738–2753. https://doi.org/10.1111/pce.12353
Bochu W, Xin C, Zhen W et al (2003) Biological effect of sound field stimulation on paddy rice seeds. Colloids Surf B Biointerfaces 32:29–34. https://doi.org/10.1016/S0927-7765(03)00128-0
Braam J (2005) In touch: plant responses to mechanical stimuli. New Phytol 165:373–389
Braam J, Sistrunk ML, Polisensky DH et al (1997) Plant responses to environmental stress: regulation and functions of the Arabidopsis TCH genes. Planta 203:35–41. https://doi.org/10.1007/pl00008113
Buer CS, Wasteneys GO, Masle J (2003) Ethylene modulates root-wave responses in Arabidopsis. Plant Physiol 132:1085–1096. https://doi.org/10.1104/pp.102.019182
Cao L, Zhang P, Lu X, Wang G, Wang Z, Zhang Q, Zhang X, Wei X, Mei F, Wei L, Wang T (2020) Systematic analysis of the maize OSCA genes revealing ZmOSCA family members involved in osmotic stress and ZmOSCA2.4 confers enhanced drought tolerance in transgenic Arabidopsis. Int J Mol Sci 21(1):351. https://doi.org/10.3390/ijms21010351
Capiati DA, País SM, Téllez-Iñón MT (2006) Wounding increases salt tolerance in tomato plants: evidence on the participation of calmodulin-like activities in cross-tolerance signalling. J Exp Bot 57:2391–2400. https://doi.org/10.1093/jxb/erj212
Chalfie M (2009) Neurosensory mechanotransduction. Nat Rev Mol Cell Biol 10:44–52. https://doi.org/10.1038/nrm2595
Chauvin A, Caldelari D, Wolfender JL, Farmer EE (2013) Four 13-lipoxygenases contribute to rapid jasmonate synthesis in wounded Arabidopsis thaliana leaves: a role for lipoxygenase 6 in responses to long-distance wound signals. New Phytol 197(2):566–575
Chehab EW, Eich E, Braam J (2009) Thigmomorphogenesis: a complex plant response to mechano-stimulation. J Exp Bot 60:43–56
Chen YH, Chao YY, Hsu YY, Hong CY, Kao CH (2012) Heme oxygenase is involved in nitric oxide-and auxin-induced lateral root formation in rice. Plant Cell Rep 31:1085–1091
Chen M, She Z, Aslam M, Liu T, Wang Z, Qi J, Niu X (2022) Genomic insights of the WRKY genes in kenaf (Hibiscus cannabinus L.) reveal that HcWRKY44 improves the plant’s tolerance to the salinity stress. Front Plant Sci 13:984233
Cheng S, Zhou DX, Zhao Y (2016) WUSCHEL-related homeobox gene WOX11 increases rice drought resistance by controlling root hair formation and root system development. Plant Signal Behav 11:e1130198. https://doi.org/10.1080/15592324.2015.1130198
Correa-Aragunde N, Graziano M, Lamattina L (2004) Nitric oxide plays a central role in determining lateral root development in tomato. Planta 218:900–905. https://doi.org/10.1007/s00425-003-1172-7
Coutand C, Dupraz C, Jaouen G et al (2008) Mechanical stimuli regulate the allocation of biomass in trees: demonstration with young Prunus avium trees. Ann Bot 101:1421–1432. https://doi.org/10.1093/aob/mcn054
Coutand C, Julien JL, Moulia B, Mauget JC, Guitard D (2000) Biomechanical study of the effect of a controlled bending on tomato stem elongation: global mechanical analysis. J Exp Bot 51(352):1813–1824. https://doi.org/10.1093/jexbot/51.352.1813
Cox CD, Bae C, Ziegler L et al (2016) Removal of the mechanoprotective influence of the cytoskeleton reveals PIEZO1 is gated by bilayer tension. Nat Commun 7(1):10366. https://doi.org/10.1038/ncomms10366
Darwish E, Ghosh R, Bentzer J, Tsardakas Renhuldt N, Proux-Wera E, Kamal N, Spannagl M, Hause B, Sirijovski N, Van Aken O (2023) The dynamics of touch-responsive gene expression in cereals. Plant J 116:282–302
Demidchik V, Shabala S (2018) Mechanisms of cytosolic calcium elevation in plants: the role of ion channels, calcium extrusion systems and NADPH oxidasemediated ‘ROS-Ca2+ Hub.’ Funct Plant Biol 45:9–27
Denness L, Mckenna JF, Segonzac C, Wormit A, Madhou P, Bennett M, Mansfield J, Zipfel C, Hamann T (2011) Cell wall damage-induced lignin biosynthesis is regulated by a reactive oxygen species- and jasmonic acid-dependent process in Arabidopsis. Plant Physiol 156:1364–1374
DIetrichPangKobayashi DLA et al (2017) Root hydrotropism is controlled via a cortex-specific growth mechanism. Nat Plants 3:1–8. https://doi.org/10.1038/nplants.2017.57
Dinneny JR (2019) Developmental responses to water and salinity in root systems. Annu Rev Cell Dev Biol 35:239–257. https://doi.org/10.1146/annurev-cellbio-100617-062949
Ditengou FA, Teale WD, Kochersperger P, Flittner KA, Kneuper I, van der Graaff E, Nziengui H, Pinosa F, Li X, Nitschke R, Laux T (2008) Mechanical induction of lateral root initiation in Arabidopsis thaliana. Proc Natl Acad Sci USA 105(48):18818–18823
Dong Q, Zhang Z, Liu Y, Tao L, Liu H (2019) FERONIA regulates auxin-mediated lateral root development and primary root gravitropism. FEBS Lett 593:97–106
Duan W, Lu B, Liu L et al (2022) Effects of exogenous melatonin on root physiology, transcriptome and metabolome of cotton seedlings under salt stress. Int J Mol Sci 23(16):9456
Dünser K, Gupta S, Herger A et al (2019) Extracellular matrix sensing by FERONIA and Leucine-Rich repeat extensins controls vacuolar expansion during cellular elongation in Arabidopsis thaliana. EMBO J 38:e100353. https://doi.org/10.15252/embj.2018100353
