Abstract
The connection between classical phytohormone-ethylene and two signaling molecules, nitric oxide (NO) and hydrogen cyanide (HCN), was investigated in dormancy removal and germination “sensu stricto” of apple (Malus domestica Borkh.) embryos. Deep dormancy of apple embryos was removed by short-term (3–6 h) pre-treatment with NO or HCN. NO- or HCN-mediated stimulation of germination was associated with enhanced emission of ethylene by the embryos, coupled with transient increase in ROS concentration in embryos. Ethylene vapors stimulated germination of dormant apple embryos and eliminated morphological anomalies characteristic for young seedlings developed from dormant embryos. Inhibitors of ethylene receptors completely impeded beneficial effect of NO and HCN on embryo germination. NO- and HCN-induced ethylene emission by apple embryo was only slightly reduced by inhibitor of 1-aminocyclopropane-1-carboxylic acid (ACC) oxidase activity during first 4 days of germination. Short-term pre-treatment of the embryos with NO and HCN modified activity of both key enzymes of ethylene biosynthetic pathway: ACC synthase and ACC oxidase. Activity of ACC synthase declined during first 4 days of germination, while activity of ACC oxidase increased markedly at that time. Additional experiments point to non-enzymatic conversion of ACC to ethylene in the presence of ROS (H2O2). The results indicate that NO and HCN may alleviate dormancy of apple embryos “via” transient accumulation of ROS, leading to enhanced ethylene emission which is required to terminate germination “sensu stricto”. Therefore, ethylene seems to be a trigger factor in control of apple embryo dormancy removal and germination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ethylene is an important phytohormone involved in many processes in plants, such as senescence, ripening, reaction to various stresses (Hall et al. 2007; Pierik et al. 2007). It is commonly accepted that ethylene plays a crucial role in the control of seed dormancy removal and early germination “sensu stricto” (Kucera et al. 2005; Matilla and Matilla-Vazquez 2008). Germination “sensu stricto” may be defined as the process associated with the initiation and completion of embryo emergence; it refers to the progress of a seed from imbibition through radicle emergence (Bewley and Black 1978; Nonogaki et al. 2007). Ethylene production during dormancy release and germination was reported for seeds of various plants: clover (Trifolium subterraneum) (Esashi and Leopold 1969), cocklebur (Xantium pennsylvanicum) (Katoh and Esashi 1975), peanut (Arachis hypogaea) (Ketring and Morgan 1971), redroot pigweed (Amaranthus retroflexus) (Kępczyński et al. 1996), apple (Malus domestica) (Kępczyński et al. 1977), and sunflower (Helianthus annuus) (Corbineau et al. 1990) (review Kępczyński and Kępczyńska 1997; Matilla and Matilla-Vazquez 2008). Ethylene biosynthesis is also an important regulatory mechanism during the breaking of dormancy and germination in beech (Fagus sylvatica) seeds (Calvo et al. 2004a, b). On the other hand, it has been determined that ethylene is not required for germination of some seeds e.g., lettuce (Lactuca sativa) and cocklebur even though they produce this hormone (Abeles and Lonski 1969; Satoh and Esashi 1984; Kępczyński and Karssen 1985). In some seeds, e.g., tomato (Lycopersicon esculentum), red rice (Oryza punctata), ethylene is rather involved in the events leading to induction of seedling growth than in the action linked to germination “sensu stricto” (Kępczyński and Kępczyńska 1997; Matilla and Matilla-Vazquez 2008). However, Machabee and Saini (1991) suggested that the transition from dormancy to germination of chickpea seeds may involve two steps: an ethylene requiring transition to the non-dormant state, followed by germination that does not have an absolute requirement for ethylene.
In general, the amount of ethylene evolution is positively correlated with seed vigor (Kucera et al. 2005) and is proposed as a marker of seed quality (Khan 1994; Chojnowski et al. 1997). Unfavourable conditions, e.g., high temperature, during imbibition can provoke inhibition of seed germination and ethylene plays a part in the elimination of this effect (Matilla 2000; Matilla and Matilla-Vazquez 2008). The participation of key enzymes, 1-aminocyclopropane-1-carboxylic acid synthase (ACS) and/or oxidase (ACO), involved in ethylene biosynthetic pathway has been found in many germinating seeds (Matilla and Matilla-Vazquez 2008). In chickpea (Cicer arietinum) seeds the maximum ACO activity reaches in the early phase of germination (Gomez-Jimenez et al. 1998, 2001).
It has been reported recently that nitric oxide (NO) induces dormancy breakage and stimulates germination of apple embryos by induction of ethylene biosynthesis (Gniazdowska et al. 2007). This effect is comparable with response of apple embryos observed after short-term pre-treatment with cyanide (HCN) (Bogatek et al. 1991; Bogatek and Gniazdowska 2006). NO and HCN are also known to stimulate germination of seeds of other plant species, e.g., lettuce (Zagórski and Lewak 1985), Arabidopsis (reviewed by Bethke et al. 2007), switchgrass (Panicum virgatum) (Sarath et al. 2006). The emission of HCN has been detected during the pre-germination period of many seeds, including those not containing any cyanogenic glycosides (Esashi et al. 1991; reviewed by Siegień and Bogatek 2006). The role of NO as a regulatory factor of seed germination is also emphasized by its emission during early germination of seeds (Simontacchi et al. 2004; Sarath et al. 2006; Borysjuk et al. 2007). Moreover, the mode of action of both molecules in seed germination depends on transient accumulation of reactive oxygen species (ROS) mainly in the embryonic axis, taking place in the early phases of germination (Oracz et al. 2007, 2009; Gniazdowska et al. 2010).
