Abstract
Main conclusion
In this study, we report that peroxynitrite is necessary for ethylene-mediated aerenchyma formation in rice roots under waterlogging conditions.
Abstract
Plants under waterlogging stress face anoxygenic conditions which reduce their metabolism and induce several adaptations. The formation of aerenchyma is of paramount importance for the survival of plants under waterlogging conditions. Though some studies have shown the involvement of ethylene in aerenchyma formation under waterlogging conditions, the implication of peroxynitrite (ONOO−) in such a developmental process remains elusive. Here, we report an increase in aerenchyma formation in rice roots exposed to waterlogging conditions under which the number of aerenchyma cells and their size was further enhanced in response to exogenous ethephon (a donor of ethylene) or SNP (a donor of nitric oxide) treatment. Application of epicatechin (a peroxynitrite scavenger) to waterlogged plants inhibited the aerenchyma formation, signifying that ONOO− might have a role in aerenchyma formation. Interestingly, epicatechin and ethephon co-treated waterlogged plants were unable to form aerenchyma, indicating the necessity of ONOO− in ethylene-mediated aerenchyma formation under waterlogging conditions. Taken together, our results highlight the role of ONOO− in ethylene-mediated aerenchyma formation in rice and could be used in the future to develop waterlogging stress-tolerant varieties of rice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water is important for the survival of living beings and the same holds true for plants that need water for their growth, nutrient transport, seed germination, photosynthesis, and transpiration, among others. However, excess of water in the soil is harmful to plants as it results in oxygen deficiency around the root zone, a condition termed as waterlogging (Bailey-Serres et al. 2012a, b). Changing environmental conditions like heavy rainfall or floods due to global warming saturate the water-holding capacity of the soil, resulting in waterlogging (Bailey-Serres et al. 2012a, b; Mondal 2020). During waterlogging conditions, the air pores of the soil get filled with water which inhibits the exchange of gasses between soil and the surroundings, leading to low oxygen availability for plants. These anoxygenic conditions around the root zone majorly affect the respiratory process of plants leading to low energy production which affects their growth and limits their productivity (Xuewen et al. 2014; Zhou et al. 2020). Waterlogging conditions also induce the closure of stomata and degradation of chlorophyll, which leads to senescence because of the reduced photosynthesis (Kuai et al. 2014; Yan et al. 2018). Additionally, waterlogging conditions also lead to the accumulation of toxic substances like aldehydes, alcohols, and ROS in plants (Xuewen et al. 2014; Zhang et al. 2017). Plants exposed to frequent waterlogging have evolved many morphological and anatomical features to maximize their fitness under such conditions. These adaptive features include formation of adventitious roots, barriers to lateral oxygen loss, gas film formation on the cuticle (Yamauchi et al. 2017; Qi et al. 2019), elongation of the apical meristem (Kuroha et al. 2018), and programmed cell death (PCD) of cortical tissues in roots, leading to the formation of aerenchyma (Yamauchi et al. 2013). Plants perform glycolysis and ethanol fermentation at a faster rate to compensate for the energy loss due to anaerobic conditions under waterlogging stress (Baxter-Burrell et al. 2002; Pan et al. 2021).
Alpuerto et al. (2016) reported the production of ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC) during waterlogging conditions. The ACC so produced is utilized for the synthesis of ethylene with the help of ACC oxidase which demands the presence of oxygen for which ACC needs to be transferred to aerobic parts of the plant where ethylene is produced (Rauf et al. 2013). Ethylene, a gaseous hormone, has a slower diffusion rate in water, so it gets accumulated in root cells and mediates the PCD along with ROS in the root cortical cells, leading to the formation of lysogenous aerenchyma (Yamauchi et al. 2017; Mignolli et al. 2021). Ethylene is also known to be responsible for fruit ripening and growth, and senescence in plants (Ahammed and Li 2022). Moreover, activation of ethylene biosynthetic genes has also been shown in plants grown under waterlogging conditions (Rauf et al. 2013), which is accompanied by an increase in the expression of respiratory burst oxidase homologs (RBOHs), leading to the formation of aerenchyma and adventitious roots (Qi et al. 2019; Pan et al. 2021). The production of ethylene acts as an opponent of abscisic acid-mediated growth of submerged parts to enhance photosynthesis (Bailey-Serres et al. 2012a, b).
