Abstract
Main conclusion
Pinus sylvestris responds to insect egg deposition by ROS accumulation linked with reduced activity of the ROS scavenger catalase. Egg mortality in needles with hypersensitive response (HR)-like symptoms is enhanced.
Aggressive reactive oxygen species (ROS) play an important role in plant defence against biotic stressors, including herbivorous insects. Plants may even generate ROS in response to insect eggs, thus effectively fighting against future larval herbivory. However, so far nothing is known on how ROS-mediated plant defence against insect eggs is enzymatically regulated. Neither do we know how insects cope with egg-induced plant ROS. We addressed these gaps of knowledge by studying the activities of ROS-related enzymes in Pinus sylvestris deposited with eggs of the herbivorous sawfly Diprion pini. This species cuts a slit into pine needles and inserts its eggs into the needle tissue. About a quarter of egg-deposited needles show chlorotic tissue at the oviposition sites, indicating hypersensitive response-like direct defence responses resulting in reduced larval hatching from eggs. Hydrogen peroxide and peroxidase sensitive staining of sections of egg-deposited pine needles revealed the presence of hydrogen peroxide and peroxidase activity in needle tissue close to the eggs. Activity of ROS-producing NADPH-oxidase did not increase after egg deposition. However, the activity of the ROS-detoxifying enzyme catalase decreased after egg deposition and ovipositional wounding of needles. These results show that local ROS accumulation at the oviposition site is not caused by increased NADPH-oxidase activity, but reduced activity of pine needle catalase may contribute to it. However, our data suggest that pine sawflies can counteract the egg deposition-induced hydrogen peroxide accumulation in pine needles by high catalase activity in their oviduct secretion which is released with the eggs into pine tissue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reactive oxygen species (ROS) play an important role in plant responses to abiotic and biotic stressors (O’Brien et al. 2012). ROS are generated under both stressful and stress-free conditions. Plant metabolism steadily leads to partial reduction of oxygen which results in formation of, for example, the superoxide radical, hydrogen peroxide or hydroxyl radicals as metabolic byproducts. Under normal growth conditions, the level of cell toxic ROS is kept in homeostasis by an effective antioxidant system. However, enhanced ROS concentrations are detectable under stressful conditions (Mittler 2002; Apel and Hirt 2004). High ROS concentrations may arise due to the imbalances of metabolic pathways, to increased activity of ROS-generating enzymes, such as NADPH oxidase, and/or to downregulation of scavenging/antioxidant systems (Mittler et al. 2004). Under stressful conditions, ROS can serve as signals that mediate stress responses which induce defensive transcriptional changes that result—in concert with direct ROS effects—in obvious cellular changes, including reinforcement of the plant cell wall by oxidative cross-linking, lignification and cell death (Lamb and Dixon 1997; Mittler et al. 2011; Baxter et al. 2014; Barros et al. 2015; Reczek and Chandel 2015). Stress-induced cell death is a well-known plant response to phytopathogens. The programmed cell death isolates and traps invading (hemi)biotrophic pathogens which require living plant tissue (Reape et al. 2008; Bozhkov and Lam 2011). These plant reactions have also been referred to as hypersensitive response (HR) to phytopathogens (Mur et al. 2008; Coll et al. 2011). ROS are known as positive regulators of HR in many, albeit not all plant–phytopathogen interactions (Torres 2010).
HR-like symptoms have also been observed in plant responses to egg depositions by herbivorous insects (Hilker and Fatouros 2015, 2016). A HR-like plant response to eggs laid onto leaves may lead to the formation of necrotic plant tissue at the site of oviposition. Insect eggs on necrotic leaf tissue quickly desiccate or fall off from the necrotic, dry leaf tissue. Thus, a plant can effectively defend against herbivorous insects in a very early stage of infestation. HR-like responses to insect eggs have been shown in several brassicaceous plants deposited with butterfly eggs (Little et al. 2007; Fatouros et al. 2012, 2015) but also in other herbaceous plants (Poaceae, Fabaceae, Apocynaceae, Solanaceae) in response to the eggs laid by planthoppers, beetles or moths (Hilker and Fatouros 2016). Histochemical staining of Arabidopsis thaliana leaves treated with the extracts of butterfly eggs revealed production of ROS at the site of treatment (Little et al. 2007; Gouhier-Darimont et al. 2013; Reymond 2013).
While enzymes involved in HR to phytopathogen infection have intensively been studied (e.g. Almagro et al. 2009; Coll et al. 2011), no knowledge is available on the activities of enzymes involved in HR-like plant responses to insect eggs.
ROS generation in response to phytopathogens requires several enzymes, including the most intensively studied enzyme NADPH oxidase (Morel and Dangl 1997; Meng and Zhang 2013). The superoxide radicals generated by e.g. NADPH oxidase are dismutated to hydrogen peroxide by superoxide dismutase (SOD). When hydrogen peroxide meets iron or copper ions, hydroxyl radicals are formed (Navrot et al. 2007). Plants can control once generated ROS by a set of antioxidant enzymes, including catalase (CAT) which removes hydrogen peroxide by converting it into water and oxygen, and several peroxidases which can reduce hydrogen peroxide as well. Among the peroxidases, ascorbate peroxidase (APX) is considered a key player in plants (Caverzan et al. 2012) using ascorbic acid as an electron donor. Other known peroxidases catalyse this reaction by reducing agents with a thiol group (glutathion) or hydroxyl groups (phenolics) as electron donor molecules (Passardi et al. 2005).
Here, we addressed the question how insect egg deposition affects ROS-generating and scavenging enzyme activities of a plant. We focused on the enzymes mentioned above that are known to be involved in responses to phytopathogens: NADPH oxidase as ROS-generating enzyme, SOD as a ROS converting enzyme, and CAT and APX as ROS-scavenging enzymes. We used Pinus sylvestris (Scots pine) deposited with eggs of the herbivorous insect Diprion pini (pine sawfly) for our studies.
We chose this plant–insect system because we observed HR-like symptoms (brownish tissue) in pine needles with sawfly egg depositions (Fig. 1), indicating that ROS enzyme activities are affected by the egg deposition. In addition, no gymnosperm species has so far been studied with respect to insect egg-induced HR-like responses and the role of enzymes involved in these responses. Furthermore, solid knowledge is available that P. sylvestris needles can respond to D. pini egg deposition. The needles change their pattern of emitted terpenoids in response to sawfly egg deposition, and the egg-induced terpenoid odour attracts egg parasitoids, thus serving pine anti-herbivore defence (Hilker et al. 2002a; Mumm et al. 2003). The egg-induced terpenoid pine odour attracting the parasitoids is not due to the ovipositional wounding which a sawfly inflicts to a pine needle when laying eggs. The sawfly slits a pine needle longitudinally with its ovipositor and inserts the eggs in a row into the slit needle (Fig. 1a). The parasitoid-attracting odour emitted by Scots pine in response to sawfly egg deposition is elicited by the protein-rich oviduct secretion which a sawfly releases with its eggs and which is in tight contact with the internal needle tissue (Hilker et al. 2005). Scots pine does not only change its terpenoid pattern in response to sawfly egg deposition, but also its expression of genes encoding terpene synthases (Köpke et al. 2008, 2010). Furthermore, sawfly egg deposition has been suggested to be taken by pine as a warning signal of impending larval herbivory since egg-deposited pine twigs increase their defence efficiency against hatching larvae (Beyaert et al. 2012).
