Abstract
Main conclusion
The hypometabolic, stress-resistant dauer larva of Caenorhabditis elegans serves as an excellent model to study the molecular mechanisms of desiccation tolerance, such as maintenance of membrane organization, protein folding, xenobiotic and ROS detoxification in the dry state.
Many organisms from diverse taxa of life have the remarkable ability to survive extreme desiccation in the nature by entering an ametabolic state known as anhydrobiosis (life without water). The hallmark of the anhydrobiotic state is the achievement and maintenance of an exceedingly low metabolic rate, as well as preservation of the structural integrity of the cell. Although described more than three centuries ago, the biochemical and biophysical mechanisms underlying this phenomenon are still not fully comprehended. This is mainly due to the fact that anhydrobiosis in animals was studied using non-model organisms, which are very difficult, if not impossible, to manipulate at the molecular level. Recently, we introduced the roundworm (nematode) Caenorhabditis elegans as a model for anhydrobiosis. Taking advantage of powerful genetic, biochemical and biophysical tools, we investigated several aspects of anhydrobiosis in a particular developmental stage (the dauer larva) of this organism. First, our studies allowed confirming the previously suggested role of the disaccharide trehalose in the preservation of lipid membranes. Moreover, in addition to known pathways such as reactive oxygen species defense, heat-shock and intrinsically disordered protein expression, evidence for some novel strategies of anhydrobiosis has been obtained. These are increased glyoxalase activity, polyamine and polyunsaturated fatty acid biosynthesis. All these pathways may constitute a generic toolbox of anhydrobiosis, which is possibly conserved between animals and plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Caenorhabditis elegans as a model organism
Life depends highly on water. At the same time, drought is a very common environmental insult affecting terrestrial organisms. While some organisms developed ways of preserving their body water efficiently, those that are unable to do so dry out upon severe desiccation and perish. However, a group of organisms from various taxa of life have evolved an extraordinary ability to survive complete desiccation by entering a reversible ametabolic state known as anhydrobiosis (Keilin 1959). These so-called “anhydrobiotes” can practically stop their metabolism and preserve their structural integrity despite losing all of their body water. Although the phenomenon has been discovered centuries ago, its molecular mechanisms have mostly remained mysterious. This happened because anhydrobiosis was studied almost exclusively in non-model organisms, which are hard to manipulate at the molecular level. Recently, we discovered that a well-known model organism, C. elegans, is also an anhydrobiote (Erkut et al. 2011). This opened a broad avenue to study the biochemical and biophysical mechanisms of anhydrobiosis, most of which are likely conserved among all anhydrobiotes.
Caenorhabditis elegans, a.k.a. “the worm”, is a non-parasitic, hermaphroditic nematode, which lives in humus-rich soil and colonizes especially around decaying organic material (Fig. 1a). It was first isolated and identified in 1897 (Maupas 1901). So far, several wild isolates have been collected from Europe, North America, Australia, Asia and Africa, which implies that the species is widely distributed (Félix and Braendle 2010). Nevertheless, its natural habitat remains mostly elusive.
Caenorhabditis elegans, its anatomy and life cycle. a A mixed-stage population of C. elegans growing on Escherichia coli lawn on an agar plate. Scale bar 500 µm. b The basic anatomy and life cycle of a hermaphrodite C. elegans at 25 °C. Reproductive stages are labeled in blue, arrested stages are labeled in green, time between molts are indicated in orange, and environmental conditions are depicted in red. Adapted from WormAtlas (Altun and Hall 2009)
As early as 1963, C. elegans was introduced as a metazoan model to study the genetic basis of animal development (Brenner 1974). Soon after that, the developmental lineage of every single C. elegans somatic cell was mapped (Sulston and Horvitz 1977). In 1998, it became the first multicellular organism whose genome sequence was determined (The C. elegans Sequencing Consortium 1998). Today, C. elegans is one of the foremost model organisms used in animal development, neurobiology and aging research. Here, we describe some general properties of this organism that made it such a successful model.
The worm has a simple body anatomy (Fig. 1b). The digestive track, which starts with the pharynx and continues with the intestine, spans almost the entire length of the animal. It constitutes a major part of the inner region, which also harbors the gonad and uterus in adults. In the outer ring (the region beneath the cuticle), there are muscles, hypodermis, neurons and other supporting tissues. The body is entirely covered by a robust, proteinaceous cuticle. Average length of an adult worm is around 1–1.5 mm.