Nitric oxide (NO), being a vital molecule in environmental stress signal regulation, was also found to mitigate the waterlogging stress in plants (Song et al. 2011). During low oxygen availability, NO formation in plants occurs in various cell organelles like chloroplast, mitochondria, cytosol, peroxisome, and apoplast by a reductive pathway involving nitrate ion (NO3−), nitrate reductase (NR), and plasma membrane-localized NR. During limited oxygen supply, NO formation promotes seed germination and increases biomass, root formation, and tolerance to stress (Timilsina et al. 2022). Moreover, NO enhances the anaerobic survival of plants by producing ATP and renewing NADP+ anaerobically by acting as an electron acceptor (Horchani et al. 2010; Oliveira et al. 2013; Ding et al. 2020). NO reaction with superoxide free radical promotes the formation of peroxynitrite (ONOO−) without the involvement of any enzyme (Ferrer-Sueta and Radi 2009). Peroxynitrite is a reactive nitrogen species (RNS) and is considered as a biological oxidant involved in pathogenetic response by nitrating the tyrosine residue on proteins.
Although it has been reported that NO induces ethylene production in plants exposed to waterlogging–flooding stress (Yamauchi et al. 2018) and along with ROS mediates cell death in rice seedlings (Yamauchi et al. 2014; Wany et al. 2017), it is still unclear how peroxynitrite which is derived from the reaction of NO and O2−, plays a role in aerenchyma formation. In this study, we performed experiments on rice saplings grown under waterlogging conditions to determine the role of ONOO− in aerenchyma formation in rice roots. Results reported here highlight an interrelation and crosstalk of ONOO− and ethylene in aerenchyma formation under waterlogging conditions in rice roots.
Materials and methods
Plant material and growth conditions
Seeds of Oryza sativa L. var. Vijaya were purchased from a certified supplier of the local market of district Prayagraj, India. Seeds were surface-sterilized in 10% (v/v) sodium hypochlorite solution for 10 min, then washed thoroughly and soaked in distilled water for 24 h. Uniform-sized seeds were wrapped in the wet muslin cloth and incubated in the dark for 2 days to germinate. After the start of germination, seeds were transferred to Petri plates lined with moistened (half-strength Hoagland nutrient solution) Whatman filter paper 42. When the germination reached the maximum, the Petri plates were transferred to a plant growth chamber (Impact, Model IIC 129D, New Delhi, India) under photosynthetically active radiation (PAR) of 300 µmol photons m−2 s−1 and 60% relative humidity with 13:11 h day–night regime for a time period until secondary leaves emerged. Following the growth period, similar-sized saplings were gently harvested for experimental setups which were performed hydroponically. After harvesting, various treatments of ethephon (Et, 10 μM, a donor of ethylene), sodium nitroprusside (SNP, a donor of NO, 15 μM), and epicatechin (Epi, 10 μM, a scavenger of peroxynitrite) were given in different combinations in half-strength Hoagland nutrient solution. The following combinations were made: control containing only half-strength Hoagland solution, WL (waterlogging conditions), WL + Et, WL + SNP, WL + Epi, and WL + Et + Epi. After this, cups having different treatments were plugged with non-absorbent cotton to block gaseous exchange and thus, creating waterlogging conditions except the control setup which was aerated daily with the help of an aquarium air pump SB-548A. The mentioned treatments were applied for 7 days after which the seedlings were analyzed for biochemical and morphological analyses.
Evaluation of length and fresh weight of rice saplings
After getting treated, growth parameters like root length, shoot length, and fresh weight of samples were determined. The shoot and root lengths of seedlings were measured in centimeters with the help of a scale, and the weight of fresh root and shoot of each setup was determined with the help of digital balance (Wensar, Model PGB220, Lucknow, India).