While the occurrence of ethylene in regulation of seed germination seems to be evident, it is still controversial whether ethylene is required for seed transition from dormancy to germination or it is just the result (co-product) of dormancy alleviation and germination persistence.
The aim of our work was to investigate the influence of dormancy removing factors, NO and HCN, on the pathway of ethylene biosynthesis during “sensu stricto” germination of apple embryos. In order to demonstrate the involvement of ethylene in NO- and HCN-mediated germination of apple embryos, we examined the activity of key enzymes of ethylene biosynthesis pathway. We also report that the apple embryo dormancy removal by NO and HCN probably depends partly on non-enzymatic conversion of ethylene precursor (ACC) to ethylene occurring in the early phase of germination, characterized by enhanced accumulation of ROS, mainly H2O2. Moreover, our results posit that ethylene emission is not only the result of germination (radical protrusion), but also that phytohormone is required for seed transition from dormancy to germination.
Materials and methods
Plant material
The experiments were carried out on apple (M. domestica Borkh., cv. Antonówka) seeds harvested in 2006–2008. Apples were provided by Kordel fruit producer at Tarczyn (Poland). Seeds were isolated from fruits at harvest time and stored in dark glass containers at 5°C. The seed coat and endosperm were removed from seeds after 24-h imbibition in distilled water at room temperature (20°C). The isolated embryos were taken for determination.
Germination tests
Embryos isolated from dormant seeds were exposed to 1 mM gaseous HCN for 6 h in the light as described by Bogatek et al. (1991). The influence of NO on embryo germination was determined using: sodium nitroprusside (SNP) (5 mM for 3 h) (Gniazdowska et al. 2007), and vapors of acidified nitrite (3 h), in light. Acidified nitrite was prepared using 20 mM sodium nitrite (NaNO2) and 0.1 M HCl as described previously by Gniazdowska et al. (2010). After those treatments, embryos were rinsed in distilled water and placed in 9 cm glass Petri dishes containing filter paper wetted with water. Control (non-treated) and NO, SNP, HCN pre-treated embryos germinated on Petri dishes (20 embryos per dish) at 25°C with 12/12 h (light/dark) photoperiod, under 150 μmol PAR m−2 s−1.
Additional experiments were carried out with ethylene donor: ethephon (2-chloro-ethylphosphonic acid) (0.5, 1.0, 3.0 mM) and ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC) (0.5, 1.0, 3.0 mM). Dormant embryos were fumigated with vapors of ethephon or germinated in water solution of ACC, as described by Gniazdowska et al. (2010). Complementary tests were done with ethephon vapors and 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO) used as a scavenger of NO. Dormant embryos were placed in a closed glass container (20 embryos per container) on filter paper wetted with cPTIO (0.3 mM) and exposed to vapors of ethephon (1 mM) for 4 days. To check the embryo sensitivity to ethylene, control (non-treated) and NO and HCN pre-treated embryos were continuously exposed to ethephon vapors in various concentrations (0.1–3.0 mM) and germinated for 4 days. On each day of the experiment, a fresh solution of tested chemicals (ethephon, ACC, cPTIO) were applied. To analyse sensitivity of embryos to ethylene, typical anomalies of seedlings developed from dormant embryos (asymmetric growth and greening of cotyledons) were counted.
To test embryo sensitivity to abscisic acid (ABA), control and NO or HCN pre-treated embryos were placed in Petri dishes containing filter paper wetted with ABA water solution at various concentration (0.1, 0.3, 1.0, 3.0 μM) and cultured for 4 days. ABA solution was exchanged every 2 days for the fresh one. To test the interaction between NO and HCN pre-treatment of the embryo and ABA, accompanying experiment was performed with fluridone—inhibitor of ABA biosynthesis. Control and pre-treated embryos were germinated in the presence of 5 μM fluridone for 4 days.
Supplementary germination experiments were carried out in the presence of 2,5-norbornadien (NBD). Control (non-treated) or pre-treated embryos were placed in a closed glass container (20 embryos per container) on the filter paper wetted with distilled water and exposed to vapors of NBD (50 μl of NBD per 500 ml per 24 h) for 4 days. Each day of the experiment, fresh volume of liquid NBD (50 μl) was added 15 min after the old NBD had been removed. Additional experiment was conducted with silver thiosulfate (STS) used as an inhibitor of ethylene receptors. Embryos (non-treated and pre-treated) germinated continuously in the solution containing 1 mM STS. For an experiment with aminoethoxyvinylglycine (AVG) inhibitor of ACS activity, control (non-treated) as well as NO, SNP and HCN pre-treated apple embryos were placed for 4 days in Petri dishes containing 1 mM AVG. Furthermore, to check the influence of inhibition of ACO activity on embryo germination control or NO, SNP and HCN pre-treated embryos were cultured for 4 days in 1 mM α-amino-isobutyric acid (Aib). On each day of the experiment, a fresh solution of tested chemicals (STS, AVG, Aib) was used.
Embryos were considered to have germinated when radicle were 2–3 mm long and exhibited characteristic geotropic bending. Germination of the embryos was counted for 4 days after sowing. All germination tests were done in three to five independent experiments, with three repetitions in each.