El Amrani B (2023) Exploring the importance of root architecture plasticity in plant adaptation to environmental constraints. Plant Species Biol 38(5):234–244
Engelsdorf T, Gigli-Bisceglia N, Veerabagu M et al (2018) The plant cell wall integrity maintenance and immune signaling systems cooperate to control stress responses in Arabidopsis thaliana. Sci Signal 11:eaao3070. https://doi.org/10.1126/scisignal.aao3070
Fang X, Liu B, Shao Q, Huang X, Li J, Luan S, He K (2021) AtPiezo plays an important role in root cap mechanotransduction. Int J Mol Sci 22(1):467. https://doi.org/10.3390/ijms22010467
Feng W, Kita D, Peaucelle A, Cartwright HN, Doan V, Duan Q, Liu MC, Maman J, Steinhorst L, Schmitz-Thom I et al (2018) The FERONIA receptor kinase maintains cell-wall integrity during salt stress through Ca2+ signaling. Curr Biol 28:666–675
Frachisse JM, Thomine S, Allain JM (2020) Calcium and plasma membrane force-gated ion channels behind development. Curr Opin Plant Biol 53:57–64
Furuichi T, Iida H, Sokabe M, Tatsumi H (2012) Expression of Arabidopsis MCA1 enhanced mechanosensitive channel activity in the Xenopus laevis oocyte plasma membrane. Plant Signal Behav 7:1022–1026. https://doi.org/10.4161/psb.20783
Gagliano M, Mancuso S, Robert D (2012) Towards understanding plant bioacoustics. Trends Plant Sci 17:323–325. https://doi.org/10.1016/j.tplants.2012.03.002
Gagliano M, Grimonprez M, Depczynski M, Renton M (2017) Tuned in: plant roots use sound to locate water. Oecologia 184:151–160. https://doi.org/10.1007/s00442-017-3862-z
Gandullo J, Ahmad S, Darwish E et al (2021) Phenotyping tomato root developmental plasticity in response to salinity in soil rhizotrons. Plant Phenomics 2021:2760532. https://doi.org/10.34133/2021/2760532
Ganguly A, Zhu C, Chen W, Dixit R (2020) FRA1 kinesin modulates the lateral stability of cortical microtubules through cellulose synthase–microtubule uncoupling proteins. Plant Cell 32:2508–2524
Gao F, Han X, Wu J et al (2012) A heat-activated calcium-permeable channel - Arabidopsis cyclic nucleotide-gated ion channel 6 - is involved in heat shock responses. Plant J 70:1056–1069. https://doi.org/10.1111/j.1365-313X.2012.04969.x
Gao Q, Wang C, Xi Y et al (2023) RALF signaling pathway activates MLO calcium channels to maintain pollen tube integrity. Cell Res 33:71–79. https://doi.org/10.1038/s41422-022-00754-3
Ghalkhani E, Hassanpour H, Niknam V (2020) Sinusoidal vibration alleviates salt stress by induction of antioxidative enzymes and anatomical changes in Mentha pulegium (L.). Acta Physiol Plant 42:1–13. https://doi.org/10.1007/s11738-020-3017-4
Ghosh R, Barbacci A, Leblanc-Fournier N (2021) Mechanostimulation: a promising alternative for sustainable agriculture practices. J Exp Bot 72:2877–2888. https://doi.org/10.1093/jxb/erab036
Ghosh S, Bheri M, Bisht D, Pandey GK (2022) Calcium signaling and transport machinery: potential for development of stress tolerance in plants. Curr Plant Biol 29:100235
Ghosh R, Mishra RC, Choi B, et al (2016) Exposure to sound vibrations lead to transcriptomic, proteomic and hormonal changes in arabidopsis. Sci Rep 6:1–15. https://doi.org/10.1038/srep33370
Gobert A, Isayenkov S, Voelker C, Czempinski K, Maathuis FJ (2007) The two-pore channel TPK1 gene encodes the vacuolar K+ conductance and plays a role in K+ homeostasis. Proc Natl Acad Sci USA 104(25):10726–10731
Gonneau M, Desprez T, Martin M et al (2018) Receptor kinase THESEUS1 is a rapid alkalinization factor 34 receptor in Arabidopsis. Curr Biol 28:2452–2458. https://doi.org/10.1016/j.cub.2018.05.075
Goodman AM, Ennos AR (1998) Responses of the root systems of sunflower and maize to unidirectional stem flexure. Ann Bot 82:347–357. https://doi.org/10.1006/anbo.1998.0693
Grzesiak MT (2009) Impact of soil compaction on root architecture, leaf water status, gas exchange and growth of maize and triticale seedlings. Plant Root 3:10–16. https://doi.org/10.3117/plantroot.3.10
Guichard M, Thomine S, Frachisse JM (2022) Mechanotransduction in the spotlight of mechano-sensitive channels. Curr Opin Plant Biol 68:102252
Guidotti BB, Gomes BR, de Cássia S-S, Soares AR, Ferrarese-Filho O (2013) The effects of dopamine on root growth and enzyme activity in soybean seedlings. Plant Signal Behav 8(9):e25477. https://doi.org/10.4161/psb.25477
Hamant O, Heisler MG, Jonsson H, Krupinski P, Uyttewaal M, Bokov P, Corson F, Sahlin P, Boudaoud A, Meyerowitz EM, Couder Y (2008) Developmental patterning by mechanical signals in Arabidopsis. Science 322(5908):1650–1655
Hamilton ES, Jensen GS, Maksaev G et al (2015) Mechanosensitive channel MSL8 regulates osmotic forces during pollen hydration and germination. Science 350:438–441. https://doi.org/10.1126/science.aac6014
Hartmann FP, Tinturier E, Julien J-L, Leblanc-Fournier N (2021) Between stress and response: Function and localization of mechanosensitive Ca2+ channels in herbaceous and perennial plants. Int J Mol Sci 22(20):11043. https://doi.org/10.3390/ijms222011043