Caenorhabditis elegans undergoes 4 larval stages before becoming an adult (Fig. 1b). Under optimal conditions, a mother can self-fertilize and lay more than 300 eggs (Begasse et al. 2015). Each egg develops ex utero before it hatches into the first larval stage (L1). This is the first developmental checkpoint of the worm. In case the L1 larva hatches into an environment with no food source, it does not develop further but it stays as an arrested larva (Baugh 2013). Otherwise, it develops subsequently into L2, L3 and L4 stages before it finally becomes an adult. Each larval stage is separated by a molt, during which a new cuticle is synthesized beneath the older one, and the old cuticle is shed. The duration of egg-to-adult development depends on the ambient temperature. For the most commonly used wild-type Bristol isolate, the life cycle is completed within 3 days at 25 °C (Byerly et al. 1976).
The popularity of C. elegans as a genetic model is mainly due to the availability of powerful genetic and biochemical tools compatible with this organism. First of all, it is fairly easy to create mutants of C. elegans (Jorgensen and Mango 2002) and map these mutations (Doitsidou et al. 2010). During the last four decades, different laboratories and consortia all around the world created thousands of mutants. These mutant lines are stored as frozen stocks and made available for the community upon request.
To study gene function in C. elegans without using mutant lines, one can take advantage of targeted gene silencing via RNA interference (RNAi). This is achieved by microinjecting double-stranded RNA (dsRNA) into the germ line of a worm (Fire et al. 1998), feeding the worm with bacteria expressing the dsRNA (Timmons and Fire 1998) or soaking the worm in a solution of dsRNA (Tabara et al. 1998). RNAi is especially important when the mutant line cannot be maintained (e.g., due to embryonic lethality) or when a systematic, targeted screen is aimed. Thousands of dsRNA-expressing bacterial clones are commercially available.
There are also established methods for transgenesis in C. elegans (Sarov et al. 2006). Transgenic animals are very useful for visualizing the intracellular localization of proteins under physiological expression levels in vivo. They can also be used to overexpress a protein to investigate its function at elevated levels. More than 16,000 GFP-tagged genes inserted in expression vectors are available for the community (Sarov et al. 2012).
Recently, a targeted genome-editing method was developed based on a certain type of (archae)bacterial adaptive immune response called clustered, regularly interspaced, short palindromic repeats (CRISPR) and CRISPR-associated (Cas) system (Wiedenheft et al. 2012). Because it relies only on customized RNA sequences, CRISPR/Cas method has profound superiority over preexisting genome-editing tools such as zinc finger or transcription activator-like effector nucleases. Furthermore, it allows targeted mutagenesis without introducing background mutations, which is a disadvantage of classical methods. CRISPR/Cas system has already been applied successfully to many organisms, including C. elegans (Friedland et al. 2013). Current research focuses on increasing the specificity and efficiency of this method.
These genetic tools become even more powerful when they are used in a systems approach. Modern transcriptomics (high-density microarray designs and RNA deep sequencing), as well as mass spectrometry-based proteomics and metabolomics techniques can provide substantial amount of information in relatively short time. Combining forward and reverse genetics with these methods eliminates most of the technical limitations that have so far prevented us from understanding the C. elegans biology more deeply.
Finally, all information available on C. elegans, including genome and proteome annotations, literature, results of transcriptomics, knockout and knockdown studies, and many more can be accessed online at WormBase (Yook et al. 2012). This database is indispensable to all the researchers in the field.
The dauer larva, a stress-resistant hypometabolic stage
In nature, the worm regularly encounters various environmental stresses such as heat, cold, high salinity or the lack of a food source. In the long run, such insults are either lethal for the worm or they reduce fecundity. However, C. elegans has evolved a remarkable mechanism to solve this problem. When an L1 larva senses unfavorable environmental conditions, such as scarce food, elevated temperature and high population density, it develops into an alternative L2 stage (L2d), which molts and arrests as the dauer larva until the conditions become favorable again (Cassada and Russell 1975) (Fig. 1b). The name dauer originates from the German word, which means “enduring” or “permanent”. As the name implies, the dauer larva can endure harsh environmental conditions and its lifespan is extended several folds (up to 4 months). It has a specialized, sealed, mostly impermeable cuticle (Riddle et al. 1981; Cox et al. 1981). The dauer larva does not feed; therefore, it relies entirely on internal energy sources, such as fats. Moreover, the time a worm spends in the dauer stage does not affect its adult life span, which makes the dauer a non-aging stage (Klass and Hirsh 1976).