Determination of content and fluorescence of photosynthetic pigments
The Fv/Fm value, a measure of the maximum photochemical efficiency of photosystem II and the NPQ (non-photochemical quenching) parameters for the measurement of chlorophyll fluorescence, was recorded in the rice leaves with the dark adaptation of 30 min, using a handheld fluorometer (Fluoro Pen FP 100, Photon Systems Instruments). Lichtenthaler’s (1987) protocol was followed to evaluate the total chlorophyll and carotenoid of rice leaves.
Anatomical visualization of aerenchyma in rice roots
Rice seedlings were cultivated in aerated control and waterlogging conditions under varied treatments, and root cross sections were cut from the root tip, i.e., 8–10 mm from the root–shoot junction. Cross sections of roots were made manually using a razor blade. Photographs of each segment were taken using a microscope (Model CX43, Olympus) attached to the PC.
Detection of nitric oxide via fluorescent histochemical staining in the rice root section
By following the process reported by Xie et al. (2013) as described by Singh et al. (2021), we visualized endogenous NO in root tips using a fluorescent dye DAF-2DA (4,5-diaminofluorescein diacetate). The root tips were incubated in the dye for 1–2 min and then washed 3–4 times with distilled water before observing under a fluorescence microscope (Model CX43, Olympus) at an emission of 515 nm and excitation of 495 nm.
Fluorescent visualization of peroxynitrite in rice root sections
The peroxynitrite was detected following the method of Saito et al. (2006). The roots were incubated in the 2.5 mM aminophenyl fluorescein (APF) dye for 1 min and then washed with potassium phosphate buffer (pH 7.4, 0.2 mM) before the observation at an emission of 515 nm and excitation of 490 nm using a fluorescence microscope.
Determination of GSNOR activity
The S-nitrosoglutathione reductase (GSNOR) activity was determined following the method of Sakamoto et al. (2002), as described in detail by Frungillo et al. (2013). Samples were crushed in 0.1 M of phosphate buffer (pH 7.2) and then centrifuged at 10,000 g at 4 °C for 10 min. The reaction mixture consisted of 1 mM GSNO, 0.4 mM NADH, and enzyme extract. The oxidation of NADH was followed at 340 nm for 1–2 min at 25 °C by using a spectrophotometer (Systronics UV–VIS spectrophotometer 119, Ahmedabad, India). The extinction coefficient of 6.22 mM.L−1 cm−1 was used for calculating GSNOR activity.
Determination of S-nitrosoglutathione content
Total S-nitrosoglutathione (SNO) content was evaluated as described by Frungillo et al. (2013). The nitrous acid was produced by hydrolysis of S-nitrosothiols in mercuric salt. This nitrous acid upon reacting with N-(1-naphthyl) ethylenediamine and sulphanilamide gets converted into brilliant azo dye. After crushing root samples in phosphate buffer (0.1 M, pH 7.2) having EDTA (0.1 M) and EGTA (ethylene glycol-bis(2-aminoethylether)-N, N, N′, N′-tetra acetic acid, 0.1 M), the supernatant was incubated for 10 min in solution A (0.5 M HCl with 1% sulfanilamide) and solution B (0.2% HgCl2 and 1% sulfanilamide in 0.5 M HCl) separately to produce diazonium salt. Further, these incubated solutions A and B were reacted to an equal volume of 0.02% of NEDA (N-(1-naphthyl) ethylenediamine dihydrochloride) in 0.5 M of HCl for 10 min to form azo dye. The absorbance was recorded at 550 nm with the help of Systronics UV–VIS spectrophotometer 119. The SNO content in the extract was determined by the changes in the absorbance between solutions A and B. The difference values obtained were calculated with help of the GSNO standard curve.