Haswell ES (2007) MscS-like proteins in plants. In: Hamill OP (ed) Mechanosensitive ion channels, part A. Elsevier and Academic Press, San Diego, CA, pp 329–359
Haswell ES, Meyerowitz EM (2006) MscS-like proteins control plastid size and shape in Arabidopsis thaliana. Curr Biol 16:1–11. https://doi.org/10.1016/j.cub.2005.11.044
Haswell ES, Peyronnet R, Barbier-Brygoo H et al (2008) Two MscS homologs provide mechanosensitive channel activities in the Arabidopsis root. Curr Biol 18:730–734. https://doi.org/10.1016/j.cub.2008.04.039
Haswell ES, Phillips R, Rees DC (2011) Mechanosensitive channels: what can they do and how do they do it? Structure 19(10):1356–1369
He CJ, Morgan PW, Drew MC (1996) Transduction of an ethylene signal is required for cell death and lysis in the root cortex of maize during aerenchyma formation induced by hypoxia. Plant Physiol 112:463–472. https://doi.org/10.1104/pp.112.2.463
He X, Zeng J, Cao F et al (2015) HvEXPB7, a novel β-expansin gene revealed by the root hair transcriptome of Tibetan wild barley, improves root hair growth under drought stress. J Exp Bot 66:7405–7419. https://doi.org/10.1093/jxb/erv436
Hejnowicz Z, Rusin A, Rusin T (2000) Tensile tissue stress affects the orientation of cortical microtubules in the epidermis of sunflower hypocotyl. J Plant Growth Regul 19:31–44
Hou C, Tian W, Kleist T et al (2014) DUF221 proteins are a family of osmosensitive calcium-permeable cation channels conserved across eukaryotes. Cell Res 24:632–635. https://doi.org/10.1038/cr.2014.14
Houston K, Tucker MR, Chowdhury J, Shirley N, Little A (2016) The plant cell wall: a complex and dynamic structure as revealed by the responses of genes under stress conditions. Front Plant Sci 7:984
Iida H (2014) Mugifumi, a beneficial farm work of adding mechanical stress by treading to wheat and barley seedlings. Front Plant Sci 5:453. https://doi.org/10.3389/fpls.2014.00453
Isayenkov S, Isner JC, Maathuis FJ (2011) Rice two-pore K+ channels are expressed indifferent types of vacuoles. Plant Cell 23(2):756–768
Jacobsen AG, Jervis G, Xu J, Topping JF, Lindsey K (2021) Root growth responses to mechanical impedance are regulated by a network of ROS, ethylene and auxin signalling in Arabidopsis. New Phytol 231:225–242
Jaffe MJ (1973) Thigmomorphogenesis: the response of plant growth and development to mechanical stimulation. Planta 114:143–157. https://doi.org/10.1007/BF00387472
Jaffe MJ, Biro R (1979) Thigmomorphogenesis: the effect of mechanical perturbation on the growth of plants, with special reference to anatomical changes, the role of ethylene, and interaction with other environmental stresses. In: Mussell H, Staples RC (eds) Stress physiology in crop plants. Wiley & Sons, New York, pp 26–59
Jeong MJ, Il CJ, Park SH et al (2014) Sound frequencies induce drought tolerance in rice plant. Pak J Bot 46:2015–2020
Jin K, Shen J, Ashton RW et al (2013) How do roots elongate in a structured soil? J Exp Bot 64:4761–4777. https://doi.org/10.1093/jxb/ert286
Kapoor RT, Ahmad A, Shakoor A, Paray BA, Ahmad P (2023) Nitric oxide and strigolactone alleviate mercury-induced oxidative stress in Lens culinaris L. by modulating glyoxalase and antioxidant defense system. Plants 12(9):1894
Katta S, Krieg M, Goodman MB (2015) Feeling force: Physical and physiological principles enabling sensory mechanotransduction. Annu Rev Cell Dev Biol 31:347–371. https://doi.org/10.1146/annurev-cellbio-100913-013426
Kaya C, Ugurlar F, Ashraf M, Alam P, Ahmad P (2023) Nitric oxide and hydrogen sulfide work together to improve tolerance to salinity stress in wheat plants by upraising the AsA-GSH cycle. Plant Physiol Biochem 194:651–663
Keller E, Steffen KL (1995) Increased chilling tolerance and altered carbon metabolism in tomato leaves following application of mechanical stress. Physiol Plant 93:519–525. https://doi.org/10.1111/j.1399-3054.1995.tb06852.x
Khait I, Lewin-Epstein O, Sharon R, Saban K, Goldstein R, Anikster Y, Zeron Y, Agassy C, Nizan S, Sharabi G, Perelman R (2023) Sounds emitted by plants under stress are airborne and informative. Cell 186(7):1328–1336
Kimbrough JM, Salinas-Mondragon R, Boss WF et al (2004) The fast and transient transcriptional network of gravity and mechanical stimulation in the Arabidopsis root apex. Plant Physiol 136:2790–2805. https://doi.org/10.1104/pp.104.044594
Kloda A, Martinac B (2002) Mechanosensitive channels of bacteria and archaea share a common ancestral origin. Eur Biophys J 31:14–25
Kobaissi AN, Kanso AA, Kanbar HJ, Kazpard V (2013) Morpho-physiological changes caused by soil compaction and irrigation on Zea mays. Eurasian J Soil Sci 2:114–121. https://doi.org/10.18393/EJSS.36878
Koevoets IT, Venema JH, Elzenga JTM, Testerink C (2016) Roots withstanding their environment: exploiting root system architecture responses to abiotic stress to improve crop tolerance. Front Plant Sci 7:1335. https://doi.org/10.3389/fpls.2016.01335