The genetics of dauer formation has been intensively studied. There are four main signaling cascades that are involved in the decision to form dauer larvae or pursue reproductive development: the guanylyl cyclase, TGF-β, insulin and steroid hormone (dafachronic acid) pathways (Fielenbach and Antebi 2008). These cascades are intermingled and they converge on transcription factors, which control many dauer-specific genes.
Metabolic suppression in the dauer stage
The dauer larva is hypometabolic due to its distinct metabolic properties, such as reduced oxygen consumption and heat dissipation as compared to reproductive larvae (O’Riordan and Burnell 1989, 1990; Vanfleteren and DeVreese 1996; Houthoofd et al. 2002; Holt and Riddle 2003; Burnell et al. 2005). Hypometabolism is a metabolic state, in which overall metabolic rate is strongly reduced (Clegg et al. 1996; Patil et al. 2013). It is often manifested by shutting down non-essential metabolic activities, such as growth and reproduction, to redirect the limited resources to the most essential functions, such as preserving vitality and structural integrity in the long run (Lant and Storey 2010).
Regardless of how starkly metabolic activities are slowed down and rearranged in the dauer stage, hypometabolism is not an extreme metabolic state. Many organisms can almost entirely shut down all of their metabolic activities when they face an environmental insult that is harsher than tolerable. This phenomenon is known as cryptobiosis, and it comes in various forms such as anhydrobiosis (in drought), cryobiosis (in cold) or anoxybiosis (in anoxia) (Keilin 1959). Cryptobiotic organisms can achieve an ametabolic state, in which they practically do not differ from dead matter. However, unlike death, ametabolism is reversible. Remarkably, the hypometabolic dauer larva of C. elegans can be induced to withstand almost complete desiccation, which implies that it may enter ametabolism upon desiccation stress (Erkut et al. 2011).
Some organisms have cryptobiotic/anhydrobiotic potential throughout their entire life cycle, whereas some others exhibit it only at a certain developmental stage. In the case of C. elegans, only the dauer larva is desiccation tolerant. What can be the reason for the stage specificity of this ability? Reaching ametabolism from a fully active metabolic state may be demanding due to the necessity of turning off all metabolic activity in a coordinated way. However, the dauer larva has already turned down those parts of its metabolism that are not essential for stress resistance and long-term survival. Therefore, turning off the rest of the metabolism may be more sustainable when the starting point is hypometabolism. In other words, metabolic depression (hypometabolism) and anhydrobiosis (ametabolism) are inseparable (Fig. 2). Thus, the dauer larva is an excellent paradigm to study transitions between full metabolism, hypometabolism and ametabolism.
The three metabolic domains of Caenorhabditis elegans. Normometabolic larvae feed, grow, reproduce and age normally. The dauer larva, on the other hand, is hypometabolic and does not display these properties. Furthermore, it is very tolerant against various stress factors. When the dauer larva senses a desiccative environment, it starts to enter ametabolism and meanwhile it activates anhydrobiosis-related genes and proteins. Ametabolic and hypometabolic states of the dauer larva are reversible
Anhydrobiotic strategies of the dauer larva
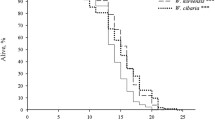
The transition of the dauer larva from hypometabolism to ametabolism does not happen immediately. This is because C. elegans is a slow-desiccation strategist. Unlike fast-desiccation strategists, such as tardigrades, slow-desiccation strategists cannot be dried out immediately. Instead, they require an adaptation period to achieve desiccation tolerance (Womersley 1987). This period is commonly known as preconditioning. We found that, under laboratory conditions, the optimal preconditioning of the dauer larva occurred at 98 % relative humidity (RH) within 3–4 days (Erkut et al. 2011). At this humidity level, worms are exposed to 27 atm of osmotic pressure, which is enough to remove more than 80 % of their body water. However, the kinetics of water loss is very slow (unpublished data), which enables worms to activate all necessary anhydrobiosis-related genes and proteins until cellular hydration levels become too low for biochemical reactions to proceed. Worms can survive preconditioning almost perfectly, even in prolonged exposures up to a month (unpublished data).
When preconditioned worms are transferred to a harsher desiccative environment (20–90 % RH), they lose even more water, yet their viability is only slightly affected. In contrast, non-preconditioned worms die very fast below 90 % RH (Erkut et al. 2011). This demonstrates the importance of the anhydrobiotic adaptation in C. elegans.