Determination of NOS (nitric oxide synthase)-like activity
The l-arginine-dependent nitric oxide synthase (NOS)-like activity in rice roots was determined following the method of Sun et al. (2018) as described by Singh et al. (2021). In this method, 200 mg of fresh root samples was crushed in potassium phosphate buffer (pH 7.0, 100 mM) and centrifuged for 10 min at 10,000 g at 4 °C. Now, the reaction mixture was prepared with potassium phosphate buffer (pH 7.0, 100 mM), MgCl2 (2 mM), FAD (1 µM), 1 mM l-arginine, 4 µM BH4, CaCl2 (0.3 mM), NADPH (0.2 mM), DTT (0.2 mM), FMN (1 µM), and enzyme extract (0.2 ml). Upon adding the enzyme extract to the reaction mixture, the oxidation of NADPH gets started showing a decrease in absorbance at 340 nm (Systronics UV–VIS spectrophotometer 119). One unit of NOS-like activity is described as nmol NADPH oxidized per min and calculated by using the extinction coefficient (6.22 mM−1 cm−1).
Statistical analyses
For the analysis of data, we used ANOVA (one-way analysis of variance) using the software SPSS 6.0. For the experimental setup, data are presented in three biological replicates (n = 3) acquiring a completely randomized design (CRD). The significant differences in the treatment were at P < 0.05 significant level which was measured by Duncan’s multiple range test.
Results and discussion
Ethylene and NO both protect rice seedlings under WL by stimulating aerenchyma formation
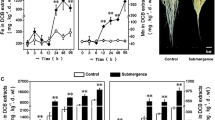
Rice plants were hydroponically grown in the nutrient medium for 21 days which upon treatment with waterlogging conditions display an increase in NO and ethylene levels in contrast to normally grown plants indicating that NO and ethylene are the inducer of waterlogging response. Previously, NO and ethylene were shown to induce cell death in plants in response to pathogen attack, revealing a connection between NO and ethylene (Wany et al. 2017). Further studies reported the role of ethylene in aerenchyma formation triggered by NO under waterlogging (Yamauchi et al. 2014; Wany and Gupta 2018). We also noticed aerenchyma formation under WL (Fig. 1). Further exogenous application of the ethylene donor Et to WL resulted in more prominent aerenchyma formation as shown in cross sections of roots (Fig. 1) compared to WL alone. In addition, SNP (a donor of NO) treatment to WL also increased aerenchyma formation. However, aerenchyma formation was always accompanied by a decline in growth attributes under WL (Fig. 2). NO level in the roots was measured using a NO-specific fluorescence dye DAF-2DA that reflected an intense green fluorescence encircling epidermis, endodermis, and pericycle when observed under a fluorescence microscope (Fig. 3a). This green color of fluorescence shows maximum intensity in the case of exogenous SNP compared to the individual WL, verifying that NO is produced for protecting rice seedlings from WL (Fig. 3a, b).
Formation of aerenchyma in rice roots under waterlogging conditions, and its modulation by ethylene, nitric oxide (NO), and peroxynitrite (ONOO −). Experiments were repeated three times (n = 3). Pictures of stained roots were taken at 20X magnification. Scale bar = 500 μm. C control, WL waterlogging conditions, Et ethephon (a donor of ethylene), SNP sodium nitroprusside (a NO donor), and Epi epicatechin (a scavenger of peroxynitrite)
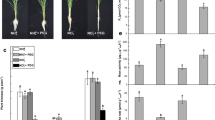
Regulation of growth, shoot/root length (a), and fresh weight (b) by ethylene, nitric oxide (NO), and peroxynitritein (ONOO −) in rice seedlings under waterlogging conditions. C control, WL waterlogging conditions, Et ethephon (a donor of ethylene), SNP sodium nitroprusside (a NO donor), and Epi epicatechin (a scavenger of peroxynitrite). The data are means ± standard error of three biological replicates (n = 3). Bars followed by different letters are statistically significant at P < 0.05 significance level according to the Duncan’s multiple range test
a Regulation of endogenous accumulation of nitric oxide (NO) in rice roots by ethylene and SNP under waterlogging conditions. b NO fluorescence intensity in various treatments. Scale bar = 500 μm. Data are means ± standard error of three biological replicates (n = 3). Bars followed by different letters are statistically different at P < 0.05 significance level according to the Duncan’s multiple range test. C control, WL waterlogging conditions, Et ethephon (a donor of ethylene) and SNP sodium nitroprusside (a NO donor). Pictures of stained roots were taken at 20× magnification; bar =
Further, we observed a reduction in fresh weight and shoot–root length of WL plants when compared with that of control plants (Fig. 2). However, the addition of Et or SNP to WL resulted in normal plant growth, same as in control condition. This shows WL stress alleviatory role of Et and SNP by facilitating programmed cell death (PCD) that leads to aerenchyma formation under WL (Fig. 2). A 12% decrease in Fv/Fm value in WL to that of control plants was also observed indicating insufficient use of light energy leading to low photosynthetic rate. However, the exogenous application of Et or SNP to WL brought back the Fv/Fm value to the normal level as in control plants demonstrating the role of endogenous ethylene and NO in maintaining the metabolic functioning of WL grown plants (Table 1). A reduction in the contents of total chlorophyll and carotenoids was also observed under WL which was reversed following the Et or SNP treatment, signifying that Et and NO have capabilities of inducing WL adaptive responses.