Needles of Pinus sylvestris with Diprion pini eggs. a Schematic drawing of an egg-deposited needle with yellow eggs laid in a row. Eggs are enclosed in oviduct secretion (blue) and covered on top by secretion of the accessory reproductive glands (ARG) of D. pini females (orange). Sampling denotes the site of sampling of tissue from egg-deposited needles for further measurement of enzyme activities. b–d Photos of pine needles with D. pini eggs covered by brownish ARG secretion. Each single egg in a row is marked by an orange arrow. Whitish needle tissue indicates HR-like pine responses (marked by white arrows)
Since D. pini is highly specialised on Pinus species as host plants, we also asked whether it has developed counter-adaptations to putative egg-induced plant ROS. Hence, we studied the question whether the eggs or secretions released with the eggs exhibit ROS-scavenging CAT activity which would protect the eggs from plant produced ROS.
Materials and methods
Plants and insects
All experiments were conducted with Pinus sylvestris L. Plant material was collected from trees growing in forests in the surroundings of Berlin, Germany. We reared the pine sawfly Diprion pini L. in our laboratory according to the published protocols for sawfly species (Bombosch and Ramakers 1976; Eichhorn 1976); only the sawfly larvae are herbivorous upon P. sylvestris, while the adults do not feed anymore.
For the experiments, we used pine branches of at least 10-year-old trees because younger trees have never been observed to be infested by D. pini (Brauns 1991). The branches were kept in the laboratory under conditions as described in our previous studies on pine responses to sawfly eggs (Mumm and Hilker 2006, and references therein) until used for the experiments.
Determination of frequency of HR-like symptoms
To determine the proportion of egg-deposited needles that show HR-like symptoms, a pine twig was offered to four female and four male D. pini individuals for mating and oviposition. The insects were removed from the twig after 24 h, and the number of needles with eggs was counted. In total, we treated 10 twigs. Needles were inspected for development of HR-like symptoms for 12–14 days (= egg incubation time).
Histochemical staining of pine needles for analysis of hydrogen peroxide accumulation and peroxidase activity in response to sawfly eggs
To obtain visible hints on hydrogen peroxide accumulation and peroxidase activity in P. sylvestris needles in response to egg deposition by D. pini, we used pine needles with 1-, 3- and 12-day-old D. pini eggs for staining experiments. We prepared cross sections of these needles by using a razor blade. The freshly hand-cut sections were directly incubated for 30 min in a staining solution composed of 1% 10 mg/ml 3,5,3′,5′-tetramethylbenzidine (TMB)–HCl (Sigma) solved in dimethyl sulfoxide (Roth) in a 50 mM Tris–acetate buffer (Roth; pH 5.0); blue staining of tissue is based on the hydrogen peroxide dependent peroxidase-mediated oxidation of TMB (Olson and Varner 1993; Ros-Barceló et al. 2002). For direct staining of hydrogen peroxide, sections were incubated for ~1 h in 4% potato starch (Sigma) in 0.1 M potassium iodide (KI, Roth; pH 5.0) solution (Ros-Barceló et al. 2002) which stains tissue with hydrogen peroxide accumulations brownish. For control, egg-free pine needles were sliced and stained by the same method.
Photos of stained sections were taken by using a photo stereomicroscope (Olympus Research Stereo SZH10; Olympus E-330 camera). Image processing (Figs. 1, 2) by zooming, mirroring and adjusting brightness to the same level was done using the software GIMP 2.8.
H2O2 detection in cross sections of Pinus sylvestris needles. a Egg-free unwounded needle without staining. E, epidermis; En, endodermis; P, parenchyma; Tr, transfusion tissue; V, vascular bundle; R, resin canal; b Egg-deposited needle without staining. The sawfly D. pini female slits a pine needle with the sclerotised ovipositor and inserts eggs deeply into the pine needle tissue after disruption of the epidermis, parenchymatic tissue and endodermis. Eggs are laid in a row into a longitudinally slit pine needle (compare Fig. 1). Eggs inside the needle are enclosed by D. pini oviduct secretion which is not distinguishable in the cross section from egg tissue. The opening of the needle at the site of egg insertion is covered by D. pini secretion from the accessory reproductive glands. This secretion is here referred to as “covering” (C) secretion and visible as brownish mass. c and d Stained cross sections of egg-free control needles (Ctrl) and egg-deposited (egg) needles. KI: starch/potassium iodide for direct H2O2 staining. TMB: 3,5,3′,5′-tetramethylbenzidine for staining of H2O2 linked to peroxidase activity. Photos of egg-deposited needles were taken 1, 3 and 12 days after oviposition, and photos of egg-free needles were taken at equivalent time points. White arrows in d point to blue/brown staining of H2O2 close to the insect eggs (1 day) and to lignified tissue around resin ducts (12 days)
Plant treatments
To determine the activities of pine enzymes, a branch was taken from 10 different trees. These 10 branches were provided with tap water and acclimated for 3 days in a climate chamber (20 °C, 18:6 h, L:D, and 100 µmol photons m−2 s−1) before the treatments. All experimental treatments were started at the beginning of the 10th hour of the long day cycle to account for possible circadian rhythm effects on ROS enzyme activity (Lai et al. 2012). For the treatments, we detached five twigs (about 30 cm long) from one large branch. A twig was used for one of the five treatments described below. Using plant material from one branch for the comparison of treatment effects within a single experimental run was a way to control for constitutive variation of traits which is well known in P. sylvestris (e.g. Gershenzon and Croteau 1991). In total, we analysed n = 10 pine twigs for each enzyme, treatment and time point.
We studied the effect of the following plant treatments on activities of ROS-processing enzymes in pine needles:
-
1.
Egg deposition Four female and four male D. pini were added to a pine twig for a period of 24 h. After this treatment period, a twig carried at least four egg masses, at maximum 12.
In addition to this treatment of pine needles by natural insect deposition, we conducted several artificial treatments that mimicked different aspects of the insect oviposition:
-
2.
Wounding Because a pine sawfly does not only release eggs on a needle, but slits a pine needle longitudinally during oviposition and inserts the eggs in a row into the slit needle, we were interested in elucidating the effects of only this ovipositional wounding on pine ROS-processing enzymes. Therefore, we slit pine needles with an insect pin in a similar way as a D. pini female slits a pine needle while ovipositing (compare Hilker et al. 2002a). We wounded eight pine needles per twig.
-
3.
Oviduct secretion + wounding Because a pine sawfly encloses its eggs in oviduct secretion, the pine needle tissue is not in direct contact with the insect egg shell, but with this secretion. Therefore, we studied whether the secretion affects pine ROS-processing enzyme activity and applied 1 μl of a sample of D. pini sawfly oviduct secretion dissolved in Ringer solution (Merck; pH 7.2) on each artificially wounded needle [see treatment (2) “wounding”; eight needles per twig]. The oviduct secretion samples were obtained by dissection of D. pini females as described earlier (Hilker et al. 2005). In total, each twig received oviduct secretion dissected from four females.
-
4.
Ringer + wounding To control for the effect of Ringer solution that has been used for treatment (3)”oviduct secretion” on pine enzyme activity, we wounded eight pine needles per twig as described for treatment (2) and applied 1 μl Ringer into each wound.
-
5.
For control, we also determined enzyme activities of needles from untreated pine twigs.
Sampling pine needle tissue
We harvested the treated needles and respective needles from control twigs in liquid nitrogen for enzyme activity analysis and kept them frozen at −80 °C until analysis. While we used entire needles of treatment (2)–(5) for the enzyme assays, we cut out and sampled egg-free tissue of locally egg-laden needles when studying enzyme activity of needles of treatment (1)”egg deposition” (Fig. 1a). We did so for the treatment “egg deposition” to focus on pine tissue enzyme activities, and to exclude enzyme activities that might be also attributable to the eggs.
For determination of pine needle enzyme activities in dependence of the time after treatments, needles of differently treated twigs were collected at following time points: 6 h, 1, 3 and 12 days after treatment (n = 10 twigs for each enzyme, treatment and each time point) except for the “egg deposition” treatment. Pine needles from egg-deposited twigs were harvested 1, 3 and 12 days after start of the treatment. We did not sample pine tissue of this treatment after 6 h because pine sawflies needed about a day to produce several egg depositions per twig. The incubation time of D. pini eggs lasts about two weeks. Hence, larvae are close to hatch from eggs at the sampling time point 12 days.