Kohorn BD, Hoon D, Minkoff BB et al (2016) Rapid oligo-galacturonide induced changes in protein phosphorylation in arabidopsis. Mol Cell Proteom 15:1351–1359. https://doi.org/10.1074/mcp.M115.055368
Kung C (2005) A possible unifying principle for mechanosensation. Nature 436(7051):647–654. https://doi.org/10.1038/nature03896
Kurusu T, Yamanaka T, Nakano M et al (2012) Involvement of the putative Ca2+-permeable mechanosensitive channels, NtMCA1 and NtMCA2, in Ca2+ uptake, Ca2+-dependent cell proliferation and mechanical stress-induced gene expression in tobacco (Nicotiana tabacum) BY-2 cells. J Plant Res 125:555–568. https://doi.org/10.1007/s10265-011-0462-6
Landrein B, Kiss A, Sassi M, Chauvet A, Das P, Cortizo M, Laufs P, Takeda S, Aida M, Traas J et al (2015) Mechanical stress contributes to the expression of the STM homeobox gene in Arabidopsis shoot meristems. Elife 4:e07811
Lee D, Polisensky DH, Braam J (2005) Genome-wide identification of touch- and darkness-regulated Arabidopsis genes: a focus on calmodulin-like and XTH genes. New Phytol 165:429–444. https://doi.org/10.1111/j.1469-8137.2004.01238.x
Lee JS, Wilson ME, Richardson RA, Haswell ES (2019) Genetic and physical interactions between the organellar mechanosensitive ion channel homologs MSL1, MSL2, and MSL3 reveal a role for inter-organellar communication in plant development. Plant Direct 3:e00124. https://doi.org/10.1002/pld3.124
Lee HJ, Kim HS, Park JM et al (2020) PIN-mediated polar auxin transport facilitates root−obstacle avoidance. New Phytol 225:1285–1296. https://doi.org/10.1111/nph.16076
Leng B, Wang X, Yuan F et al (2021) Heterologous expression of the Limonium bicolor MYB transcription factor LbTRY in Arabidopsis thaliana increases salt sensitivity by modifying root hair development and osmotic homeostasis. Plant Sci 302:110704. https://doi.org/10.1016/j.plantsci.2020.110704
Li Z, Gong M (2008) Mechanical stimulation-induced heat tolerance of suspension culture cells in tobacco (Nicotiana tobacum L.) and its relation to H2O2. Plant Physiol Commun 44:42
Li ZG, Yan SZ, Xie H (2011) Effect of mechanical stimulation on salt and heavy metal tolerance in tobacco suspension cultured cells. J Yunnan Norm Univ Nat Sci 31:16–20
Li Y, Yuan F, Wen Z, Li Y, Wang F, Zhu T et al (2015) Genome-wide survey and expression analysis of the OSCA gene family in rice. BMC Plant Biol 15(1):261
Li Y, Hu Y, Wang J, Liu X, Zhang W, Sun L (2020) Structural insights into a plant mechanosensitive ion channel MSL1. Cell Rep 30(13):4518–4527. https://doi.org/10.1016/j.celrep.2020.03.026
Li H, Chen H, Chen L, Wang C (2022) The role of hydrogen sulfide in plant roots during development and in response to abiotic stress. Int J Mol Sci 23:1024. https://doi.org/10.3390/ijms23031024
Li H, Testerink C, Zhang Y (2021) How roots and shoots communicate through stressful times. Trends Plant Sci 26:940–952. https://doi.org/10.1016/j.tplants.2021.03.005
Liu Z, Persson S, Zhang Y (2015) The connection of cytoskeletal network with plasma membrane and the cell wall. J Integr Plant Biol 57:330–340
Lockhart JA (1965) An analysis of irreversible plant cell elongation. J Theor Biol 8:264–275. https://doi.org/10.1016/0022-5193(65)90077-9
Lombardo MC, Lamattina L (2012) Nitric oxide is essential for vesicle formation and trafficking in Arabidopsis root hair growth. J Exp Bot 63:4875–4885
Long Y, Cheddadi I, Mosca G, Mirabet V, Dumond M, Kiss A et al (2020) Cellular heterogeneity in pressure and growth emerges from tissue topology and geometry. Curr Biol 30:1504–1516. https://doi.org/10.1016/j.cub.2020.02.027
Lopez R, Badel E, Peraudeau S, Leblanc-Fournier N, Beaujard F, Julien JL, Cochard H, Moulia B (2014) Tree shoot bending generates hydraulic pressure pulses: a new long-distance signal? J Exp Bot 65(8):1997–2008. https://doi.org/10.1093/jxb/eru045
López-Ribera I, Vicient CM (2017) Drought tolerance induced by sound in Arabidopsis plants. Plant Signal Behav 12:e1368938. https://doi.org/10.1080/15592324.2017.1368938
Louf JF, Guéna G, Badel E, Forterre Y (2017) Universal poroelastic mechanism for hydraulic signals in biomimetic and natural branches. Proc Natl Acad Sci USA 114(42):11034–11039
Lourenço TF, Serra TS, Cordeiro AM et al (2015) Rice e3-ubiquitin ligase high expression of osmotically responsive gene1 modulates the expression of ROOT MEANDER CURLING, a gene involved in root mechanosensing, through the interaction with two ethylene-response factor transcription factors. Plant Physiol 169:2275–2287. https://doi.org/10.1104/pp.15.01131
Lynch JP (2013) Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Ann Bot 112:347–357. https://doi.org/10.1093/aob/mcs293
Lynch JP (2018) Rightsizing root phenotypes for drought resistance. J Exp Bot 69:3279–3292. https://doi.org/10.1093/jxb/ery048
Maathuis FJM (2011) Vacuolar two-pore K+ channels act as vacuolar osmosensors. New Phytol 191:84–91. https://doi.org/10.1111/j.1469-8137.2011.03664.x