Peroxynitrite is essential in ethylene-mediated aerenchyma formation under WL
Recent research has shown the in vivo production of ONOO− in plants under stress conditions which facilitates protein nitration by post-translational modifications (Alvarez and Radi 2003; Gaupels et al. 2011). Alamillo and Garcia-Olmedo (2001) reported that ONOO− facilitates cell death in Arabidopsis leaf infected by Pseudomonas syringae, but the role of ONOO− in aerenchyma formation under WL is still unclear. Epi, a flavonoid (Ahammed et al. 2023), is being used as a scavenger of ONOO ̄. Epi treatment to WL grown rice plants suppressed the formation of aerenchyma and a tremendous decrease in morphological attributes showing approximately 40% reduction in shoot–root length and 24% reduction in fresh weight when compared to the control (Fig. 2). Moreover, a 27% decline in the total chlorophyll and 30% decline in the carotenoids content were observed in the WL plants along with a 28% reduction in the Fv/Fm value under normal growth condition, indicating reduced photosynthesis in WL plants (Table. 1). Similar results of reduction in growth parameters were obtained when exogenous Et was added to Epi + WL condition, signifying that without ONOO− ethylene was not able to improve growth parameters under WL.
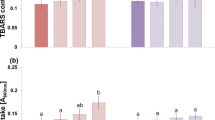
Further, to measure the ONOO− content, we used an ONOO−-specific fluorescence dye, aminophenyl fluorescein (APF), in rice root sections, and the fluorescence was observed under a microscope. An illuminating green color indicates the presence of ONOO− in root sections (Fig. 4a). Under WL + Et, maximum luminescence of green color was observed indicating a high level of ONOO− than the WL while in the case of WL + Epi and WL + Epi + Et, the luminescence intensity was negligible, indicating the absence of ONOO− which signifies that WL brings about endogenous ONOO− formation (Fig. 4a). These results are also supported by the quantitative data of ONOO− (Fig. 4b). The GSNOR is a reductase enzyme, which mediates the reduction of GSNO, which is considered as a pool of NO derived by S-nitrosylation of glutathione (Rodriguez-Ruiz et al. 2017). Under normal growth conditions, the GSNO content as well as the NOS-like activity was found to be low due to high GSNOR activity (Fig. 5a–c). But in the case of WL, a 70% increase in the GSNO content was observed due to low GSNOR activity. The NOS-like activity also showed an enormous increase of 105% to the normal growth condition responsible for a high level of NO in plants (Fig. 5a–c). Under exogenous application of Et or SNP to WL, again an increase in GSNO content with reduced GSNOR activity was observed; however, the NOS-like activity was found to be reduced in the case of SNP + WL, signifying that there must be some regulatory factors which are affecting the NOS-like activity to maintain the balance of NO production as shown in Fig. 5. In the case of WL + Epi and WL + Epi + Et, the GSNO content, NOS-like activity, and GSNOR activity were found to be approximately similar to the WL (Fig. 5a–c), indicating a compromised NO pool and its protective activity.