Sampling insect tissue
To determine ROS-scavenging CAT activity in different tissues and secretions of adult D. pini females, 1 to 5-day-old females were dissected. All samples were transferred into Ringer solution and frozen at –80 °C until measured.
Eggs were dissected from the oviduct to determine the enzymatic activity of eggs without any secretions. One egg sample contained 75 eggs dissected from five females (15 eggs taken from each female). A sample of oviduct secretion which is enveloping the eggs was obtained by dissecting the oviducts of five D. pini females. In addition to “wrapping” her eggs with oviduct secretion, a D. pini female applies a further secretion on top of the longitudinally slit needle with the eggs inside. We refer to this secretion as “covering secretion” (Fig. 1a) which is produced in the accessory reproductive glands of the females. We dissected this abdominal accessory reproductive gland of an unmated D. pini female (= one sample). Haemolymph control samples were collected because all dissected samples were inevitably contaminated with haemolymph. The third pair of legs of a D. pini female was cut off at the coxa. Haemolymph that emerged from the experimentally inflicted injuries was sucked into a glass capillary which was attached to a pipette with a small rubber bulb at the end (haemolymph from five females = one sample). As tissue controls, head and thorax were dissected from five D. pini females (= one sample).
Enzyme assays: general
All measurements of pine enzyme activities were performed in statistical blocks, i.e. all treatments of one plant of a specific collection time point were measured on the same 96 well plate. Enzyme activities were recorded spectrophotometrically by using the Multiscan® GO Microplate Spectrophotometer (Thermo Scientific). Except for measurements of APX activity, all sample extracts that have been prepared as described below were kept frozen at −80 °C until measurement; freezing does not affect the activity of enzymes of the antioxidant system (Murias et al. 2005). Sample extracts that were used for the determination of APX activity were measured immediately after extraction (without freezing them) because of the lability of the enzyme (Asada 1992).
NADPH oxidase activity in pine needle tissue
Plant extracts were prepared according to the method of Rojas et al. (2012). Needles were powdered under liquid nitrogen in a precooled mortar. The needle powder (100 mg) was used for further analysis and extracted in 2 ml extraction buffer consisting of 0.25 M sucrose (Roth), 50 mM Hepes–KOH (Roth; pH 7.2), 3 mM Na2 EDTA (Roth), 1 mM dithiothreitol (Roth), 0.6% polyvinylpyrrolidone (Sigma), 3.6 mM l-cysteine (Roth), 0.1 mM MgCl2 (Roth), and SigmaFast™ complete EDTA–free proteinase inhibitor cocktail tablets (Sigma) and filtered through cheesecloth. The filtrate was centrifuged at 4 °C at 10,000×g for 45 min. Supernatants were ultracentrifuged at 203,000×g for 60 min. The resulting pellets were resuspended in 200 µl 10 mM Tris–HCl (Roth; pH 7.4).
NADPH oxidase activity (EC 1.6.3.1) of pine needles was measured by monitoring absorbance in the course of reduction of 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilid–sodium (XTT) (Life Technologies) over a period of 60 min. Absorbance was measured every 20 min at 492 nm starting at time point zero. The reaction mixture consisted of 240 µl of 50 mM Tris–HCl (pH 7.4), 0.5 mM XTT and 100 mM NADPH (Roth) and 10 µl pine extract. A blank sample consisted of 10 µl 10 mM Tris–HCl (pH 7.4). All measurements were performed as technical triplicates. Rates of superoxide production were calculated with an extinction coefficient of 2.16 × 104 M−1 cm−1, blank corrected, and expressed per minute per milligram of pine needle protein.
SOD activity in pine needle tissue
To measure SOD (EC 1.15.1.1) activity, needle extracts were powdered under liquid nitrogen in a precooled mortar. The needle powder (50 mg) was extracted by addition of 500 µl 100 mM potassium phosphate buffer (Roth; pH 7.8), 5% polyvinylpolypyrrolidone and 0.5% Triton X-100 (Roth). The extract was vortexed and placed on ice for 15 min; this step was repeated once. Thereafter, the extract was centrifuged at 20,817×g, 4 °C for 20 min. The collected supernatant was centrifuged again for 10 min under the same conditions to remove particulate matter. The final supernatant was desalted using Sephadex G-25 columns (GE Healthcare) which were equilibrated with the same buffer as used for extraction, but polyvinylpolypyrrolidone was omitted. The desalted crude extract was diluted 1:5 with the buffer used for equilibration of Sephadex columns and then used for determination of SOD activity.
The activity measurement was adapted to 96 well plates from a recently published protocol (Popović et al. 2016). A 5 µl aliquot of Sephadex desalted crude pine needle extract was mixed with 245 µl reaction buffer consisting of 50 mM potassium phosphate buffer (pH 7.8), 0.66 mM diethylenetriaminepentaacetic acid (DTPA; Roth), 10 mM l-methionine (Roth), 33 µM nitroblue tetrazolium (Roth) and 3.3 µM riboflavin (Roth). Plates were kept in the dark until measurement. The reaction was initiated by transferring the plates under fluorescent lamps (100 µm photons m−2 s−1) for 3 min. Absorbance was measured at 560 nm. To calculate SOD activity in pine needles from absorbance data, an absorbance standard curve was ascertained by pipetting 5 µl of an SOD standard ranging from 1000 to 0 U/ml on each plate. SOD standard was prepared from recombinant bovine SOD (Sigma) in equilibration buffer as described above for the pine needle extract for these measurements. All measurements were performed as technical triplicates. SOD activities of pine needles were calculated by use of the standard curve and expressed as units per gram fresh weight.
APX activity in pine needle tissue
To measure APX (EC 1.11.1.11) activity, needle extracts were prepared as described for the SOD assays, except for addition of 5 mM ascorbic acid (Roth) to the extraction and equilibration buffer.
For measurements we adapted a protocol described by Gillespie et al. (2011) to 96 well plates. 10 µl undiluted, desalted crude pine extract were mixed with 80 µl assay buffer consisting of 50 mM potassium phosphate buffer (pH 7.8), 0.5 mM ascorbic acid and 0.2 mM DTPA. A blank sample consisted of 10 µl equilibration buffer. The measurements began immediately after the addition of 10 µl 20 mM hydrogen peroxide (Roth). Oxidation of ascorbate was monitored at 290 nm for 90 s, measured in 5-s intervals. Due to a high turnover, each row on the 96 well plate was measured sequentially with its own blank to account for pipetting time errors. All measurements were performed as technical triplicates. Ascorbic acid turnover was calculated using a molar extinction coefficient of 2800 M−1 cm−1, blank corrected and expressed as nmol hydrogen peroxide turnover per minute per milligram of protein.
CAT activity in pine needle tissue
To measure CAT (EC 1.11.1.6) activity, needle extracts were prepared as described above for measuring SOD activity and diluted 1:5 with equilibration buffer.
CAT activity was determined according to the procedure described by Noctor et al. (2016). Desalted crude extract (10 µl) was mixed with 170 µl reaction buffer (100 mM potassium phosphate pH 7.5, 1 mM DTPA). A blank sample consisted of 10 µl equilibration buffer. The reaction was started by addition of 20 µl 0.2 M hydrogen peroxide solution. Decrease in hydrogen peroxide content was monitored for 2 min at 240 nm. All measurements were performed as technical triplicates. CAT activity was calculated using a molar extinction coefficient of 40 M−1 cm−1, blank corrected and expressed as µmol hydrogen peroxide degradation per minute per milligram of protein.
CAT activity in insect tissue
Egg extracts were prepared by homogenizing a sample (with 75 eggs) in 50 µl Ringer solution using a pestle. The homogenate was centrifuged (3350×g, 10 min, 4 °C), and the supernatant of fresh extracts was used for the enzyme assay.