Maity K, Heumann JM, McGrath AP et al (2019) Cryo-EM structure of OSCA1.2 from Oryza sativa elucidates the mechanical basis of potential membrane hyperosmolality gating. Proc Natl Acad Sci USA 116:14309–14318. https://doi.org/10.1073/pnas.1900774116
Maksaev G, Haswell ES (2012) MscS-Like10 is a stretch-activated ion channel from Arabidopsis thaliana with a preference for anions. Proc Natl Acad Sci USA 109:19015–19020. https://doi.org/10.1073/pnas.1213931109
Manoli A, Begheldo M, Genre A et al (2014) NO homeostasis is a key regulator of early nitrate perception and root elongation in maize. J Exp Bot 65:185–200. https://doi.org/10.1093/jxb/ert358
Marconi M, Wabnik K (2021) Shaping the organ: a biologist guide to quantitative models of plant morphogenesis. Front Plant Sci 12:746183. https://doi.org/10.3389/fpls.2021.746183
Markovic D, Colzi I, Taiti C, Ray S, Scalone R, Gregory Ali J, Mancuso S, Ninkovic V (2019) Airborne signals synchronize the defenses of neighboring plants in response to touch. J Exp Bot 70:691–700
Marschner H (2012) Marschner’s mineral nutrition of higher plants, 3rd edn. Academic Press, London, UK
Martin L, Leblanc-Fournier N, Julien JL et al (2010) Acclimation kinetics of physiological and molecular responses of plants to multiple mechanical loadings. J Exp Bot 61:2403–2412. https://doi.org/10.1093/jxb/erq069
Martinac B (2012) Mechanosensitive ion channels: an evolutionary and scientific tour de force in mechanobiology. Channels (austin) 6:211–213
Martinac B, Kloda A (2003) Evolutionary origins of mechanosensitive ion channels. Prog Biophy Mol Biol 82(1–3):11–24
Martinac B, Adler J, Kung C (1990) Mechanosensitive ion channels of E. coli activated by amphipaths. Nature 348:261–263. https://doi.org/10.1038/348261a0
Martinac B, Nomura T, Chi G, Petrov E, Rohde PR et al (2014) Bacterial mechanosensitive channels: models for studying mechanosensory transduction. Antioxid Redox Signal 20(6):952–969
Martínez-Alcántara B, Jover S, Quiñones A, et al (2012) Flooding affects uptake and distribution of carbon and nitrogen in citrus seedlings. J Plant Physiol 169:1150–1157. https://doi.org/10.1016/j.jplph.2012.03.016
Masle J (2002) High soil strength: Mechanical forces at play on root morphogenesis and in root. In: Waisel Y, Eshel A, Beeckman T, Kafkafi U (eds) Plant roots: the hidden half. CRC Press, New York, pp 807–819
Meyerhoff O, Müller K, Roelfsema MRG et al (2005) AtGLR3.4, a glutamate receptor channel-like gene is sensitive to touch and cold. Planta 222:418–427. https://doi.org/10.1007/s00425-005-1551-3
Meyers MA, Chawla KK (2008) Mechanical behavior of materials, 2nd edn. Cambridge University Press, New York, USA
Miller ND, Brooks TLD, Assadi AH, Spalding EP (2010) Detection of a gravitropism phenotype in glutamate receptor-like 3.3 mutants of Arabidopsis thaliana using machine vision and computation. Genetics 186:585–593. https://doi.org/10.1534/genetics.110.118711
Mohammadi MA, Cheng Y, Aslam M, Jakada BH, Wai MH, Ye K, He X et al (2021) ROS and oxidative response systems in plants under biotic and abiotic stresses: revisiting the crucial role of phosphite triggered plants defense response. Front Microbiol 12:631318
Monshausen GB, Gilroy S (2009a) The exploring root-root growth responses to local environmental conditions. Curr Opin Plant Biol 12:766–772. https://doi.org/10.1016/j.pbi.2009.08.002
Monshausen GB, Gilroy S (2009b) Feeling green: mechanosensing in plants. Trends Cell Biol 19:228–235. https://doi.org/10.1016/j.tcb.2009.02.005
Monshausen GB, Haswell ES (2013) A force of nature: molecular mechanisms of mechanoperception in plants. J Exp Bot 64(15):4663–4680
Monshausen GB, Bibikova TN, Weisenseel MH, Gilroy S (2009) Ca2+ regulates reactive oxygen species production and pH during mechanosensing in Arabidopsis roots. Plant Cell 21:2341–2356. https://doi.org/10.1105/tpc.109.068395
Mori K, Renhu N, Naito M et al (2018) Ca2+-permeable mechanosensitive channels MCA1 and MCA2 mediate cold-induced cytosolic Ca2+ increase and cold tolerance in Arabidopsis. Sci Rep 8:1–10. https://doi.org/10.1038/s41598-017-17483-y
Moulia B, Der Loughian C, Bastien R et al (2011) Integrative mechanobiology of growth and architectural development in changing mechanical environments. In: Wojtaszek P (ed) Mechanical integration of plant cells and plants. Signaling and communication in plants, vol 9. Springer, Berlin, Heidelberg, pp 269–302. https://doi.org/10.1007/978-3-642-19091-9_11
Mousavi SAR, Dubin AE, Zeng WZ et al (2021) PIEZO ion channel is required for root mechanotransduction in Arabidopsis thaliana. Proc Natl Acad Sci USA 118(20):e2102188118. https://doi.org/10.1073/pnas.2102188118
Murthy SE, Dubin AE, Whitwam T et al (2018) OSCA/TMEM63 are an evolutionarily conserved family of mechanically activated ion channels. Elife 7:e41844
Nakagawa Y, Katagiri T, Shinozaki K et al (2007) Arabidopsis plasma membrane protein crucial for Ca2+ influx and touch sensing in roots. Proc Natl Acad Sci USA 104:3639–3644. https://doi.org/10.1073/pnas.0607703104