Determination of ethylene emission by embryos
Isolated embryos (control or pre-treated with SNP, NO and HCN) (0.5 g) were transferred to 8 ml glass vials containing 0.5 ml distilled water. After 30 min of preincubation at 30°C in darkness, vials were tightly closed and incubated for 1 h at 30°C in darkness. One milliliter of the headspace gas sample was withdrawn and injected into gas chromatograph Agilent 6890 (Hewlett Packard, Agilent, Santa Clara, CA, USA) equipped with a flame ionization detector and a stainless steel column (3 m × 3.5 mm) packed with Poropack Q (as described by Gniazdowska et al. 2007). In addition, ethylene emission by embryos in the presence of 1 mM Aib (inhibitor of ACO activity) was detected. Experiments were conducted 2 and 4 days after embryo sowing and repeated five times in three independent tests.
Quantification of ACC (1-aminocyclopropane-1-carboxylic acid)
Isolated embryos (control or pre-treated with NO, SNP or HCN) (0.5 g) were homogenized in 2 ml 80% (v/v) ethanol. The extracts were centrifuged at 12,000g for 15 min at 4°C using High Speed Centrifuge MPW-350R (Warsaw, Poland) and ethanol was fully removed. Residues were dissolved in distilled water and ACC content was determined by chemical conversion to ethylene using Lizada and Yang (1979) protocol. After 3 min of incubation at room temperature, ethylene evolution from ACC was measured using an Agilent 6890 gas chromatograph, as described above. The ACC conversion efficiency was determined using ACC (Sigma-Aldrich) as a standard. Determination was done for isolated embryos (just after seed coat removal, before pre-treatment) and 1 and 4 days after sowing. Presented data are average of five repetitions obtained in three independent experiments.
Determination of ACC synthase and ACC oxidase activities
ACC synthase (EC 4.4.1.14) activity was measured according to Yip et al. (1991) with some modifications. Apple embryos (control and pre-treated) (0.5 g) were homogenized in 4 ml homogenization buffer 0.1 M HEPES–KOH pH 8.5, 5 mM dithiothreitol (DTT), 0.01 mM phenylmethanesulfonyl fluoride (PMSF), 10 μM pyridoxal phosphate (PLP), 2% (w/v) polyvinylpolypyrrolidone (PVPP) in ice bath. Obtained homogenate was mixed on Vortex (5 s), and centrifuged (15,000g for 15 min, 4°C using High Speed Centrifuge MPW-350R, Poland). The supernatant was filtered and used in further determinations. Crude enzymatic extract (0.4 ml) was incubated in airtight glass flasks with 0.6 ml reaction buffer: 0.1 M HEPES–KOH pH 8.5, 10 μM PLP, 200 μM S-adenosyl methionine (SAM) at 37°C for 1 h. The reaction was stopped by adding 0.2 ml 10 mM HgCl2. The product of the reaction (ACC) was determined as ethylene emission according to Lizada and Yang (1979) protocol. The ACS activity was expressed as the amount of ethylene produced (nl) per mg protein per hour.
ACC oxidase (E.C. 1.14.17.4) was extracted from the embryo according to Mathooko et al. (1993) with some modifications. Plant material (0.5 g) was homogenized in 4 ml of extraction buffer (0.1 M Tris–HCl pH 7.2, 10% (v/v) glycerol, 4% (v/v) Triton X-100, 5 mM DTT, 30 mM Na-ascorbate, 0.15 mM PMSF) in ice bath. After centrifugation (15,000g for 15 min at 4°C), the obtained supernatant was filtered and 0.5 ml (crude enzymatic extract) was added to 1.5 ml of incubation buffer (0.1 M Tris–HCl pH 7.2, 2 mM ACC, 1 mM DTT, 30 mM NaHCO3, 30 mM Na-ascorbate, 20 μM FeSO4) into the reaction glass flasks. The ACO was assayed using gas chromatography to measure ethylene produced after incubation (1 h at 30°C). The ACO activity was expressed as the amount of ethylene produced (nl) per mg protein per hour. Ethylene evolution was detected by gas chromatograph Agilent 6890.
Presented data are average of four repetitions obtained in three independent experiments.
Protein was determined according to Bradford (1976) with BSA (Sigma-Aldrich) used as a standard.
Determination of ethylene production in non-enzymatic reaction
Non-enzymatic conversion of ACC to ethylene, without any plant tissue, was measured using 500 mM H2O2, 200 mM NaNO2, 350 mM KCN, 200 mM HCl, and increasing concentrations of ACC (50, 100, 200 μM). Into glass vials containing 0.5 ml 50, 100 or 200 μM ACC, 0.5 ml of 500 mM H2O2, 200 mM NaNO2, 350 mM KCN or 200 mM HCl were added. Vials were closed and incubated at 25°C for 20 h in darkness. After incubation, 1 ml of the headspace gas sample was withdrawn and injected into gas chromatograph Agilent 6890. Experiments were repeated five times in three independent tests.
Quantification of hydrogen peroxide (H2O2)
Isolated embryos were used for H2O2 determination according to Sergiev et al. (1997). Plant tissue (0.2 g) was homogenized with 0.1% (w/v) cold trichloroacetic acid (TCA) in ice. The obtained extract was centrifuged at 15,000g for 15 min at 4°C using High Speed Centrifuge MPW-350R. The supernatant (0.5 ml) was added to 0.5 ml 10 mM potassium phosphate buffer (pH 7.0) and freshly prepared 1.0 ml 1 M KI in 10 mM potassium phosphate buffer (pH 7.0). The H2O2 concentration was determined using Shimadzu UV 1700 spectrophotometer with absorption measured at 390 nm. H2O2 at concentration 10–100 μM was used to prepare a standard curve.