a Regulation of endogenous accumulation of peroxynitrite (ONOO−) in rice roots by waterlogging conditions and epicatechin. b Peroxynitrite fluorescence intensity under various treatments. Scale bar = 500 μm. Data are means ± standard error of three biological replicates (n = 3). Bars followed by different letters are statistically different at P < 0.05 significance level according to the Duncan’s multiple range test. Pictures of stained roots were taken at 20× magnification. C control, WL waterlogging conditions, Et ethephon (a donor of ethylene) and Epi epicatechin (a scavenger of peroxynitrite)
Regulation of activity of S-nitrosoglutathione reductase (GSNOR, a), content of S-nitrosoglutathione (GSNO, b and activity of L-arginine-dependent nitric oxide synthase-like activity (NOS-like activity, c in rice roots under waterlogging conditions by ethylene, nitric oxide (NO) and peroxynitrite (ONOO −). Data are means ± standard error of three biological replicates (n = 3). Bars followed by different letters are statistically different at P < 0.05 significance level according to the Duncan’s multiple range test. C control, WL waterlogging conditions, Et ethephon (a donor of ethylene), SNP sodium nitroprusside (a NO donor), and Epi epicatechin (a scavenger of peroxynitrite)
Conclusions
This experimental evidence provides a strong support for the role of ONOO− in aerenchyma formation in rice roots under WL mediated by a correlation between NO, ONOO−, and ethylene. In this context, we need to look over the range up to which the ONOO− can influence Et in aerenchyma formation. Further, the presence of ONOO− was reported in Et-mediated aerenchyma formation in rice roots signifying that ONOO− is necessary for Et-mediated aerenchyma formation under WL as in the absence of ONOO−, aerenchyma formation does not take place even though Et was present. More work is needed to determine the regulatory mechanism of ONOO− in inducing the aerenchyma formation under WL stress which may be used to generate stress-resilient rice plants.
Author contribution statement
VPS conceptualized the work. PS, SJ, AK, PG, and VM performed experiments and wrote the first draft of the manuscript. DKT, SPS, RG, and VPS corrected and finalized the manuscript.
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Abbreviations
- Epi:
-
Epicatechin
- Et:
-
Ethephon
- Fv/Fm:
-
Maximum photochemical efficiency of photosystem II
- GSNOR:
-
S-Nitrosoglutathione reductase
- NO:
-
Nitric oxide
- NOS-like activity:
-
Nitric oxide synthase-like activity
- ONOO− :
-
Peroxynitrite
- GSNO:
-
S-Nitrosoglutathione
- SNP:
-
Sodium nitroprusside
- WL:
-
Waterlogging conditions
References
Ahammed GJ, Li X (2022) Elevated carbon dioxide-induced regulation of ethylene in plants. Environ Exp Bot 202:105025
Ahammed GJ, Wu Y, Wang Y, Guo T, Shamsy R, Li X (2023) Epigallocatechin-3-Gallate (EGCG): a unique secondary metabolite with diverse roles in plant-environment interaction. Environ Exp Bot 209:105299
Alamillo JM, García-Olmedo F (2001) Effects of urate, a natural inhibitor of peroxynitrite-mediated toxicity, in the response of Arabidopsis thaliana to the bacterial pathogen Pseudomonas syringae. Plant J 25(5):529–540
Alpuerto JB, Hussain RMF, Fukao T (2016) The key regulator of submergence tolerance, SUB1A, promotes photosynthetic and metabolic recovery from submergence damage in rice leaves. Plant Cell Environ 39(3):672–684
Alvarez B, Radi R (2003) Peroxynitrite reactivity with amino acids and proteins. Amino Acids 25:295–311
Bailey-Serres J, Fukao T, Gibbs DJ, Holdsworth MJ, Lee SC, Licausi F, Perata P, Voesenek LA, van Dongen JT (2012a) Making sense of low oxygen sensing. Trends Plant Sci 17(3):129–138