What happens during preconditioning at the molecular level? We first approached this question from a biochemical point of view. One hallmark of preconditioning in C. elegans appears to be the several fold accumulation of trehalose, a non-reducing disaccharide (a dimer of glucose) commonly found in many anhydrobiotes (Watanabe 2006). Based on in vitro studies, this sugar was suggested to protect membranes against desiccation by forming a hydrogen bond network with phospholipids, thus imitating the hydration effect of water (water replacement hypothesis), or by forming an intracellular glass to immobilize macromolecules (vitrification theory) (Crowe 2002).
We asked whether the accumulation of trehalose in C. elegans has implications for desiccation tolerance. Worms synthesize trehalose from glucose 6-phosphate and UDP-glucose in a two-step reaction (Behm 1997). C. elegans genome encodes two genes (tps-1 and tps-2) for the enzyme trehalose 6-phosphate synthase (TPS), which catalyzes the first step of this reaction. Mutants lacking both of these genes can synthesize neither trehalose nor trehalose-based glycolipids (Penkov et al. 2010). Furthermore, these mutants cannot survive desiccation below 60 % RH even after preconditioning (Erkut et al. 2011). Thus, our data clearly show that trehalose is an essential molecule for C. elegans during anhydrobiosis. Recently, trehalose was demonstrated to be an important factor for desiccation tolerance in the mosquito Anopheles gambiae and the budding yeast Saccharomyces cerevisiae, as well (Liu et al. 2013; Tapia and Koshland 2014).
How does trehalose exert its function? Desiccated trehalose-deficient mutants exhibit substantial membrane damage upon rehydration (Erkut et al. 2011), which agrees with the proposed roles of trehalose in protecting phospholipids against desiccation-induced physical changes (Crowe 2002). However, as mentioned above, this function of trehalose has been studied only in vitro, using model lipids with predetermined compositions. It was unclear how the native phospholipids of an anhydrobiotic organism would respond to trehalose upon desiccation. We addressed this problem using infrared spectroscopy on preconditioned dauer larvae, taking advantage of the TPS knockout strain. Our data suggest that desiccation disturbs the lipidic order of C. elegans membranes in two major ways: it reduces lipid-packing density (i.e., gain of volume by acyl chains) and causes lipid chain packing heterogeneity (i.e., lipids are packed differently along the membrane) (Erkut et al. 2011, 2012). Although these perturbations occur already in the desiccated state, no membrane damage is manifested until fast influx of water (rehydration). Therefore, we also studied the effects of rehydration on C. elegans lipids in vitro.
Preconditioning induces phospholipid remodeling by specifically depleting phosphatidylcholine (PC) without affecting the levels of phosphatidylethanolamine (PE) or other minor phospholipids (Abusharkh et al. 2014). This modification increases the trehalose affinity of phospholipids. It also allows trehalose to cover a larger area and intercalate deeper into the sub-headgroup region of the membrane, all favoring water replacement hypothesis. Thus, phospholipid remodeling and trehalose biosynthesis act synergistically during preconditioning (Abusharkh et al. 2014).
As mentioned above, desiccation induces acyl chain disorder in membranes. If the native lipid packing of membranes is not regained quickly during rehydration, membranes are damaged. Our data indicate uniquely that preconditioned phospholipids, in cooperation with trehalose, can be reordered much more quickly during fast rehydration (Abusharkh et al. 2014). The reordering is so fast that it happens even before water replaces trehalose in higher hydration levels. This appears to be the core membrane-protective mechanism of this sugar in C. elegans anhydrobiosis.
Trehalose accumulation and membrane remodeling are clearly not the only mechanisms involved in desiccation tolerance. To have a more complete understanding of the anhydrobiotic strategies of the dauer larva, we surveyed the changes in its transcriptome and proteome during preconditioning (Erkut et al. 2013). Due to the high genetic redundancy of C. elegans, we found it more efficient to focus on biochemical pathways rather than individual genes and proteins involved in them.
Our data show that several pathways might be involved in desiccation tolerance. To validate some of these pathways functionally, we measured the desiccation tolerance levels of selected mutants from each pathway. We unambiguously show that heat-shock and intrinsically disordered protein (IDP) expression, reactive oxygen species (ROS) and xenobiotic detoxification mechanisms are involved in anhydrobiosis. All these agree with previously described findings. Furthermore, we found evidence for novel anhydrobiotic pathways such as increased glyoxalase activity as well as polyamine and polyunsaturated fatty acid (PUFA) biosynthesis (Erkut et al. 2013).