Results
NO and HCN stimulate germination and increase sensitivity to ethylene of dormant apple embryos
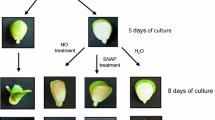
Dormant apple seeds were unable to germinate even in favorable conditions, 4 days after sowing only 2% germinated embryos were observed (Table 1). Nitric oxide and HCN removed dormancy of apple embryos and stimulated germination (Table 1). After 4 days of imbibition, dormant embryos (control) differed visually from non-dormant (pre-treated) ones, although both of them did not germinate. Pre-treated embryos started to be more green (light pistachio), their cotyledons did not lie tightly on each other and begun to half-open.
After 7 days of culture about 70% of NO, SNP or HCN pre-treated embryos germinated as it was described in our previous papers (Gniazdowska et al. 2007, 2010). At the same time, germination of control (non-treated) embryos was less than 10%. Moreover, seedlings developing from pre-treated embryos did not exhibit morphological anomalies (asymmetric growth and greening of cotyledons) typical for seedlings developed from dormant (non-treated) embryos as it was reported later (Gniazdowska et al. 2007, 2010).
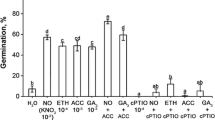
Ethylene precursor (ACC) stimulated germination of dormant embryos (Fig. 1). 7% of dormant embryos germinated after 4 days imbibitions in 0.5 mM ACC. ACC in higher concentration was less effective, embryos germinated in 4 or 3% in 1 mM or 3.0 mM ACC, respectively. The best effect of ethephon on germination of dormant apple embryos was detected in the presence of 1 mM ethephon vapors (5% of germination), while after treatment with 0.5 and 3 mM ethephon vapors, only slight stimulation was noticed (Fig. 1).
Germination of 4 days old control (non-treated) apple embryos imbibed continuously in ACC (0.5–3 mM) or exposed to ethephon vapors (0.5–3 mM). Germination of control embryos imbibed in water without ACC or ethephon vapors is as shown in Table 1. Values are mean ± SD of three replicates from three independent experiments of approx. 60 embryos each
Both inhibitors of ethylene receptors NBD and STS repressed germination of embryos pre-treated by NO, SNP or HCN. None of the embryos (control or pre-treated) fumigated with NBD germinated till the 4th day of experiment. STS was not only less effective than NBD, but also inhibited embryo germination (Table 1). Inhibitor of ACO activity (Aib) only slightly influenced germination of NO, SNP and HCN pre-treated embryos. Germination of control dormant embryos was arrested by Aib (Table 1). Inhibitor of ACS activity (AVG) totally blocked the germination of dormant (control) and pre-treated embryos (Table 1).
NO, SNP and HCN enhanced embryo sensitivity to ethephon (Table 2), defined as the number of the embryos without typical morphological anomalies described for dormant embryos (Bogatek et al. 1991; Gniazdowska et al. 2010).
NO and HCN lowered embryo sensitivity to exogenous ABA (Table 3) similarly as it was reported earlier for SNP (Gniazdowska et al. 2007). Germination of dormant (control) embryos was completely inhibited even by low concentration of ABA (0.1–0.3 μM). However, imbibition of NO or HCN pre-treated embryos in ABA decreased their germination in 50%. The higher concentration of ABA (1 or 3 μM) had to be used to inhibit totally germination of NO and HCN pre-treated embryos (Table 3).
Inhibition of ABA biosynthesis during imbibition in fluridone-stimulated germination of control and pre-treated embryos but the effect of fluridone was more pronounced for pre-treated embryos (Table 4; Fig. 2). All pre-treated embryos germinated after 4 days imbibition in fluridone solution (Table 4; Fig. 2).
Growth and development of apple seedlings. Photograph showing the differences in growth of seedlings developed from: control (non-treated) dormant embryos, embryos pre-treated with acidified nitrite (NO, 3 h), sodium nitroprusside (SNP, 5 mM, 3 h), hydrogen cyanide (HCN, 1 mM, 6 h) after 4 days culture in water or 5 μM fluridone
NO scavenger (cPTIO) inhibited completely germination of control (still dormant) embryos (Table 5). Moreover, the beneficial effect of ethephon vapors on embryo germination was reversed by cPTIO (Table 5).
NO and HCN modify ethylene emission by apple embryos
Neither ethylene emission by dormant control nor pre-treated embryos was detected 1 day after sowing. Ethylene was emitted by the embryos 24 h later, but only by the ones pre-treated with NO, SNP and HCN (Fig. 3). At that time, no ethylene emission by control (non-treated) embryos was observed (Fig. 3). NO, SNP and HCN enhanced ethylene emission 4 days after sowing, it was more than threefold higher than in the control embryos. The enhancement in ethylene emission was associated with stimulation of embryo germination by NO, SNP or HCN detected 4 days after treatment (Table 1). Ethylene emission by the embryos was only slightly influenced by ACO inhibitor—Aib in embryos pre-treated by NO, SNP or HCN. In NO pre-treated embryos, Aib had no effect on ethylene emission (Table 6). In SNP or HCN pre-treated embryos, inhibition of ethylene emission by Aib was less than 30% 2 days after sowing and around 20% 4 days after sowing (Table 6). To the contrary, Aib repressed ethylene emission in 4 days old control embryos in more than 60% (Table 6).