Oviduct extracts were obtained by transferring dissected oviducts of five females to 20 μl ice-cold Ringer solution. To remove oviduct cell fragments, samples were centrifuged (20,814×g, 20 min, 4 °C), and the supernatant containing the oviduct secretion was used for the analysis. The pellet with oviduct cell fragments was resuspended in 100 µl 70 mM potassium phosphate buffer (pH 6.6) containing 0.1% Triton X-100, and the cells were broken by using a syringe. Fresh extracts were used for the assay.
Furthermore, we addressed the question whether oviduct secretion keeps CAT activity after release by the sawfly female. Therefore, we prepared the extracts as described above, but prior to the enzyme assay we kept them stored in open vials for 3 days under the conditions used for pine needle treatment with fresh oviduct extract. We chose a time period of 3 days since previous studies have shown that application of oviduct secretion kept in Ringer solution and stored frozen can elicit a change of pine needle volatiles 3 days after application into slit pine needles; the pine needles treated with this oviduct secretion released volatiles that were attractive to egg parasitoids (Hilker et al. 2005). Here, we were interested in elucidating whether the oviduct secretion also displays ROS processing activity 3 days after release from the females.
To prepare an extract of the accessory reproductive gland of a D. pini female, the dissected gland was transferred to 50 µl ice-cold Ringer solution and centrifuged as described for oviduct secretion sample. The supernatant was used for analysis.
A haemolymph extract was prepared by adding 20 µl ice-cold Ringer solution to the haemolymph collected from five D. pini females. The sample was centrifuged as described for the oviduct secretion sample, and the supernatant was analysed.
For control, five heads (thoraxes) of D. pini females were homogenised in 300 (500) µl ice-cold Ringer using a pestle. The homogenate was centrifuged (3350×g, 10 min, 4 °C), and the supernatant was used for the assays.
We determined insect CAT activity by a different method than the one applied for pine needle CAT activity, and used the procedure described by Johansson and Borg (1988). We did so because the method described by Johansson and Borg does not apply the chelating agent DTPA which was used in the plant CAT assay. As yet we do not know whether addition of a chelating agent would affect the insect's enzyme activities. The D. pini oviduct secretion has so far only been shown to elicit defence responses in pine needles when it was kept in Ringer solution without any further chelating agents (e.g. Hilker et al. 2005).
For the determination of insect CAT activity, each of the insect extracts (5 µl) described above was mixed with 50 µl methanol (100% v/v) (Roth) and 55 µl 250 mM potassium phosphate/sodium hydroxide buffer (Roth; pH 7.0). The reaction was initiated by addition of 10 µl 90 mM hydrogen peroxide. The mixture was shaken for 20 min, and the reaction was terminated by the addition of 50 µl 7.8 M KOH (Roth). We added 100 µl 34.2 mM Purpald® (Sigma) in 480 mM HCl (Roth), and the mixture was incubated for 10 min on a shaker. Furthermore, 50 µl 65.2 mM potassium periodate (Sigma) was added, and the sample was incubated for 5 min. The absorbance of the purple formaldehyde adduct was measured at 550 nm. All measurements were performed as technical triplicates. CAT activity was calculated using a standard curve with formaldehyde and was expressed as µmol hydrogen peroxide degradation per minute per milligram of protein.
Protein determination of pine and insect samples
To refer the pine and insect enzyme activities mentioned above to the respective total protein contents, we determined protein concentrations of pine needles and insect samples by the method described by Smith et al. (1985) using Pierce® BCA Protein Assay Kit (Thermo Scientific) and following the protocol of this kit. Extraction buffers or buffers that were applied to solve membrane pellets were also used for preparation of the corresponding standards.
Protein extracts to which ascorbate oxidase measurements were referred to were diluted tenfold with extraction buffer without ascorbate; thus ascorbate in pine needles was diluted to a final concentration of 0.5 mM which was important because ascorbate interferes with BCA Kit measurements. Extraction buffer containing 0.5 mM ascorbic acid was used for the preparation of standards for these measurements.
Statistics
We used R 3.2.0 (R Development Core Team 2015) for all statistical analyses. The packages MASS, multcomp, nlme and PMCMR from the CRAN server (R Development Core Team 2015) were additionally installed.
Pine needle enzyme activities were statistically evaluated separately for each time point. Normal distribution of data was analysed by the Shapiro–Wilk test.
First, we performed a multiple comparison of pine needle enzyme activities across all treatments (including control needles). Differences between treatments of normally distributed data were analysed by a linear mixed model with treatment and activity as fixed effects and trees as random effects. The estimation method was set to maximum likelihood. If a treatment had a significant effect on enzyme activities, differences between treatments were detected by the post hoc Tukey test. Non-normally distributed data were evaluated by using a Friedman ANOVA. If significant effects between treatments were detected, they were further evaluated by a post hoc pairwise Wilcoxon–Wilcoxon test with a Benjamini–Hochberg correction for multiple comparisons.
Furthermore, we conducted pairwise comparisons of pine needle enzyme activities in egg-deposited samples and in untreated control needle samples by a Wilcoxon signed-rank test. We conducted this additional pairwise comparison because pine tissue taken from the naturally egg-deposited needles was located next to the eggs (i.e. directly next to the treated site, compare Fig. 1a), whereas pine needles with the artificial treatments were always analysed as whole needles. By taking the needle tissue next to the egg-deposited site we avoided co-analysis of pine and insect egg enzyme activities, but excluded pine tissue located directly at the egg insertion site (treatment site) of the needle.
Sawfly CAT activity data were not normally distributed (Shapiro–Wilk test). Therefore, differences between CAT activities in different tissues were statistically analysed by a Kruskal–Wallis H test. The Dunn test with Benjamini–Hochberg correction was used for post hoc comparisons.
Results
Pine needles respond to sawfly egg deposition by locally limited hydrogen peroxide production
HR-like symptoms were observed in about a quarter of P. sylvestris needles infested with D. pini eggs (Table 1). While pine needles with freshly laid eggs first showed no specific symptoms (Table 1, Fig. 1b), they formed some yellowish spots (desiccated tissue) along the egg row about 4 days after egg deposition (Fig. 1c). Later at the end of the egg incubation time (12 days after egg deposition) the yellowish, desiccated tissue is visible along the entire egg row (Fig. 1d). Hence, the intensity of these egg-induced pine responses increases during the egg incubation time (Table 1). No larval hatching was observed in egg-deposited needles showing HR-like symptoms.
Cross sections of egg-free and egg-deposited P. sylvestris needles were prepared which visualised production of ROS in response to D. pini egg deposition (Fig. 2a, b). The secretion covering the eggs on top is brownish even without staining (Fig. 2b); the secretion shows this brownish colour as soon as it is released by the female. The chemistry of this sawfly secretion, which is produced in the insect female accessory reproductive glands, has not been studied yet. Application of TMB or starch/KI solutions at different time points after egg deposition or at respective time points in the controls revealed that egg-free control needles show no blue/brownish (TMB-reaction) or brown (starch/KI reaction) staining that would indicate hydrogen peroxide production (Fig. 2c). However, the needles showed hydrogen peroxide positive staining very locally in pine tissue that is surrounding 1- and 3-day-old sawfly eggs (Figs. 2d, 1 and 3 days). Twelve days after egg deposition hydrogen peroxide positive staining was additionally visible in vascular tissue and in the resin ducts of egg-deposited P. sylvestris needles (Fig. 2d, 12 days).