Bailey-Serres J, Lee SC, Brinton E (2012b) Waterproofing crops: effective flooding survival strategies. Plant Physiol 160(4):1698–1709
Baxter-Burrell A, Yang Z, Springer PS, Bailey-Serres J (2002) RopGAP4-dependent Rop GTPase rheostat control of Arabidopsis oxygen deprivation tolerance. Science 296(5575):2026–2028
Ding J, Liang P, Wu P, Zhu M, Li C, Zhu X, Gao D, Chen Y, Guo W (2020) Effects of waterlogged on grain yield and associated traits of historic wheat cultivars in the middle and lower reaches of the Yangtze River. China Field Crops Res 246:107695
Ferrer-Sueta G, Radi R (2009) Chemical biology of peroxynitrite: kinetics, diffusion, and radicals. ACS Chem Biol 4(3):161–177
Frungillo L, De Oliveira JFP, Saviani EE, Oliveira HC, Martínez MC, Salgado I (2013) Modulation of mitochondrial activity by S-nitrosoglutathione reductase in Arabidopsis thaliana transgenic cell lines. Biochim Biophys Acta-Bioenerg 1827(3):239–247
Gaupels F, Spiazzi-Vandelle E, Yang D, Delledonne M (2011) Detection of peroxynitrite accumulation in Arabidopsis thaliana during the hypersensitive defense response. Nitric Oxide 25(2):222–228
Horchani F, Aschi-Smiti S, Brouquisse R (2010) Involvement of nitrate reduction in the tolerance of tomato (Solanum lycopersicum L.) plants to prolonged root hypoxia. Acta Physiol Plant 32(6):1113–1123
Kuai J, Liu Z, Wang Y, Meng Y, Chen B, Zhao W, Zhou Z, Oosterhuis DM (2014) Waterlogging during flowering and boll forming stages affects sucrose metabolism in the leaves subtending the cotton ball and its relationship with boll weight. Plant Sci 223:79–98
Kuroha T, Nagai K, Gamuyao R, Wang DR, Furuta T, Nakamori M, Kitaoka T, Adachi K, Minami A, Mori Y, Mashiguchi K (2018) Ethylene-gibberellin signaling underlies adaptation of rice to periodic flooding. Science 361(6398):181–186
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Mignolli F, Barone JO, Vidoz ML (2021) Root submergence enhances respiration and sugar accumulation in the stem of flooded tomato plants. Plant Cell Environ 44(11):3643–3654
Mondal D (2020) Floodplain alteration of the Bagmari–Bansloi–Pagla river system. In: Das BC, Ghosh S, Islam A, Roy S (eds) Anthropogeomorphology of bhagirathi-hooghly river system in India. CRC Press, pp 123–133
Oliveira HC, Salgado I, Sodek L (2013) Involvement of nitrite in the nitrate-mediated modulation of fermentative metabolism and nitric oxide production of soybean roots during hypoxia. Planta 237:255–264
Pan J, Sharif R, Xu X, Chen X (2021) Mechanisms of waterlogging tolerance in plants: research progress and prospects. Front Plant Sci 11:627331
Qi X, Li Q, Ma X, Qian C, Wang H, Ren N, Shen C, Huang S, Xu X, Xu Q, Chen X (2019) Waterlogging-induced adventitious root formation in cucumber is regulated by ethylene and auxin through reactive oxygen species signalling. Plant Cell Environ 42(5):1458–1470
Rauf M, Arif M, Fisahn J, Xue GP, Balazadeh S, Mueller-Roeber B (2013) NAC transcription factor speedy hyponastic growth regulates flooding-induced leaf movement in Arabidopsis. Plant Cell 25(12):4941–4955
Rodríguez-Ruiz M, Mioto P, Palma JM, Corpas FJ (2017) S-nitrosoglutathione reductase (GSNOR) activity is down-regulated during pepper (Capsicum annuum L.) fruit ripening. Nitric Oxide 68:51–55
Saito S, Yamamoto-Katou A, Yoshioka H, Doke N, Kawakita K (2006) Peroxynitrite generation and tyrosine nitration in defense responses in tobacco BY-2 cells. Plant Cell Physiol 47(6):689–697
Sakamoto A, Ueda M, Morikawa H (2002) Arabidopsis glutathione-dependent formaldehyde dehydrogenase is an S-nitrosoglutathione reductase. FEBS Lett 515(1–3):20–24