Desiccation affects protein structure, which is an obstacle that anhydrobiotic organisms have to overcome (Prestrelski et al. 1993). Although trehalose can protect proteins against desiccation-induced protein aggregation (Tapia and Koshland 2014), more prominent factors are thought to be HSPs and IDPs (Tompa and Kovacs 2010; Hand et al. 2011). The most commonly known IDPs are late embryogenesis abundant (LEA) proteins. These proteins were first identified in plants, and then found in the anhydrobiotic nematode Aphelenchus avenae (Browne et al. 2002). Some domains of LEA proteins can transform from coiled coils into α helices as the surrounding water is removed (i.e., they are dried). This peculiar characteristic of LEA proteins makes them perfectly suited molecular chaperones in the desiccated state, where HSPs are also affected by reduced water content (Hand et al. 2011). Previously, it was shown that desiccation stress induced lea-1 transcription in the C. elegans dauer larva (Gal et al. 2004). In the same study, it was also suggested that silencing lea-1 gene could cause sensitivity to mild desiccation stress. Our results elaborate these findings by showing that lea-1 gene is upregulated both at the mRNA and protein levels during desiccation. However, silencing lea-1 gene sensitizes the worm against harsh desiccation only. Thus, LEA-1 activity in C. elegans is required only in case of extensive water loss. In a similar study, it was shown that LEA1 RNAi dramatically reduced the desiccation and freezing tolerance of the anhydrobiotic cysts of the brine shrimp Artemia franciscana (Toxopeus et al. 2014). Furthermore, some anhydrobiotic organisms (e.g., bdelloid rotifers), which cannot synthesize trehalose, rely solely on LEA proteins for desiccation tolerance (Tunnacliffe et al. 2005).
Often, oxidative stress accompanies dehydration (França et al. 2007). Anhydrobiotic organisms have various mechanisms to detoxify ROS. Our results indicate that C. elegans uses cytoplasmic superoxide dismutases to eliminate superoxide, and glutathione peroxidases as well as catalases to convert peroxide into water (Erkut et al. 2013).
Caenorhabditis elegans also elevates glyoxalases during preconditioning. These enzymes convert toxic 2-oxoaldehydes glyoxal and methylglyoxal, which arise as by-products of lipid oxidation and glycolysis, into harmless carboxylic acids glycolate and lactate, respectively (Lee et al. 2012; Sousa Silva et al. 2013). We showed that the end-products of glyoxalases are required for the maintenance of the mitochondrial membrane potential in worms after desiccation and rehydration (Toyoda et al. 2014). Recently, it was also shown that in Panagrolaimus superbus, another anhydrobiotic nematode, glyoxalase serves as a scavenger for the ROS hydrogen peroxide that form during desiccation (Culleton et al. 2015). These results altogether suggest that glyoxalases may serve multiple purposes during desiccation.
Is trehalose the only small metabolite involved in anhydrobiosis? Probably it is not. Polyamines such as putrescine and spermidine may also be essential for desiccation tolerance, because mutations in polyamine biosynthetic pathway result in strong desiccation sensitivity (Erkut et al. 2013). However, the molecular function of polyamines in anhydrobiosis remains unknown.
Caenorhabditis elegans can synthesize many species of PUFAs de novo (Watts 2009). These PUFAs can become part of phospholipids, and thereby fine-tune the physicochemical properties of membranes. Our results indicated an overall induction of the PUFA biosynthetic pathway, probably for the production of arachidonic acid. Mutants unable to synthesize this particular PUFA are extremely sensitive to desiccation stress (Erkut et al. 2013).
Another function of PUFAs is to act as signaling molecules on vanilloid-type transient receptor potential (TRPV) channels, which are known to be responsible for osmosensation in C. elegans (Kahn-Kirby and Bargmann 2006). Therefore, PUFAs may additionally be involved in sensing the desiccative environment (hygrosensation). Although C. elegans hygrosensation remains mostly elusive, we have indications that it may be coordinated by head neurons located on a sensory region called amphid, and bear similarities to osmosensation (Erkut et al. 2013).
Concluding remarks
The metabolically depressed stress-resistant dauer larva of the nematode C. elegans can withstand complete desiccation possibly by entering an ametabolic state. It serves as an excellent model to study the molecular mechanisms of this adaptation, thanks to many powerful genetic and biochemical tools available for this organism. Moreover, studying ametabolism in a hypometabolic state (the dauer larva) brings its own advantages and therefore C. elegans may answer several essential questions: How are macromolecules such as lipids, nucleic acids and proteins, preserved in the dry state? Is the metabolism entirely turned off during desiccation or is there still some energy requirement to survive in the long term? What is the sequence of events that take place during entry and exit from ametabolism? And more importantly, what maintains the boundary between life and death in an ametabolic state? Finding answers to these questions will not only extend our understanding of life, but also lay the foundation for advanced technologies in the future.