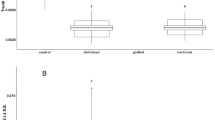
Catalase activity in freshly sampled Diprion pini tissues and secretions (median, interquartile range, minimum/maximum). Statistical differences evaluated by Kruskal–Wallis H test. Different letters above boxes indicate significant differences (P < 0.05) evaluated by Dunn-test with Benjamini–Hochberg correction
Pine needle activities of SOD, APX and CAT are affected by sawfly oviposition-associated processes
Pine needle NADPH oxidase activity was not significantly affected by either treatment (Table 2). Neither sawfly natural egg deposition nor any of the processes related to the egg deposition (wounding, release of oviduct secretion) did affect the activity of this ROS-generating enzyme.
SOD activity in wounded pine needles was significantly lower compared to untreated control needles when determined 6 h after treatment. Even though wounded pine needles treated with Ringer solution or oviduct secretion also showed slightly lower SOD activities than untreated controls, their activities did not significantly differ from the control. In contrast, 3 and 12 days after treatments SOD activity was significantly higher in wounded needles treated with Ringer and oviduct secretion. The tissue samples from egg-deposited needles showed also slightly higher SOD activity than the controls at day 3 and 12 after treatment, but these activities did not significantly differ from the untreated and artificial controls when performing a multiple comparison including all sample types (Table 2). However, when conducting a pairwise comparison of SOD activities in egg-deposited needles and untreated control needles, we see a significant increase in SOD activity 3 and 12 days after egg deposition.
APX activity was significantly reduced 12 days after all artificial pine needle treatments when compared to control needles. Naturally egg-deposited needles showed a moderate decrease in APX activity as well; however, this did not significantly differ from the APX activities in the other samples, neither when conducting a multiple comparison across all sample types nor in a pairwise comparison with the control (Table 2).
A multiple comparison of CAT activities across all sample types revealed that the activity of this enzyme was significantly reduced in artificially wounded needles and in egg-deposited needles 1 day after treatment when compared to untreated control needles. Three days after treatment, CAT activity was also significantly reduced in wounded needles with oviduct secretion or with Ringer in a multiple comparison. Egg-deposited needles showed a decrease in CAT activity at this time (3 days) only when compared pairwise with the untreated control needle sample (Table 2).
For all analysed enzymes we observed that especially the activity levels in untreated controls determined after 6 h differed from those measured after 1, 3 and 12 days in control plants. This may be due to diurnal activity changes of ROS-processing enzymes (Lai et al. 2012; Carvalho et al. 2013). All treatments started at the 10th hour of an 18 h-long light cycle in the climate chamber. Thus, samples taken 1, 3 and 12 days after treatments were harvested nearly at ‘noon’ of the day. However, samples taken 6 h after treatment (and at respective time in the controls) were collected almost in the end of the light period. The very low CAT activity detected in control pine needles at the sampling time point 6 h and the higher CAT activities in these needles at time points 1, 3 and 12 days matches results of CAT activity measurements in A. thaliana. This plant changes its activity during a day with a peak of activity at noon and lower activities at sunset and sunrise as well as at night (Lai et al. 2012).
Pine sawfly oviduct secretion has strong ROS-scavenging CAT activity
To determine whether D. pini females protect their eggs from hydrogen peroxide produced by pine needles in response to egg deposition, we measured CAT activity in the eggs, in the secretion enveloping the eggs (the oviduct secretion), in the tissue producing the secretion (the oviduct), and in the covering secretion applied on top of the eggs on the needle outside as well as other body tissues. Both freshly dissected oviduct tissue and oviduct secretion showed strong CAT activity, while CAT activity was significantly lower in the covering secretion and in the eggs dissected from the ovaries. The CAT activity in eggs dissected from the ovaries did not differ from CAT activities in other control tissue of D. pini females (head, thorax, haemolymph) (Fig. 3).
We also addressed the question whether oviduct secretion keeps its CAT activity after having been released from the female. Therefore, we studied the CAT activity of oviduct secretion 3 days after dissection from females; after dissection, the secretion had been kept under the same abiotic conditions as the treated pine needles. No CAT activity was detectable in these secretion samples.
Discussion
Plants can defend themselves against insect egg deposition, an initial step of herbivore attack, by the formation of necrotic tissue at the oviposition site, thus causing desiccation or detachment of eggs from the plant (Hilker and Fatouros 2016). No knowledge has been available as yet on the activities of ROS-processing enzymes involved in the formation of these HR-like symptoms. Our study shows that P. sylvestris responds to sawfly egg deposition by a change in the activity of ROS-processing enzymes. One and three days after egg deposition, CAT activity was significantly reduced in P. sylvestris needles deposited with eggs of the sawfly D. pini. Reduction in activity of this hydrogen peroxide degrading enzyme was obviously not specifically triggered by the eggs or egg-associated oviduct secretion, but by the ovipositional wounding that a sawfly inflicts with its ovipositor to a needle while laying the eggs. In contrast, neither natural egg deposition nor ovipositional wounding elicited changes in the activity of the ROS generating NADPH oxidase (Fig. 4).
Proposed model of hydrogen peroxide turnover in Pinus sylvestris tissue in response to Diprion pini egg deposition/ovipositional wounding. APX, ascorbate peroxidase; CAT, catalase; SOD, superoxide dismutase producing H2O2 from the superoxide anion; NOX, NADPH oxidase. The bold green line in the schematic green needle indicates the ovipositional wounding inflicted to pine needle tissue by the sharp sclerotised ovipositor of D. pini females
Wounding of plants has been shown to result in ROS accumulation and increased NADPH oxidase activity (Bi and Felton 1995; Wu and Baldwin 2010; Minibayeva et al. 2015). However, wounding of a plant by insects feeding continuously on the entire leaf or canopy is different from the spatially and temporally restricted wounding associated with insect egg deposition. Similar to an oviposition-associated wounding, also wounding of plants by gall-inducing insects is locally restricted to a distinct, small site where the gall is formed. Plants can defend against gall-inducing insects by the formation of necrotic tissue at the site where the galling larva starts feeding and by lignification of the gall (Hilker et al. 2002b; Chen 2008); these defensive responses against gall inducers have been suggested to be a result of ROS accumulation (Oliveira et al. 2016). When Liu et al. (2010) studied responses of wheat to attack by a gall midge, they found that ROS accumulation of this plant in response to the gall midge infestation was not due to increased NADPH oxidase activity, but probably to class III peroxidases. A study of responses of the model plant A. thaliana to extracts of crushed Pieris brassicae eggs revealed that mutants deficient of the NADPH oxidases RBOHD or RBOHF show similar accumulation of ROS as wild-type A. thaliana plants, thus indicating NADPH oxidase independent production of ROS in response to this treatment (Gouhier-Darimont et al. 2013). Accumulation of hydrogen peroxide in P. sylvestris in response to D. pini egg deposition (and associated ovipositional wounding) could neither be attributed to increased NAPDH oxidase activity.
A possible explanation for hydrogen peroxide accumulation in egg-deposited P. sylvestris needles is that formation of this ROS is increased via an NAPDH oxidase independent pathway. Hydrogen peroxide is generated by several enzymes which include—in addition to the key enzyme NADPH oxidase—e.g. peroxisomal glycolate oxidase, xanthine oxidase, urate oxidase, sulphite oxidase, oxalate oxidase and amine oxidase (Lane 2002; Gupta et al. 2016). Amine oxidases are also well known to play a role in the formation of hypersensitive cell death; these enzymes oxidise polyamines, such as putrescine or spermidine, which accumulate in response to phytopathogen infection; hydrogen peroxide is produced by these oxidations (Angelini et al. 2007; Jiménez-Bremont et al. 2014). Furthermore, a study by Roach et al. (2015) indicates that di-amine oxidases also play a significant role in plant responses to wounding.