Ethylene emission by control (non-treated) apple embryos or embryos pre-treated with NO (20 mM NaNO2 + 100 mM HCl, 3 h), SNP (5 mM, 3 h) or HCN (1 mM, 6 h) 2 or 4 days after sowing. No ethylene emission by control, non-treated embryos was observed 2 days after sowing. Values are mean ± SD of five replicates from three independent experiments. Ethylene emission by embryos pre-treated by SNP was presented previously by Gniazdowska et al. (2007)
NO and HCN modify activity of ACS and ACO in apple embryos
ACS activity in control (non-treated) apple embryos was relatively low (less than 0.1 nl h−1 mg−1 protein), and decreased slightly during the culture (Fig. 4a). Four days after sowing ACS activity in control, still dormant embryos was only 0.05 nl h−1 mg−1 protein. ACS activity measured 24 h after NO, SNP and HCN embryo pre-treatment was twice higher in comparison to the control. In NO pre-treated embryos, it remained at constant level for next day, and dropped down 4 days after sowing. In SNP and HCN pre-treated embryos, ACS activity decreased continuously during the culture to the level detected in non-treated embryos (Fig. 4a).
ACO activity was low (less than 0.25 nl h−1 mg−1 protein) at the beginning of the experiment both in dormant and pre-treated embryos (Fig. 3b). It increased rapidly 4 days after sowing. At that time, ACO activity in NO, SNP and HCN pre-treated embryos was more than twice higher than that of one detected in control embryos (Fig. 4b).
ACC concentration in apple embryo was only slightly modified by NO or HCN pre-treatment ACC concentration in dormant apple embryos determined at the beginning of the experiment, just after removing of seed coat and before any treatment was high and amounted 4.6 μmol g−1 FW. ACC concentration both in control (non-treated) and pre-treated embryos declined during germination (Table 7). ACC concentration in control embryos detected 1 day after sowing was 3.5 μmol g−1 FW. It was almost constant during culture period and decreased only in 15% for next 3 days. ACC concentration in NO, SNP and HCN pre-treated embryos was lower (around 2.2 μmol g−1 FW) than in control, still dormant ones, and did not change significantly during the experiment (Table 7).
Pre-treatment with NO or HCN enhanced H2O2 concentration in apple embryos
H2O2 concentration in the tissue was determined 1 and 4 days after embryo treatment with HCN, SNP and NO (Table 7). The concentration of H2O2 in control apple embryos after 1 day of imbibition in water was more than twice lower than in all pre-treated embryos. Additional 3 days of culture did not change significantly H2O2 concentration in NO, SNP and HCN pre-treated embryos. In contrast, in control embryos, H2O2 concentration increased during that time to the level detected before for pre-treated embryos (Table 7).
Ethylene is produced in chemical reaction between ACC and ROS, NO or HCN
ACC was converted to ethylene in chemical reaction without any plant material. Ethylene was detected after incubation of ACC with 500 mM H2O2, 200 mM NaNO2, 350 mM KCN (Fig. 5) without any tissue. Ethylene emission raised with ACC concentration and was the highest in the presence of ROS (H2O2). No ethylene was detected in vials in which ACC was mixed with HCl, regardless of the ACC concentration.
Conversion of ACC (50, 100, 200 μM) to ethylene in the presence of ROS (500 mM H2O2), NO donor (200 mM NaNO2), HCN donor (350 mM KCN). No ethylene was detected in the mixture of ACC and 200 mM HCl, independently of the ACC concentration. Values are mean ± SD of five replicates from three independent experiments
Discussion
The involvement of ethylene in regulation of seed germination is widely described by many authors (for review, see Kępczyński and Kępczyńska 1997; Matilla 2000; Kucera et al. 2005). However, its role in the removal of dormancy and transduction to germination still remains controversial (Matilla 2000; Matilla and Matilla-Vazquez 2008). In our study, it has been demonstrated that the ethylene acts by strengthening the influence of NO and HCN pre-treatment during promotion of “sensu stricto” germination in apple embryos. We confirmed that both molecules NO and HCN stimulate embryo germination in the ethylene-dependent manner (Fig. 3). Ethylene vapors (released during ethephon decomposition) or ACC, the direct ethylene precursor alleviated deep embryonic dormancy and accelerated development of young apple seedlings (Fig. 1). Embryos fumigated with NO and HCN synthesized ethylene earlier than control (still dormant) ones. Ethylene was emitted by pre-treated embryos in the early phase of germination, just before initiation of radicle growth. Similar observation was reported for germinating Virginia-type peanut seeds (Ketring and Morgan 1971). Contribution of ethylene in regulation of early phases of germination in NO and HCN pre-treated apple embryos was also supported by the findings that NBD, a widely used inhibitor of ethylene receptors (Sisler and Serek 2003), totally repressed beneficial effect of NO or HCN (Table 1). Equivalent effect was detected in the presence of Ag+ ions released from STS (Table 1). Such limitation of seed germination by inhibitors can be partially abated by simultaneous treatment with ethylene or ACC (Leubner-Metzger et al. 1998; Kucera et al. 2005). Similarly, NBD suppressed HCN-induced germination of sunflower embryos (Oracz et al. 2008), or germination of barley grains and seedlings development (Locke et al. 2000). Experiments with inhibitors of ethylene receptors (Table 1) suggest that in apple embryos ethylene is compulsory for germination “sensu stricto”. In other words, pre-treated seeds, after dormancy breakage, are unable to germinate while ethylene receptors are blocked.