Author contribution statement
Both authors wrote the manuscript.
Abbreviations
- Cas:
-
CRISPR-associated
- CRISPR:
-
Clustered, regularly interspaced, short palindromic repeats
- dsRNA:
-
Double-stranded RNA
- GFP:
-
Green fluorescent protein
- HSP:
-
Heat-shock protein
- IDP:
-
Intrinsically disordered protein
- LEA:
-
Late embryogenesis abundant
- PC:
-
Phosphatidylcholine
- PE:
-
Phosphatidylethanolamine
- PUFA:
-
Polyunsaturated fatty acid
- RH:
-
Relative humidity
- RNAi:
-
RNA interference
- ROS:
-
Reactive oxygen species
- TGF-β:
-
Transforming growth factor beta
- TPS:
-
Trehalose 6-phosphate synthase
- TRPV:
-
Transient receptor potential, vanilloid type
References
Abusharkh SE, Erkut C, Oertel J et al (2014) The role of phospholipid headgroup composition and trehalose in the desiccation tolerance of Caenorhabditis elegans. Langmuir 30:12897–12906. doi:10.1021/la502654j
Altun ZF, Hall HD (2009) Introduction. WormAtlas. doi:10.3908/wormatlas.1.1
Baugh LR (2013) To grow or not to grow: nutritional control of development during Caenorhabditis elegans L1 arrest. Genetics 194:539–555. doi:10.1534/genetics.113.150847
Begasse ML, Leaver M, Vazquez F et al (2015) Temperature dependence of cell division timing accounts for a shift in the thermal limits of C. elegans and C. briggsae. Cell Rep 10:647–653. doi:10.1016/j.celrep.2015.01.006
Behm CA (1997) The role of trehalose in the physiology of nematodes. Int J Parasitol 27:215–229. doi:10.1016/S0020-7519(96)00151-8
Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77:71–94
Browne J, Tunnacliffe A, Burnell A (2002) Anhydrobiosis: plant desiccation gene found in a nematode. Nature 416:38. doi:10.1038/416038a
Burnell AM, Houthoofd K, O’Hanlon K, Vanfleteren JR (2005) Alternate metabolism during the dauer stage of the nematode Caenorhabditis elegans. Exp Gerontol 40:850–856. doi:10.1016/j.exger.2005.09.006
Byerly L, Cassada RC, Russell RL (1976) The life cycle of the nematode Caenorhabditis elegans. I. Wild type growth and reproduction. Dev Biol 51:23–33
Cassada RC, Russell RL (1975) The dauerlarva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev Biol 46:326–342. doi:10.1016/0012-1606(75)90109-8
Clegg JS, Drinkwater LE, Sorgeloos P (1996) The metabolic status of diapause embryos of Artemia franciscana (SFB). Physiol Zoology 69:49–66
Cox GN, Staprans S, Edgar RS (1981) The cuticle of Caenorhabditis elegans. Dev Biol 86:456–470. doi:10.1016/0012-1606(81)90204-9
Crowe LM (2002) Lessons from nature: the role of sugars in anhydrobiosis. Comp Biochem Physiol B: Biochem Mol Biol 131:505–513. doi:10.1016/S1095-6433(01)00503-7
Culleton BA, Lall P, Kinsella GK et al (2015) A role for the Parkinson’s disease protein DJ-1 as a chaperone and antioxidant in the anhydrobiotic nematode Panagrolaimus superbus. Cell Stress Chaperones 20:121–137. doi:10.1007/s12192-014-0531-6
Doitsidou M, Poole RJ, Sarin S et al (2010) C. elegans mutant identification with a one-step whole-genome-sequencing and SNP mapping strategy. PLoS ONE 5:e15435. doi:10.1371/journal.pone.0015435
Erkut C, Penkov S, Khesbak H et al (2011) Trehalose renders the dauer larva of Caenorhabditis elegans resistant to extreme desiccation. Curr Biol 21:1331–1336. doi:10.1016/j.cub.2011.06.064
Erkut C, Penkov S, Fahmy K, Kurzchalia TV (2012) How worms survive desiccation: trehalose pro water. Worm 1:61–65. doi:10.4161/worm.19040
Erkut C, Vasilj A, Boland S et al (2013) Molecular strategies of the Caenorhabditis elegans dauer larva to survive extreme desiccation. PLoS ONE 8:e82473. doi:10.1371/journal.pone.0082473
Félix M-A, Braendle C (2010) The natural history of Caenorhabditis elegans. Curr Biol 20:R965–R969. doi:10.1016/j.cub.2010.09.050