Another explanation for hydrogen peroxide accumulation in egg-deposited pine needles is that the formation of this ROS does not increase, but that its degradation decreases (Mittler et al. 2004; Torres 2010). This explanation for hydrogen peroxide accumulation in egg-deposited pine needles is supported by the finding that these needles showed reduced CAT activity 1 and 3 days after oviposition. APX activity was significantly reduced in wounded needles 12 days after treatment, while APX activity in the egg-deposited needles decreased by trend at this time point after treatment. Interestingly, the plant hormone salicylic acid (SA) is known to inhibit APX and CAT in plants (Durner and Klessig 1995, 1996). Accumulation of SA and activation of SA responsive defence genes has not been determined in egg-deposited pine needles so far, but was observed in A. thaliana locally at the site of egg deposition by P. brassicae (Gouhier-Darimont et al. 2013).
Changes in SOD activities in response to the treatments varied dependent on the treatment and time point of measurement. Induction of SOD is a general phenomenon related to stress in plants (Alscher et al. 2002). Thus, the significant increase of SOD activities in wounded needles treated with oviduct secretion or Ringer solution (3 and 12 days after treatment) were expected because of the wound stress. Additionally, when comparing egg-deposited needles directly to untreated control needles we can also observe a significant increase in SOD activity, indicating an accumulation of hydrogen peroxide at the site of the egg deposition. However, the lack of changes in SOD activities in artificially wounded samples and the decrease in SOD activity shortly after wounding (6 h) remains elusive.
Our study suggests that the differential regulation of ROS-mediating enzyme activities in response to egg deposition was very localised. When we performed a multiple comparison of enzyme activities across all sample types, several significant changes that were found in response to wounding of P. sylvestris needles were detectable only by trend in response to D. pini egg deposition. This may be due to the type of tissue sampling. While we measured enzyme activities in the entire wounded needles, we sampled only the tissue right next to the eggs in the egg-deposited needles to avoid measuring egg-inherent enzyme activities. However, when we conducted a pairwise comparison of enzyme activities in egg-deposited and untreated control needles, we can see similar significant effects of natural egg deposition and the artificial treatments, except for APX. Very local responses to egg deposition were also found in A. thaliana deposited with eggs of the butterfly P. brassicae; differential regulation of genes were most pronounced in leaf tissue directly below the eggs (Little et al. 2007). Staining of pine needle sections also showed a very localised accumulation of hydrogen peroxide at the sawfly egg—pine needle interface (Fig. 2d).
Hydrogen peroxide accumulation in egg-deposited needles may on the one hand serve defence signalling that is initiating stress-related transcription of defence gene networks (Mittler et al. 2011). On the other hand, plant ROS accumulation in response to the eggs might directly harm the developing eggs. Furthermore, increased ROS levels kill plant cells and thus, might reduce humidity of the needle tissue in which D. pini eggs are embedded. This consequence of ROS accumulation may lead to desiccation of D. pini eggs. Indeed, our study showed that those needles which form HR-like symptoms can kill all the eggs laid into them; no larvae hatched from eggs in these needles. If pine tissue does not form clearly visible HR-like symptoms and eggs survive, egg-induced changes in ROS-mediating enzyme activities may also affect the hatching larvae which start feeding at the natal site. Staining of the egg-deposited needles revealed the presence of hydrogen peroxide and peroxidase activity in the vascular tissue and resin ducts of needle sections (Fig. 2d). This is a strong indication of ongoing lignification processes which are mediated by hydrogen peroxide and peroxidase activity in xylem tissue (Barceló 1998; Ros-Barceló et al. 2002). Lignified and desiccated needle tissue may also negatively affect neonate D. pini larvae which suffer increased mortality when starting their larval development at the site where they hatch as compared to larvae which start feeding on egg-free needles (Beyaert et al. 2012). Hence, oviposition-induced formation of pine hydrogen peroxide or of other ROS may be involved in harming D. pini eggs in various ways.
Defence of P. sylvestris against insect eggs by ROS accumulation is not left unanswered by D. pini. Oviduct secretion of D. pini enclosing the eggs and oviduct tissue producing the secretion showed very high ROS-scavenging activity, while CAT activity in eggs dissected from D. pini ovaries was not higher than in non-reproductive D. pini tissue. In another hymenopteran species, the parasitic wasp Cotesia vestalis, eggs showed increased peroxidase and CAT activity after egg deposition into host larvae (Hao et al. 2012). However, it remained unclear whether these enzyme activities in Cotesia eggs are induced in response to host immune activities or whether these are due to maternal secretions that Cotesia wasps attach to their eggs when ovipositing (e.g. Glatz et al. 2004). Other studies showed that CAT plays a central role in protecting oocytes and the early embryo from ROS damage (DeJong et al. 2007; Diaz-Albiter et al. 2011; Sim and Denlinger 2011). Our results suggest that the sawfly D. pini relies on protecting the offspring from plant ROS by external egg secretions rather than by egg-inherent ROS-scavenging enzyme activity. However, enhanced CAT activity was only found in freshly released oviduct secretion, whereas oviduct secretion kept at the same experimental conditions as the treated pine needles had lost its strong CAT activity 3 days after dissection (release) from D. pini females. The high CAT activity of freshly released oviduct secretion may avert the negative effects of a ROS burst as initial response of pine to the ovipositional wounding by D. pini females (Fig. 4).
In conclusion, our study shows close ROS-related interactions between P. sylvestris and D. pini egg deposition. This plant and insect species share a long common evolutionary time since the Paleogene when the sawfly genus Diprion evolved (O’Reilly et al. 2015). Pine species need to have evolved survival mechanisms which limit sawfly populations because high population densities of the gregariously feeding D. pini larvae may severely damage entire pine forests (e.g. Lyytikäinen-Saarenmaa et al. 2002). A defensive response of pine against the initial step of attack by D. pini, the egg deposition, is accumulation of ROS. Future studies need to address whether in addition to the detected decreased activity of CAT also ROS-generating enzymes other than NADPH oxidase are involved in ROS accumulation in egg-deposited pine needles. The pine response to egg deposition is counteracted by high CAT activity in D. pini oviduct secretion that is associated with the eggs and ovipositional wounding. The sawfly eggs and associated secretions may contain in addition to CAT further ROS-scavenging enzymes (Felton and Summers 1995; Mathews et al. 1997) which contribute to limiting the negative effects of ROS produced by pine needles.
Author contribution statement
NB designed the experiments, performed all plant enzyme measurements and related data analysis. UTK performed all insect tissue enzyme measurements and related data analysis. NB and UTK took the photos. MH conceptualized and supervised the study, participated in its design and coordination. NB drafted the manuscript, and MH and UTK assisted in drafting and revising the manuscript. All authors read and approved the final manuscript.