On the other hand, it has been suggested that ethylene is not able to break seed dormancy by itself only (Kucera et al. 2005). Treatment of apple embryos together with cPTIO (NO scavenger) and ethylene vapors decreased germination and seedling development (Table 5). It was also shown that cPTIO reduced the stimulatory effect of NO on apple embryo germination and seedling growth (Gniazdowska et al. 2007). Therefore, we propose the existence of cross-talk between NO and ethylene in regulation of dormancy breakage and germination. Moreover, NO donors and cyanide seem to increase tissue responsiveness to ethylene (Table 2). Not only ethylene biosynthesis, but also sensitivity of plant tissues to this hormone appears an important factor for germination of Arabidopsis seed (Beaudoin et al. 2000; Ghassemian et al. 2000; Kucera et al. 2005). It is suggested that in Arabidopsis, ethylene can possibly act not as a positive regulator of germination itself, but rather by interfering with ABA signaling and synthesis. Ketring and Morgan (1972) reported that ABA reduced ethylene production in peanut seeds and kept them in dormant state. We demonstrated in earlier research that short-term pre-treated of dormant apple embryos with SNP (most commonly used NO donor) lowers their sensitivity to ABA (Gniazdowska et al. 2007). Comparable results were obtained with acidified nitrite (NO) and HCN (Table 3). It should be emphasized that decreased sensitivity to ABA correlates well with increased sensitivity to ethylene in apple embryos shortly pre-treated with NO and HCN (Table 2). Pre-treatment of dormant embryos with HCN resulted in transient decrease in ABA concentration during first 3 days of germination, while in the next days of culture ABA level increased rapidly, as growth of embryos’ root was detected (Bogatek et al. 2003). It is possible that NO pre-treatment may also affect the embryo’s ABA level or, alternatively, ethylene synthesized in reaction to NO pre-treatment may counteract the ABA-induced inhibition of germination by interfering with ABA signaling. Such interaction between ethylene and ABA was suggested just recently by Linkies et al. (2009). In our experiment, apple embryos imbibed in ABA did not germinate, they were kept dormant, and ethylene emission by such embryos was undetectable (data not shown). On the other hand, fluridone which inhibits ABA biosynthesis during imbibition rapidly stimulated germination of both control and pre-treated embryos (Table 4; Fig. 2).
Seed germination not only depends on action of classical plant hormones, but also is regulated by signaling molecules, primary ROS. It has been shown that ROS accumulation is necessary for dormancy alleviation and seed germination of various plant species (Bailly et al. 2008; Oracz et al. 2009). Dormancy release of sunflower embryos by pre-treatment with HCN resulted in ROS accumulation, occurring concomitantly with carbonylation of specific embryo proteins (Oracz et al. 2007). Authors suggested that changes in proteome oxidation may be crucial for embryo activation leading to dormancy breakage and finally germination. HCN- and NO-induced germination of apple embryos was also connected with transient ROS accumulation, occurring 24 h after sowing and later on after additional 3 days of culture (Table 7). It was well correlated with changes in ACC concentration in apple embryos during first days of culture. High endogenous content of ACC was progressively reduced, especially during germination “sensu stricto” of pre-treated embryos (Table 7). Consequently, we suggest a close connection between stimulation of ethylene emission by NO and HCN and signaling ROS accumulation in the embryos.
Short-term pre-treatment of dormant apple embryos with HCN increased the activity of ACS and ACO in 5-day-old seedlings (Bogatek et al. 2004). In this study, ACS activity was relatively low during germination “sensu stricto” of apple embryos (Fig. 4a). HCN and NO induced fast and transient rise of ACS activity only at the first day after sowing, afterwards ACS activity declined (Fig. 4a). However, AVG, an inhibitor of ACS activity, repressed germination “sensu stricto” of NO- and HCN-pre-treated embryos (Table 1), suggesting that even low ACS activity can stimulate dormancy removal.
S-Adenosylmethionine, a substrate for ACS activity is also a component of other important physiological pathways, e.g., polyamine biosynthesis (Puga-Hermida et al. 2006; Matilla and Matilla-Vazquez 2008). Activity of enzymes responsible for methionine and SAM synthesis in seeds was detected mainly during terminal phase of germination defined by radical protrusion (Gallardo et al. 2000). It can explain such a low ACS activity observed in apple embryos during first (initial) phases of germination. ACO activity in apple embryos was low during first days of germination “sensu stricto” (Fig. 4b). Short-term pre-treatment with HCN and NO donors increased activity of this enzyme after 2 days of culture (Fig. 4b). Ethylene stimulated ACO activity in the late phase of germination in pea (Pisum sativum) seeds (Petruzzelli et al. 2000). Our results presented above indicate that in the early phases of germination, ACC conversion to ethylene may be partially independent of ACO. This suggestion comes from experiment with Aib (an inhibitor of ACO activity) which had little effect on germination and ethylene emission of HCN- and NO-treated embryos (Table 1). This production of ethylene is probably localized mostly in the embryonic axis (Petruzzelli et al. 2000). Even though the amount of ethylene produced during this reaction may be extremely low due to the high sensitivity of embryo axis to ethylene, it appears sufficient for initiation of the processes fundamental for germination. The possibility of such non-enzymatic production of ethylene from ACC was postulated more than 20 years ago (Kacperska and Kubacka-Zębalska 1989a, b). Those authors proposed that parallel to enzymatic oxidation of ACC to ethylene in some cases, e.g., under desiccation stress, non-enzymatic conversion can take place. Those findings were based on “in vitro” data of Lynch et al. (1985) that demonstrated lipoxygenase-generated lipid hydroperoxides mediating ethylene production from ACC. In our study it has been shown that ACC in water solution can be oxidized by H2O2, NO or HCN in the absence of any plant material (Fig. 5).