Fielenbach N, Antebi A (2008) C. elegans dauer formation and the molecular basis of plasticity. Genes Dev 22:2149–2165. doi:10.1101/gad.1701508
Fire A, Xu S, Montgomery MK et al (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391:806–811. doi:10.1038/35888
França MB, Panek AD, Eleutherio ECA (2007) Oxidative stress and its effects during dehydration. Comp Biochem Physiol B: Biochem Mol Biol 146:621–631. doi:10.1016/j.cbpa.2006.02.030
Friedland AE, Tzur YB, Esvelt KM et al (2013) Heritable genome editing in C. elegans via a CRISPR-Cas9 system. Nat Methods 10:741–743. doi:10.1038/nmeth.2532
Gal TZ, Glazer I, Koltai H (2004) An LEA group 3 family member is involved in survival of C. elegans during exposure to stress. FEBS Lett 577:21–26. doi:10.1016/j.febslet.2004.09.049
Hand SC, Menze MA, Toner M et al (2011) LEA proteins during water stress: not just for plants anymore. Annu Rev Physiol 73:115–134. doi:10.1146/annurev-physiol-012110-142203
Holt SJ, Riddle DL (2003) SAGE surveys C. elegans carbohydrate metabolism: evidence for an anaerobic shift in the long-lived dauer larva. Mech Ageing Dev 124:779–800. doi:10.1016/S0047-6374(03)00132-5
Houthoofd K, Braeckman BP, Lenaerts I et al (2002) Ageing is reversed, and metabolism is reset to young levels in recovering dauer larvae of C. elegans. Exp Gerontol 37:1015–1021. doi:10.1016/S0531-5565(02)00063-3
Jorgensen EM, Mango SE (2002) The art and design of genetic screens: Caenorhabditis elegans. Nat Rev Genet 3:356–369. doi:10.1038/nrg794
Kahn-Kirby AH, Bargmann CI (2006) TRP channels in C. elegans. Annu Rev Physiol 68:719–736. doi:10.1146/annurev.physiol.68.040204.100715
Keilin D (1959) The Leeuwenhoek Lecture: the problem of anabiosis or latent life: history and current concept. Proc Roy Soc B: Biol Sci 150:149–191. doi:10.1098/rspb.1959.0013
Klass M, Hirsh D (1976) Non-ageing developmental variant of Caenorhabditis elegans. Nature 260:523–525. doi:10.1038/260523a0
Lant B, Storey KB (2010) An overview of stress response and hypometabolic strategies in Caenorhabditis elegans: conserved and contrasting signals with the mammalian system. Int J Biol Sci 6:9–50. doi:10.7150/ijbs.6.9
Lee JY, Song J, Kwon K et al (2012) Human DJ-1 and its homologs are novel glyoxalases. Human Mol Genetics 21:3215–3225. doi:10.1093/hmg/dds155
Liu K, Dong Y, Huang Y et al (2013) Impact of trehalose transporter knockdown on Anopheles gambiae stress adaptation and susceptibility to Plasmodium falciparum infection. Proc Natl Acad Sci USA 110:17504–17509. doi:10.1073/pnas.1316709110
Maupas E (1901) Modes et formes de reproduction des nematodes. doi:10.1234/12345678
O’Riordan VB, Burnell AM (1989) Intermediary metabolism in the dauer larva of the nematode Caenorhabditis elegans—1. Glycolysis, gluconeogenesis, oxidative phosphorylation and the tricarboxylic acid cycle. Comp Biochem Phys B-Biochem Mol Biol 92:233–238. doi:10.1016/0305-0491(89)90271-X
O’Riordan VB, Burnell AM (1990) Intermediary metabolism in the dauer larva of the nematode Caenorhabditis elegans—II. The glyoxylate cycle and fatty-acid oxidation. Comp Biochem Phys B-Biochem Mol Biol 95:125–130
Patil YN, Marden B, Brand MD, Hand SC (2013) Metabolic downregulation and inhibition of carbohydrate catabolism during diapause in embryos of Artemia franciscana. Physiol Biochem Zool 86:106–118. doi:10.1086/667808
Penkov S, Mende F, Zagoriy V et al (2010) Maradolipids: diacyltrehalose glycolipids specific to dauer larva in Caenorhabditis elegans. Angew Chem Int Ed Engl 49:9430–9435. doi:10.1002/anie.201004466