Abbreviations
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- HR:
-
Hypersensitive response
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
References
Almagro L, Gómez Ros LV, Belchi-Navarro S et al (2009) Class III peroxidases in plant defence reactions. J Exp Bot 60:377–390. doi:10.1093/jxb/ern277
Alscher RG, Erturk N, Heath LS (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot 53(1331–134):1. doi:10.1093/jexbot/53.372.1331
Angelini R, Tisi A, Rea G et al (2007) Involvement of polyamine oxidase in wound healing. Plant Physiol 146:162–177. doi:10.1104/pp.107.108902
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399. doi:10.1146/annurev.arplant.55.031903.141701
Asada K (1992) Ascorbate peroxidase—a hydrogen peroxide-scavenging enzyme in plants. Physiol Plant 85:235–241. doi:10.1111/j.1399-3054.1992.tb04728.x
Barceló AR (1998) Hydrogen peroxide production is a general property of the lignifying xylem. Ann Bot 82:97–103. doi:10.1006/anbo.1998.0655
Barros J, Serk H, Granlund I, Pesquet E (2015) The cell biology of lignification in higher plants. Ann Bot 115:1053–1074. doi:10.1093/aob/mcv046
Baxter A, Mittler R, Suzuki N (2014) ROS as key players in plant stress signalling. J Exp Bot 65:1229–1240. doi:10.1093/jxb/ert375
Beyaert I, Köpke D, Stiller J et al (2012) Can insect egg deposition “warn” a plant of future feeding damage by herbivorous larvae? Proc R Soc B 279:101–108. doi:10.1098/rspb.2011.0468
Bi JL, Felton GW (1995) Foliar oxidative stress and insect herbivory: primary compounds, secondary metabolites, and reactive oxygen species as components of induced resistance. J Chem Ecol 21:1511–1530. doi:10.1007/BF02035149
Bombosch S, Ramakers PMJ (1976) Zur Dauerzucht von Gilpinia hercyniae Htg. Z Pflanzenk Pflanzen 83:40–44
Bozhkov PV, Lam E (2011) Green death: revealing programmed cell death in plants. Cell Death Differ 18:1239–1240. doi:10.1038/cdd.2011.86
Brauns A (1991) Taschenbuch der Waldinsekten, 4th edn. Gustav Fischer Verlag, Stuttgart
Caverzan A, Passaia G, Rosa SB et al (2012) Plant responses to stresses: role of ascorbate peroxidase in the antioxidant protection. Genet Mol Biol 35:1011–1019. doi:10.1590/S1415-47572012000600016
Chen MS (2008) Inducible direct plant defense against insect herbivores: a review. Insect Sci 15:101–114. doi:10.1111/j.1744-7917.2008.00190.x
Coll NS, Epple P, Dangl JL (2011) Programmed cell death in the plant immune system. Cell Death Differ 18:1247–1256. doi:10.1038/cdd.2011.37
da Carvalho KS, Pinheiro HA, Festucci-Buselli RA et al (2013) Diurnal changes in leaflet gas exchange, water status and antioxidant responses in Carapa guianensis plants under water-deficit conditions. Acta Physiol Plant 35:13–21. doi:10.1007/s11738-012-1043-6
DeJong RJ, Miller LM, Molina-Cruz A et al (2007) Reactive oxygen species detoxification by catalase is a major determinant of fecundity in the mosquito Anopheles gambiae. Proc Natl Acad Sci USA 104:2121–2126. doi:10.1073/pnas.0608407104
Diaz-Albiter H, Mitford R, Genta FA et al (2011) Reactive oxygen species scavenging by catalase is important for female Lutzomyia longipalpis fecundity and mortality. PLoS One 6:e17486. doi:10.1371/journal.pone.0017486
Durner J, Klessig DF (1995) Inhibition of ascorbate peroxidase by salicylic acid and 2,6-dichloroisonicotinic acid, two inducers of plant defense responses. Proc Natl Acad Sci USA 92:11312–11316. doi:10.1073/pnas.92.24.11312
Durner J, Klessig DF (1996) Salicylic acid is a modulator of tobacco and mammalian catalases. J Biol Chem 271:28492–28501. doi:10.1074/jbc.271.45.28492
Eichhorn O (1976) Dauerzucht von Diprion pini L. (Hym.: Diprionidae) im Laboratorium unter Berücksichtigung der Fotoperiode. Anz Schädlingskd Pfl 49:38–41. doi:10.1007/BF02342165
Fatouros NE, Lucas-Barbosa D, Weldegergis BT et al (2012) Plant volatiles induced by herbivore egg deposition affect insects of different trophic levels. PLoS One 7:e43607. doi:10.1371/journal.pone.0043607
Fatouros NE, Paniagua Voirol LR, Drizou F et al (2015) Role of large cabbage white butterfly male-derived compounds in elicitation of direct and indirect egg-killing defenses in the black mustard. Front Plant Sci 6:1–9. doi:10.3389/fpls.2015.00794
Felton GW, Summers CB (1995) Antioxidant systems in insects. Arch Insect Biochem Physiol 29:187–197. doi:10.1002/arch.940290208
Gershenzon J, Croteau R (1991) Terpenoids. In: Rosenthal GA, Berenbaum MR (eds) Herbivores: their interactions with secondary plant metabolites, 2nd edn. Academic Press, San Diego, pp 165–219
Gillespie KM, Rogers A, Ainsworth EA (2011) Growth at elevated ozone or elevated carbon dioxide concentration alters antioxidant capacity and response to acute oxidative stress in soybean (Glycine max). J Exp Bot 62:2667–2678. doi:10.1093/jxb/erq435
Glatz R, Schmidt O, Asgari S (2004) Isolation and characterization of a Cotesia rubecula bracovirus gene expressed in the lepidopteran Pieris rapae. J Gen Virol 85:2873–2882. doi:10.1099/vir.0.80307-0
Gouhier-Darimont C, Schmiesing A, Bonnet C et al (2013) Signalling of Arabidopsis thaliana response to Pieris brassicae eggs shares similarities with PAMP-triggered immunity. J Exp Bot 64:665–674. doi:10.1093/jxb/ers362
Gupta K, Sengupta A, Chakraborty M, Gupta B (2016) Hydrogen peroxide and polyamines act as double edged swords in plant abiotic stress responses. Front Plant Sci 7:1–19. doi:10.3389/fpls.2016.01343
Hao Z-P, Zhao J-R, Yuan Z-Q et al (2012) Influence of photoperiod on hydrogen peroxide metabolism during diapause induction in Cotesia vestalis (Haliday) (Hymenoptera: Braconidae). J Kansas Entomol Soc 85:206–218. doi:10.2317/JKES120318.1
Hilker M, Fatouros NE (2015) Plant responses to insect egg deposition. Annu Rev Entomol 60:493–515. doi:10.1146/annurev-ento-010814-020620
Hilker M, Fatouros NE (2016) Resisting the onset of herbivore attack: plants perceive and respond to insect eggs. Curr Opin Plant Biol 32:9–16. doi:10.1016/j.pbi.2016.05.003
Hilker M, Kobs C, Varama M, Schrank K (2002a) Insect egg deposition induces Pinus sylvestris to attract egg parasitoids. J Exp Biol 205:455–461
Hilker M, Rohfritsch O, Meiners T (2002b) The plant’s response towards insect egg deposition. In: Hilker M, Meiners T (eds) Chemoecology of insect eggs and egg deposition. Blackwell Publishing Ltd, Oxford, pp 205–233
Hilker M, Stein C, Schröder R et al (2005) Insect egg deposition induces defence responses in Pinus sylvestris: characterisation of the elicitor. J Exp Biol 208:1849–1854. doi:10.1242/jeb.01578
Jiménez-Bremont JF, Marina M, de la Guerrero-González ML et al (2014) Physiological and molecular implications of plant polyamine metabolism during biotic interactions. Front Plant Sci 5:95. doi:10.3389/fpls.2014.00095
Johansson LH, Borg LA (1988) A spectrophotometric method for determination of catalase activity in small tissue samples. Anal Biochem 174:331–336
Köpke D, Schröder R, Fischer HM et al (2008) Does egg deposition by herbivorous pine sawflies affect transcription of sesquiterpene synthases in pine? Planta 228:427–438. doi:10.1007/s00425-008-0747-8
Köpke D, Beyaert I, Gershenzon J et al (2010) Species-specific responses of pine sesquiterpene synthases to sawfly oviposition. Phytochemistry 71:909–917. doi:10.1016/j.phytochem.2010.03.017
Lai AG, Doherty CJ, Mueller-Roeber B et al (2012) CIRCADIAN CLOCK-ASSOCIATED 1 regulates ROS homeostasis and oxidative stress responses. Proc Natl Acad Sci USA 109:17129–17134. doi:10.1073/pnas.1209148109