Release of dormancy in peanut seeds was correlated with internal increase of ethylene concentration (Ketring and Morgan 1972). This “germination” ethylene level must be attained to release dormancy. Endogenous ethylene is needed for stimulation of ACO gene expression, e.g., in etiolated pea seedlings (Peck and Kende 1995) or during pea seed germination (Petruzzelli et al. 2000). It was demonstrated that ethephon increased ACC content, ACO activity, and ethylene production in dormant beech seeds (Calvo et al. 2004a, b). It is possible that such positive feedback loop operates also in germinating apple embryos at early stages of germination mediated by NO or HCN. It can be hypothesized that during the early phase of germination, oxidation of ACC to ethylene by free radicals (mainly ROS) is necessary for further induction of enzymatic ethylene biosynthesis, and results, finally, in enhanced ethylene production. Such complex regulation of ethylene synthesis may be necessary for reaching stated level of this hormone required for dormancy removal (Fig. 6). A small amount of ethylene synthesized during the initial period of germination “sensu stricto” is crucial for embryo’s axis activation, that being a signaling factor for the seed’s “decision” whether to stay dormant or to commence germination.
The scheme of hypothetic alterations in ACC and ROS concentration and ethylene production during germination “sensu stricto” of apple embryos. Decreased ACC concentration during first phase of germination parallels to enhanced ROS accumulation mainly in embryonic axis (Gniazdowska et al. 2010). At that phase of germination, non-enzymatic biosynthesis of ethylene is predominant. Low ethylene emission activates enzymatic pathway of ethylene biosynthesis by positive feedback loop. High ethylene emission in terminal phase of germination “sensu stricto” reflects process of radical protrusion
Abbreviations
- ABA:
-
Abscisic acid
- ACC:
-
1-Aminocyclopropane-1-carboxylic acid
- ACO:
-
ACC oxidase
- ACS:
-
ACC synthase
- Aib:
-
α-Amino-isobutyric acid
- AVG:
-
Aminoethoxyvinylglycine
- cPTIO:
-
2-(4-Carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide
- HCN:
-
Hydrogen cyanide
- NBD:
-
Norbornadien
- NO:
-
Nitric oxide
- ROS:
-
Reactive oxygen species
- SAM:
-
S-Adenosyl-l-methionine
- SNP:
-
Sodium nitroprusside
- STS:
-
Silver thiosulfate
References
Abeles FB, Lonski J (1969) Stimulation of lettuce seed germination by ethylene. Plant Physiol 44:277–280
Bailly C, El-Maarouf-Bouteau H, Corbineau F (2008) From intracellular signaling networks to cell death: the dual role of reactive oxygen species in seed physiology. C R Biol 331:806–814
Beaudoin N, Serizet C, Gosti F, Giraudat J (2000) Interactions between abscisic acid and ethylene signaling cascades. Plant Cell 12:1103–1115
Bethke PC, Libourel IGL, Jones RL (2007) Nitric oxide in seed dormancy and germination. In: Bradford K, Nonogaki H (eds) Seed development, dormancy and germination. Blackwell, Oxford, pp 153–175
Bewley JD, Black M (1978) Physiology and biochemistry of seeds in relation to germination. Springer, New York
Bogatek R, Gniazdowska A (2006) Nitric oxide and HCN reduce deep dormancy of apple seeds. Acta Physiol Plant 28:281–287
Bogatek R, Dziewanowska K, Lewak ST (1991) Hydrogen cyanide and embryonal dormancy in apple seeds. Physiol Plant 83:417–421
Bogatek R, Gawrońska H, Oracz K (2003) Involvement of oxidative stress and ABA in CN-mediated elimination of embryonic dormancy in apple. In: Nicolas G, Bradford KJ, Come D, Pritchard HW (eds) The biology of seeds: recent research advances. CAB International Publishing, Wallingford, pp 211–216
Bogatek R, Sykała A, Krysiak C (2004) Cyanide-induced ethylene biosynthesis in dormant apple embryos. Acta Physiol Plant 26(Suppl):16
Borysjuk L, Macherel D, Benamar A, Wobus U, Rolletschek H (2007) Low oxygen sensing and balancing in plant seeds: a role for nitric oxide. New Phytol 176:813–823
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Calvo AP, Nicolas C, Lorenzo O, Nicolas G, Rodriguez D (2004a) Evidence for positive regulation by gibberellins and ethylene of ACC oxidase expression and activity during transition from dormancy to germination in Fagus sylvatica L. seeds. J Plant Growth Regul 23:44–53
Calvo AP, Nicolas C, Lorenzo O, Nicolas G, Rodriguez D (2004b) Evidence of a cross-talk regulation of a GA20-oxidase (FsGA20ox1) by gibberellins and ethylene during the breaking of dormancy in Fagus silvatica seeds. Physiol Plant 120:623–630
Chojnowski M, Corbineau F, Côme D (1997) Physiological and biochemical changes induced in sunflower seeds by osmopriming and subsequent drying, storage and aging. Seed Sci Res 7:323–332