Nakano M, Samejima R, Iida H (2014) Mechanosensitive channel candidate MCA2 is involved in touch-induced root responses in Arabidopsis. Front Plant Sci 5:421. https://doi.org/10.3389/fpls.2014.00421
Nakano M, Furuichi T, Sokabe M et al (2021) The gravistimulation-induced very slow Ca2+ increase in Arabidopsis seedlings requires MCA1, a Ca2+-permeable mechanosensitive channel. Sci Rep 11:227. https://doi.org/10.1038/s41598-020-80733-z
Nakayama Y, Fujiu K, Sokabe M, Yoshimura K (2007) Molecular and electrophysiological characterization of a mechanosensitive channel expressed in the chloroplasts of Chlamydomonas. Proc Natl Acad Sci USA 104:5883–5888
Nam BE, Park YJ, Gil KE et al (2020) Auxin mediates the touch-induced mechanical stimulation of adventitious root formation under windy conditions in Brachypodium distachyon. BMC Plant Biol 20:1–15. https://doi.org/10.1186/s12870-020-02544-8
Niklas KJ (1992) Plant biomechanics: an engineering approach to plant form and function. University of Chicago Press
Niu X, Chen M, She Z, Aslam M, Qi J, Qin Y (2022) Ectopic expression of kenaf (Hibiscus cannabinus L.) HcWRKY50 improves plants’ tolerance to drought stress and regulates ABA signaling in Arabidopsis. Agronomy 12(5):1176
Okamoto T, Tsurumi S, Shibasaki K et al (2008) Genetic dissection of hormonal responses in the roots of Arabidopsis grown under continuous mechanical impedance. Plant Physiol 146:1651–1662. https://doi.org/10.1104/pp.107.115519
Okamoto T, Takatani S, Motose H et al (2021) The root growth reduction in response to mechanical stress involves ethylene-mediated microtubule reorganization and transmembrane receptor-mediated signal transduction in Arabidopsis. Plant Cell Rep 40:575–582. https://doi.org/10.1007/s00299-020-02653-6
Patel AJ, Honoré E, Maingret F et al (1998) A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J 17:4283–4290. https://doi.org/10.1093/emboj/17.15.4283
Paul-Victor C, Rowe N (2011) Effect of mechanical perturbation on the biomechanics, primary growth and secondary tissue development of inflorescence stems of Arabidopsis thaliana. Ann Bot 107(2):209–218. https://doi.org/10.1093/aob/mcq227
Peyronnet R, Haswell ES, Barbier-Brygoo H, Frachisse JM (2008) AtMSL9 and AtMSL10: Sensors of plasma membrane tension in Arabidopsis roots. Plant Signal Behav 3(9):726–729. https://doi.org/10.4161/psb.3.9.6487
Peyronnet R, Tran D, Girault T, Frachisse JM (2014) Mechanosensitive channels: feeling tension in a world under pressure. Front Plant Sci 5:558
Pivetti CD, Yen M-R, Miller S, Busch W, Tseng Y-H, Booth IR, Saier MH (2003) Two families of mechanosensitive channel proteins. Microbiol Mol Biol Rev 67:66–85
Potocka I, Szymanowska-Pulka J (2018) Morphological responses of plant roots to mechanical stress. Ann Bot 122:711–723. https://doi.org/10.1093/aob/mcy010
Procko C, Murthy S, Keenan WT, Mousavi SAR, Dabi T, Coombs A, Procko E, Baird L, Patapoutian A, Chory J (2021) Stretch-activated ion channels identified in the touch-sensitive structures of carnivorous Droseraceae plants. Elife 10:e64250
Radin I, Richardson RA, Coomey JH et al (2021) Plant PIEZO homologs modulate vacuole morphology during tip growth. Science 373:586–590. https://doi.org/10.1126/science.abe6310
Ramesh S, Tyerman S, Xu B et al (2015) GABA signalling modulates plant growth by directly regulating the activity of plant-specific anion transporters. Nat Commun 6:7879. https://doi.org/10.1038/ncomms8879
Ranade SS, Syeda R, Patapoutian A (2015) Mechanically activated ion channels. Neuron 87:1162–1179
Richter GL, Monshausen GB, Krol A, Gilroy S (2009) Mechanical stimuli modulate lateral root organogenesis. Plant Physiol 151(4):1855–1866. https://doi.org/10.1104/pp.109.142448
Ridone P, Vassalli M, Martinac B (2019) Piezo1 mechanosensitive channels: what are they and why are they important. Biophys Rev 11(5):795–805
Roberts JA, Hussain A, Taylor IB, Black CR (2002) Use of mutants to study long-distance signalling in response to compacted soil. J Exp Bot 53:45–50. https://doi.org/10.1093/jxb/53.366.45
Robinson S, Kuhlemeier C (2018) Global compression reorients cortical microtubules in Arabidopsis hypocotyl epidermis and promotes growth. Curr Biol 28(11):1794–1802
Rodrigo-Moreno A, Bazihizina N, Azzarello E, et al (2017) Root phonotropism: early signalling events following sound perception in Arabidopsis roots. Plant Sci 264:9–15. https://doi.org/10.1016/j.plantsci.2017.08.001
Rui Y, Dinneny JR (2020) A wall with integrity: surveillance and maintenance of the plant cell wall under stress. New Phytol 225:1428–1439
Ryan PR, Delhaize E, Watt M, Richardson AE (2016) Plant roots: understanding structure and function in an ocean of complexity. Ann Bot 118:555–559. https://doi.org/10.1093/aob/mcw192
Saddhe AA, Kumar K (2015) In silico identification and expression analysis of MscS like gene family in rice. Plant Gene 1:8–17