Prestrelski SJ, Tedeschi N, Arakawa T, Carpenter JF (1993) Dehydration-induced conformational transitions in proteins and their inhibition by stabilizers. Biophys J 65:661–671. doi:10.1016/S0006-3495(93)81120-2
Riddle DL, Swanson MM, Albert PS (1981) Interacting genes in nematode dauer larva formation. Nature 290:668–671. doi:10.1038/290668a0
Sarov M, Schneider S, Pozniakovski A et al (2006) A recombineering pipeline for functional genomics applied to Caenorhabditis elegans. Nat Methods 3:839–844. doi:10.1038/nmeth933
Sarov M, Murray JI, Schanze K et al (2012) A genome-scale resource for in vivo tag-based protein function exploration in C. elegans. Cell 150:855–866. doi:10.1016/j.cell.2012.08.001
Sousa Silva M, Gomes RA, Ferreira AEN et al (2013) The glyoxalase pathway: the first hundred years… and beyond. Biochem J 453:1–15. doi:10.1042/BJ20121743
Sulston JE, Horvitz HR (1977) Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol 56:110–156
Tabara H, Grishok A, Mello CC (1998) RNAi in C. elegans: soaking in the genome sequence. Science 282:430–431
Tapia H, Koshland DE (2014) Trehalose is a versatile and long-lived chaperone for desiccation tolerance. Curr Biol 24:2758–2766. doi:10.1016/j.cub.2014.10.005
The C. elegans Sequencing Consortium (1998) Genome sequence of the nematode C. elegans: a platform for investigating biology. Science 282:2012–2018
Timmons L, Fire A (1998) Specific interference by ingested dsRNA. Nature 395:854. doi:10.1038/27579
Tompa P, Kovacs D (2010) Intrinsically disordered chaperones in plants and animals. Biochem Cell Biol 88:167–174. doi:10.1139/O09-163
Toxopeus J, Warner AH, MacRae TH (2014) Group 1 LEA proteins contribute to the desiccation and freeze tolerance of Artemia franciscana embryos during diapause. Cell Stress Chaperones 19:939–948. doi:10.1007/s12192-014-0518-3
Toyoda Y, Erkut C, Pan-Montojo F et al (2014) Products of the Parkinson’s disease-related glyoxalase DJ-1, D-lactate and glycolate, support mitochondrial membrane potential and neuronal survival. Biol Open 3:777–784. doi:10.1242/bio.20149399
Tunnacliffe A, Lapinski J, McGee B (2005) A putative LEA protein, but no trehalose, is present in anhydrobiotic bdelloid rotifers. Hydrobiologia 546:315–321. doi:10.1007/s10750-005-4239-6
Vanfleteren JR, DeVreese A (1996) Rate of aerobic metabolism and superoxide production rate potential in the nematode Caenorhabditis elegans. J Exp Zool 274:93–100. doi:10.1002/(SICI)1097-010X(19960201)274:2<93:AID-JEZ2>3.0.CO;2-8
Watanabe M (2006) Anhydrobiosis in invertebrates. Appl Entomol Zool 41:15–31. doi:10.1303/aez.2006.15
Watts JL (2009) Fat synthesis and adiposity regulation in Caenorhabditis elegans. Trends Endocrin Met 20:58–65. doi:10.1016/j.tem.2008.11.002
Wiedenheft B, Sternberg SH, Doudna JA (2012) RNA-guided genetic silencing systems in bacteria and archaea. Nature 482:331–338. doi:10.1038/nature10886
Womersley C (1987) A reevaluation of strategies employed by nematode anhydrobiotes in relation to their natural environment. In: Veech JA, Dickson DW (eds) Vistas on nematology. Society of Nematologists Inc, Hyattsville, pp 165–173
Yook K, Harris TW, Bieri T et al (2012) WormBase 2012: more genomes, more data, new website. Nucleic Acids Res 40:D735–D741. doi:10.1093/nar/gkr954
Author information
Authors and Affiliations
Corresponding author
Additional information
Special topic: Desiccation Biology. Guest editors: Olivier Leprince and Julia Buitink.
Rights and permissions
About this article
Cite this article
Erkut, C., Kurzchalia, T.V. The C. elegans dauer larva as a paradigm to study metabolic suppression and desiccation tolerance. Planta 242, 389–396 (2015). https://doi.org/10.1007/s00425-015-2300-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-015-2300-x