Lamb C, Dixon RA (1997) The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol 48:251–275. doi:10.1146/annurev.arplant.48.1.251
Lane BG (2002) Oxalate, germins, and higher-plant pathogens. IUBMB Life 53:67–75. doi:10.1080/10399710290038954
Little D, Gouhier-Darimont C, Bruessow F, Reymond P (2007) Oviposition by pierid butterflies triggers defense responses in Arabidopsis. Plant Physiol 143:784–800. doi:10.1104/pp.106.090837
Liu X, Williams CE, Nemacheck JA et al (2010) Reactive oxygen species are involved in plant defense against a gall midge. Plant Physiol 152:985–999. doi:10.1104/pp.109.150656
Lyytikäinen-Saarenmaa P, Lyytikäinen-Saarenmaa P, Tomppo E (2002) Impact of sawfly defoliation on growth of Scots pine Pinus sylvestris (Pinaceae) and associated economic losses. Bull Entomol Res 92:137–140. doi:10.1079/BER2002154
Mathews MC, Summers CB, Felton GW (1997) Ascorbate peroxidase: a novel antioxidant enzyme in insects. Arch Insect Biochem Physiol 34:57–68. doi:10.1002/(SICI)1520-6327(1997)34:1<57:AID-ARCH5>3.0.CO;2-T
Meng X, Zhang S (2013) MAPK cascades in plant disease resistance signaling. Annu Rev Phytopathol 51:245–266. doi:10.1146/annurev-phyto-082712-102314
Minibayeva F, Beckett RP, Kranner I (2015) Roles of apoplastic peroxidases in plant response to wounding. Phytochemistry 112:122–129. doi:10.1016/j.phytochem.2014.06.008
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410. doi:10.1016/B978-044482650-3/50031-6
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498. doi:10.1016/j.tplants.2004.08.009
Mittler R, Vanderauwera S, Suzuki N et al (2011) ROS signaling: the new wave? Trends Plant Sci 16:300–309. doi:10.1016/j.tplants.2011.03.007
Morel JB, Dangl JL (1997) The hypersensitive response and the induction of cell death in plants. Cell Death Differ 4:671–683. doi:10.1038/sj.cdd.4400309
Mumm R, Hilker M (2006) Direct and indirect chemical defence of pine against folivorous insects. Trends Plant Sci 11:351–358. doi:10.1016/j.tplants.2006.05.007
Mumm R, Schrank K, Wegener R et al (2003) Chemical analysis of volatiles emitted by Pinus svlvestris after induction by insect oviposition. J Chem Ecol 29:1235–1252. doi:10.1023/A:1023841909199
Mur LA, Kenton P, Lloyd AJ et al (2008) The hypersensitive response; the centenary is upon us but how much do we know? J Exp Bot 59:501–520. doi:10.1093/jxb/erm239
Murias M, Rachtan M, Jodynis-Liebert J (2005) Effect of multiple freeze-thaw cycles of cytoplasm samples on the activity of antioxidant enzymes. J Pharmacol Toxicol Methods 52:302–305. doi:10.1016/j.vascn.2005.03.002
Navrot N, Rouhier N, Gelhaye E, Jacquot JP (2007) Reactive oxygen species generation and antioxidant systems in plant mitochondria. Physiol Plant 129:185–195. doi:10.1111/j.1399-3054.2006.00777.x
Noctor G, Mhamdi A, Foyer CH (2016) Oxidative stress and antioxidative systems: recipes for successful data collection and interpretation. Plant, Cell Environ 39:1140–1160. doi:10.1111/pce.12726
O’Brien JA, Daudi A, Butt VS, Bolwell GP (2012) Reactive oxygen species and their role in plant defence and cell wall metabolism. Planta 236:765–779. doi:10.1007/s00425-012-1696-9
O’Reilly JE, dos Reis M, Donoghue PCJ (2015) Dating tips for divergence-time estimation. Trends Genet 31:637–650. doi:10.1016/j.tig.2015.08.001
Oliveira DC, Isaias RMS, Fernandes GW et al (2016) Manipulation of host plant cells and tissues by gall-inducing insects and adaptive strategies used by different feeding guilds. J Insect Physiol 84:103–113. doi:10.1016/j.jinsphys.2015.11.012
Olson PD, Varner JE (1993) Hydrogen peroxide and lignification. Plant J 4:887–892. doi:10.1046/j.1365-313X.1993.04050887.x
Passardi F, Cosio C, Penel C, Dunand C (2005) Peroxidases have more functions than a swiss army knife. Plant Cell Rep 24:255–265. doi:10.1007/s00299-005-0972-6
Popović M, Šuštar V, Gričar J et al (2016) Identification of environmental stress biomarkers in seedlings of European beech (Fagus sylvatica) and Scots pine (Pinus sylvestris). Can J For Res 46:58–66. doi:10.1139/cjfr-2015-0274
R Development Core Team (2015) R: A language and environment for statistical computing
Reape TJ, Molony EM, McCabe PF (2008) Programmed cell death in plants: distinguishing between different modes. J Exp Bot 59:435–444. doi:10.1093/jxb/erm258
Reczek CR, Chandel NS (2015) ROS-dependent signal transduction. Curr Opin Cell Biol 33:8–13. doi:10.1016/j.ceb.2014.09.010
Reymond P (2013) Perception, signaling and molecular basis of oviposition-mediated plant responses. Planta 238:247–258. doi:10.1007/s00425-013-1908-y
Roach T, Colville L, Beckett RP et al (2015) A proposed interplay between peroxidase, amine oxidase and lipoxygenase in the wounding-induced oxidative burst in Pisum sativum seedlings. Phytochemistry 112:130–138. doi:10.1016/j.phytochem.2014.06.003
Rojas CM, Senthil-Kumar M, Wang K et al (2012) Glycolate oxidase modulates reactive oxygen species-mediated signal transduction during nonhost resistance in Nicotiana benthamiana and Arabidopsis. Plant Cell 24:336–352. doi:10.1105/tpc.111.093245
Ros-Barceló A, Pomar F, López-Serrano M et al (2002) Developmental regulation of the H2O2-producing system and of a basic peroxidase isoenzyme in the Zinnia elegans lignifying xylem. Plant Physiol Biochem 40:325–332. doi:10.1016/S0981-9428(02)01376-1
Sim C, Denlinger DL (2011) Catalase and superoxide dismutase-2 enhance survival and protect ovaries during overwintering diapause in the mosquito Culex pipiens. J Insect Physiol 57:628–634. doi:10.1016/j.jinsphys.2011.01.012
Smith PK, Krohn RI, Hermanson GT et al (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85. doi:10.1016/0003-2697(85)90442-7
Torres MA (2010) ROS in biotic interactions. Physiol Plant 138:414–429. doi:10.1111/j.1399-3054.2009.01326.x
Wu J, Baldwin IT (2010) New insights into plant responses to the attack from insect herbivores. Annu Rev Genet 44:1–24. doi:10.1146/annurev-genet-102209-163500
Acknowledgements
We thank the technicians Ute Braun and Gabriele Haberberger, Institute of Biology, Freie Universität Berlin, for rearing and maintaining the sawfly population, and the students Juliane Dankwarth and Marlena Winter for dissecting sawflies and supporting the enzymatic assays. We also thank Prof. Dr. Christian Schmitz-Linneweber and Arne Hillebrand, Humboldt Universität Berlin, for technical support with ultracentrifugation and Prof. Dr. Margarete Baier, Freie Universität Berlin, for her advice in enzyme activity analysis. Furthermore, we thank two anonymous reviewers for their helpful comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animals in this study were treated according to legal guidelines of the European Union and the Federal Republic of Germany as well as according to institutional guidelines of Freie Universität Berlin.
Conflict of interest
The authors declare they have no conflict of interest and consented in submitting and publishing this study.
Funding
This study was funded by the German Research Foundation (DFG Hi 416/22-1).
Rights and permissions
About this article
Cite this article
Bittner, N., Trauer-Kizilelma, U. & Hilker, M. Early plant defence against insect attack: involvement of reactive oxygen species in plant responses to insect egg deposition. Planta 245, 993–1007 (2017). https://doi.org/10.1007/s00425-017-2654-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-017-2654-3