Saotome K, Murthy SE, Kefauver JM et al (2018) Structure of the mechanically activated ion channel Piezo1. Nature 554:481–486. https://doi.org/10.1038/nature25453
Sarquis JI, Jordan WR, Morgan PW (1991) Ethylene evolution from maize (Zea mays L.) seedling roots and shoots in response to mechanical impedance. Plant Physiol 96:1171–1177. https://doi.org/10.1104/pp.96.4.1171
Schoelynck J, Puijalon S, Meire P, Struyf E (2015) Thigmomorphogenetic responses of an aquatic macrophyte to hydrodynamic stress. Front Plant Sci 6:43. https://doi.org/10.3389/fpls.2015.00043
Scott AC, Allen NS (1999) Changes in cytosolic pH within Arabidopsis root columella cells play a key role in the early signaling pathway for root gravitropism. Plant Physiol 121:1291–1298
Shahzad Z, Canut M, Tournaire-Roux C et al (2016) A potassium-dependent oxygen sensing pathway regulates plant root hydraulics. Cell 167:87-98.e14. https://doi.org/10.1016/j.cell.2016.08.068
Shi H, Kim YS, Guo Y et al (2003) The Arabidopsis SOS5 locus encodes a putative cell surface adhesion protein and is required for normal cell expansion. Plant Cell 15:19–32. https://doi.org/10.1105/tpc.007872
Shih HW, Miller ND, Dai C, Spalding EP, Monshausen GB (2014) The receptor-like kinase FERONIA is required for mechanical signal transduction in Arabidopsis seedlings. Curr Biol 24:1887–1892. https://doi.org/10.1016/j.cub.2014.06.064
Shih HW, Depew CL, Miller ND, Monshausen GB (2015) The cyclic nucleotide-gated channel CNGC14 regulates root gravitropism in Arabidopsis thaliana. Curr Biol 25:3119–3125. https://doi.org/10.1016/j.cub.2015.10.025
Silverberg JL, Noar RD, Packer MS, et al (2012) 3D imaging and mechanical modeling of helical buckling in Medicago truncatula plant roots. Proc Natl Acad Sci U S A 109:16794–16799. https://doi.org/10.1073/pnas.1209287109
Sintaha M, Man C-K, Yung W-S et al (2022) Drought stress priming improved the drought tolerance of soybean. Plants 11:2954. https://doi.org/10.3390/plants11212954
Smithers ET, Luo J, Dyson RJ (2019) Mathematical principles and models of plant growth mechanics: from cell wall dynamics to tissue morphogenesis. J Exp Bot 70(14):3587–3600
Stephan AB, Kunz HH, Yang E, Schroeder JI (2016) Rapid hyperosmotic-induced Ca2+ responses in Arabidopsis thaliana exhibit sensory potentiation and involvement of plastidial KEA transporters. Proc Natl Acad Sci USA 113:E5242–E5249. https://doi.org/10.1073/pnas.1519555113
Stokes A, Nicoll BC, Coutts MP, Fitter AH (1997) Responses of young Sitka spruce clones to mechanical perturbation and nutrition: effects on biomass allocation, root development, and resistance to bending. Can J for Res 27:1049–1057. https://doi.org/10.1139/x97-041
Sukharev SI, Martinac B, Blount P, Kung C (1994) Functional reconstitution as an assay for biochemical isolation of channel proteins: application to the molecular identification of a bacterial mechanosensitive channel. Methods 6:51–59. https://doi.org/10.1006/meth.1994.1007
Syeda R, Florendo MN, Cox CD et al (2016) Piezo1 channels are inherently mechanosensitive. Cell Rep 17(7):1739–1746. https://doi.org/10.1016/j.celrep.2016.10.033
Tan YQ, Yang Y, Zhang A et al (2020) Three CNGC family members, CNGC5, CNGC6, and CNGC9, are required for constitutive growth of Arabidopsis root hairs as Ca2+-permeable channels. Plant Commun 1:100001. https://doi.org/10.1016/j.xplc.2019.100001
Tateno M (1991) Increase in lodging safety factor of thigmomorphogenically dwarfed shoots of mulberry tree. Physiol Plant 81:239–243. https://doi.org/10.1111/j.1399-3054.1991.tb02136.x
Telewski FW, Pruyn ML (1998) Thigmomorphogenesis: a dose response to flexing in Ulmus americana seedlings. Tree Physiol 18(1):65–68
Teng J, Loukin S, Anishkin A, Kung C (2015) The force-from-lipid (FFL) principle of mechanosensitivity, at large and in elements. Pflugers Arch Eur J Physiol 467:27–37. https://doi.org/10.1007/s00424-014-1530-2
Thor K, Jiang S, Michard E et al (2020) The calcium-permeable channel OSCA1.3 regulates plant stomatal immunity. Nature 585:569–573. https://doi.org/10.1038/s41586-020-2702-1
Tixier A, Badel E, Franchel J, Lakhal W, Leblanc-Fournier N, Moulia B, Julien JL (2014) Growth and molecular responses to long-distance stimuli in poplars: bending vs flame wounding. Physiol Plant 150(2):225–237
Tyagi A, Ali S, Park S, Bae H (2023a) Exploring the potential of multiomics and other integrative approaches for improving waterlogging tolerance in plants. Plants 12(7):1544
Tyagi A, Ali S, Park S, Bae H (2023b) Assessing the effect of sound vibrations on plant neurotransmitters in Arabidopsis. J Plant Growth Regul 42:5216–5223. https://doi.org/10.1007/s00344-023-10918-z
van den Berg T, Korver RA, Testerink C, ten Tusscher KHWJ (2016) Modeling halotropism: a key role for root tip architecture and reflux loop remodeling in redistributing auxin. Development 143:3350–3362. https://doi.org/10.1242/dev.135111
Van Zelm E, Zhang Y, Testerink C (2020) Salt tolerance mechanisms of plants. Annu Rev Plant Biol 71:403–433. https://doi.org/10.1146/annurev-arplant-050718-100005