Singh S, Husain T, Kushwaha BK, Suhel M, Fatima A, Mishra V, Singh SK, Bhatt JA, Rai M, Prasad SM, Dubey NK et al (2021) Regulation of ascorbate-glutathione cycle by exogenous nitric oxide and hydrogen peroxide in soybean roots under arsenate stress. J Hazard Mater 409:123686
Song J, Shi G, Gao B, Fan H, Wang B (2011) Waterlogging and salinity effects on two Suaeda salsa populations. Physiol Plant 141(4):343–351
Sun C, Liu L, Lu L, Jin C, Lin X (2018) Nitric oxide acts downstream of hydrogen peroxide in regulating aluminum-induced antioxidant defense that enhances aluminum resistance in wheat seedlings. Environ Exp Bot 145:95–103
Timilsina A, Dong W, Hasanuzzaman M, Liu B, Hu C (2022) Nitrate–nitrite–nitric oxide pathway: a mechanism of hypoxia and anoxia tolerance in plants. Int J Mol Sci 23(19):11522
Wany A, Gupta KJ (2018) Reactive oxygen species, nitric oxide production and antioxidant gene expression during development of aerenchyma formation in wheat. Plant Signal Behav 13(2):3002–3017
Wany A, Kumari A, Gupta KJ (2017) Nitric oxide is essential for the development of aerenchyma in wheat roots under hypoxic stress. Plant Cell Environ 40(12):3002–3017
Xie Y, Mao Y, Lai D, Zhang W, Zheng T, Shen W (2013) Roles of NIA/NR/NOA1-dependent nitric oxide production and HY1 expression in the modulation of Arabidopsis salt tolerance. J Exp Bot 64(10):3045–3060
Xuewen X, Huihui W, Xiaohua Q, Qiang X, Xuehao C (2014) Waterlogging-induced increase in fermentation and related gene expression in the root of cucumber (Cucumis sativus L.). Sci Hortic 179:388–395
Yamauchi T, Shimamura S, Nakazono M, Mochizuki T (2013) Aerenchyma formation in crop species: a review. Field Crops Res 152:8–16
Yamauchi T, Watanabe K, Fukazawa A, Mori H, Abe F, Kawaguchi K, Oyanagi A, Nakazono M (2014) Ethylene and reactive oxygen species are involved in root aerenchyma formation and adaptation of wheat seedlings to oxygen-deficient conditions. J Exp Bot 65(1):261–273
Yamauchi T, Yoshioka M, Fukazawa A, Mori H, Nishizawa NK, Tsutsumi N, Yoshioka H, Nakazono M (2017) An NADPH oxidase RBOH functions in rice roots during lysigenous aerenchyma formation under oxygen-deficient conditions. Plant Cell 29(4):775–790
Yamauchi T, Colmer TD, Pedersen O, Nakazono M (2018) Regulation of root traits for internal aeration and tolerance to soil waterlogging-flooding stress. Plant Physiol 176(2):1118–1130
Yan K, Zhao S, Cui M, Han G, Wen P (2018) Vulnerability of photosynthesis and photosystem I in Jerusalem artichoke (Helianthus tuberosus L.) exposed to waterlogging. Plant Physiol Biochem 125:239–246
Zhang P, Lyu D, Jia L, He J, Qin S (2017) Physiological and de novo transcriptome analysis of the fermentation mechanism of Cerasus sachalinensis roots in response to short-term waterlogging. BMC Genom 18:1–14
Zhou W, Chen F, Meng Y, Chandrasekaran U, Luo X, Yang W, Shu K (2020) Plant waterlogging/flooding stress responses: from seed germination to maturation. Plant Physiol Biochem 148:228–236
Acknowledgements
The authors are thankful to the UGC for financial assistance to carry out this work.
Funding
The authors are thankful to the UGC for financial assistance to carry out this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interest. The authors declare that no human and/or animal material, data, or cell lines were used in this study.
Additional information
Communicated by Dorothea Bartels.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singh, P., Jaiswal, S., Kushwaha, A. et al. Peroxynitrite is essential for aerenchyma formation in rice roots under waterlogging conditions. Planta 258, 2 (2023). https://doi.org/10.1007/s00425-023-04148-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-023-04148-6