Vincill ED, Clarin AE, Molenda JN, Spalding EP (2013) Interacting glutamate receptor-like proteins in phloem regulate lateral root initiation in Arabidopsis. Plant Cell 25:1304–1313. https://doi.org/10.1105/tpc.113.110668
Wang Y, Zhang W, Li K et al (2008) Salt-induced plasticity of root hair development is caused by ion disequilibrium in Arabidopsis thaliana. J Plant Res 121:87–96. https://doi.org/10.1007/s10265-007-0123-y
Wang X, Bi S, Wang L et al (2020) GLABRA2 regulates actin bundling protein VILLIN1 in root hair growth in response to osmotic stress. Plant Physiol 184:176–193. https://doi.org/10.1104/PP.20.00480
Weber A, Braybrook S, Huflejt M, Mosca G, Routier-Kierzkowska AL, Smith RS (2015) Measuring the mechanical properties of plant cells by combining micro-indentation with osmotic treatments. J Exp Bot 66:3229–3241
Weerasinghe RR, Swanson SJ, Okada SF et al (2009) Touch induces ATP release in Arabidopsis roots that is modulated by the heterotrimeric G-protein complex. FEBS Lett 583:2521–2526. https://doi.org/10.1016/j.febslet.2009.07.007
Wilson ME, Jensen GS, Haswell ES (2011) Two mechanosensitive channel homologs influence division ring placement in Arabidopsis chloroplasts. Plant Cell 23:2939–2949. https://doi.org/10.1105/tpc.111.088112
Wilson ME, Maksaev G, Haswell ES (2013) MscS-like mechanosensitive channels in plants and microbes. Biochemistry 52:5708–5722. https://doi.org/10.1021/bi400804z
Wu F, Chi Y, Jiang Z et al (2020) Hydrogen peroxide sensor HPCA1 is an LRR receptor kinase in Arabidopsis. Nature 578:577–581. https://doi.org/10.1038/s41586-020-2032-3
Xu SL, Rahman A, Baskin TI, Kieber JJ (2008) Two leucine-rich repeat receptor kinases mediate signaling, linking cell wall biosynthesis and ACC synthase in Arabidopsis. Plant Cell 20:3065–3079. https://doi.org/10.1105/tpc.108.063354
Yamamoto C, Sakata Y, Taji T et al (2008) Unique ethylene-regulated touch responses of Arabidopsis thaliana roots to physical hardness. J Plant Res 121:509–519. https://doi.org/10.1007/s10265-008-0178-4
Yang X (2004) Effects of different sound intensities on root development of Actinidia chinensis plantlet. Chin J Apppl Environ Biol 10:274–276
Yoshimura K, Iida K, Iida H (2021) MCAs in Arabidopsis are Ca2+-permeable mechanosensitive channels inherently sensitive to membrane tension. Nat Commun 12(1):1–9. https://doi.org/10.1038/s41467-021-26363-z
Young IM, Montagu K, Conroy J, Bengough AG (1997) Mechanical impedance of root growth directly reduces leaf elongation rates of cereals. New Phytol 135:613–619. https://doi.org/10.1046/j.1469-8137.1997.00693.x
Yuan F, Yang H, Xue Y et al (2014) OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature 514:367–371. https://doi.org/10.1038/nature13593
Zeb Q, Wang X, Hou C et al (2020) The interaction of CaM7 and CNGC14 regulates root hair growth in Arabidopsis. J Integr Plant Biol 62:887–896. https://doi.org/10.1111/jipb.12890
Zha G, Wang B, Liu J, et al (2016) Mechanical touch responses of Arabidopsis TCH1-3 mutant roots on inclined hard-agar surface. Int Agrophysics 30:105–111. https://doi.org/10.1515/intag-2015-0065
Zhan A, Schneider H, Lynch JP (2015) Reduced lateral root branching density improves drought tolerance in maize. Plant Physiol 168:1603–1615. https://doi.org/10.1104/pp.15.00187
Zhang Y, Li C, Zhang J et al (2017) Dissection of HY5/HYH expression in Arabidopsis reveals a root-autonomous HY5-mediated photomorphogenic pathway. PLoS ONE 12:e0180449
Zhang Z, Tong X, Liu SY, Chai LX, Zhu FF, Zhang XP et al (2019) Genetic analysis of a Piezo- like protein suppressing systemic movement of plant viruses in Arabidopsis thaliana. Sci Rep 9:3187
Zhang X, Yang Z, Wu D, Yu F (2020) RALF–FERONIA signaling: linking plant immune response with cell growth. Plant Commun 1:100084. https://doi.org/10.1016/j.xplc.2020.100084
Zhang Z, van Ophem D, Chelakkot R et al (2022) A mechano-sensing mechanism for waving in plant roots. Sci Rep 12:9635
Zhdanov O, Blatt MR, Zare-Behtash H, Busse A (2021) Wind-evoked anemotropism affects the morphology and mechanical properties of Arabidopsis. J Exp Bot 72(5):1906–1918. https://doi.org/10.1093/jxb/eraa541
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (NRF-2021R1F1A1060414).
Author information
Authors and Affiliations
Contributions
AT, SA, SP: Writing—original draft, review and editing.
HB: Conceptualization, supervision, and finalizing the draft manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no relevant financial or non-financial interests.
Additional information
Communicated by Dorothea Bartels.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tyagi, A., Ali, S., Park, S. et al. Deciphering the role of mechanosensitive channels in plant root biology: perception, signaling, and adaptive responses. Planta 258, 105 (2023). https://doi.org/10.1007/s00425-023-04261-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-023-04261-6