Corbineau F, Bagniol S, Côme D (1990) Sunflower (Helianthus annuus L.) seed and its regulation by ethylene. Israel J Bot 39:313–325
Esashi Y, Leopold AC (1969) Dormancy regulation in subterranean clover seeds by ethylene. Plant Physiol 44:1470–1472
Esashi Y, Isuzugawa K, Matsuyama S, Ashino H, Hasegawa R (1991) Endogenous evolution of HCN during pre-germination periods in many seed species. Physiol Plant 83:27–33
Gallardo K, Job C, Groot SPC, Puype M, Demol H, Vandekerckhove J, Job D (2000) Importance of methionine biosynthesis for Arabidopsis seeds germination and seedling growth. Physiol Plant 116:238–247
Ghassemian M, Nambara E, Cutler S, Kawaide H, Kamiya Y, McCourt P (2000) Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis. Plant Cell 12:1117–1126
Gniazdowska A, Dobrzyńska U, Babańczyk T, Bogatek R (2007) Breaking of apple embryo dormancy by nitric oxide involves stimulation of ethylene production. Planta 225:1051–1057
Gniazdowska A, Krasuska U, Czajkowska K, Bogatek R (2010) Nitric oxide, hydrogen cyanide and ethylene are required in the control of germination and undisturbed development of young apple seedlings. Plant Growth Regul 61:75–84
Gomez-Jimenez MC, Matilla AJ, Garrido D (1998) Isolation and characterization of a cDNA encoding an ACC oxidase from Cicer arietinum and its expression during embryogenesis and seed germination. Aust J Plant Physiol 25:765–773
Gomez-Jimenez MC, Garcia-Olivarez E, Matilla AJ (2001) 1-Amino-1-cyclopropane carboxylate oxidase from embryonic axis of germinating cheak-pea (Cicer arietinum L.) seeds: cellular immunolocalization and alteration in its expression by indole-3-acetic acid, abscisic acid and spermine. Seed Sci Res 11:243–253
Hall BP, Shakeel SN, Schaller GE (2007) Ethylene receptors: ethylene perceptional signal transduction. J Plant Growth Regul 26:118–130
Kacperska A, Kubacka-Zębalska M (1989a) Formation of stress ethylene depends both on ACC synthesis and on the activity of free radical-generating system. Physiol Plant 77:231–237
Kacperska A, Kubacka-Zębalska M (1989b) Stress ethylene metabolism as related to degree of tissue injury. In: Clijsters H, de Proft M, Marcelle M, van Poucke M (eds) Biochemical and physiological aspects of ethylene production in lower and higher plants. Kluwer Academic Publishers, Dordrecht, pp 211–218
Katoh H, Esashi Y (1975) Dormancy and impotency of coclebur seeds. I. CO2, C2H4, O2 and high temperature. Plant Cell Physiol 16:687–696
Kępczyński J, Karssen CM (1985) Requirement for the action of endogenous ethylene during germination of non-dormant seeds of Amaranthus caudatus. Physiol Plant 63:49–52
Kępczyński J, Kępczyńska E (1997) Ethylene in seed dormancy and germination. Physiol Plant 101:720–726
Kępczyński J, Rudnicki RM, Khan AA (1977) Ethylene requirement for germination of partly after-ripened apple embryo. Physiol Plant 40:292–295
Kępczyński J, Bihun M, Kępczyńska E (1996) Ethylene involvement in the dormancy and germination of Amaranthus seeds. In: Kanellis AK, Chang C, Kende H, Grierson D (eds) Proceedings of the international symposium on biology and biotechnology of the plant hormone ethylene. NATO ASI Series, Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 113–122
Ketring DL, Morgan PW (1971) Physiology of oil seeds II. Dormancy release in Virginia-type peanut seeds by plant growth regulators. Plant Physiol 47:488–492
Ketring DL, Morgan PW (1972) Physiology of oil seeds. IV. Role of endogenous ethylene and inhibitory regulators during natural and induced after ripening of dormant Virginia-type peanut seeds. Plant Physiol 50:382–387
Khan AA (1994) ACC-derived ethylene production, a sensitive test for seed vigor. J Am Soc Hort Sci 119:1083–1090
Kucera B, Cohn MA, Leubner-Metzger G (2005) Plant hormone interactions during seed dormancy release and germination. Seed Sci Res 15:281–307
Leubner-Metzger G, Petruzzelli L, Waldvogel R, Vögeli-Lange R, Meins F Jr (1998) Ethylene-responsive element binding protein (EREBP) expression and the transcriptional regulation of class I b-1,3-glucanase during tobacco seed germination. Plant Mol Biol 38:785–795
Linkies A, Muler K, Morris K, Tureckova V, Wenk M, Cadman CSC, Corbineau F, Strnad M, Lynn JR, Finch-Savage WE, Leubner-Metzger G (2009) Ethylene interacts with abscisic acid to regulate endosperm rupture during germination: a comparative approach using Lepidium sativum and Arabidopsis thaliana. Plant Cell 21:3803–3822
Lizada CC, Yang SF (1979) A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal Biochem 100:140–145
Locke JM, Bryce JH, Morris PC (2000) Contrasting effects of ethylene perception and biosynthesis inhibitors on germination and seedling growth of barley (Hordeum vulgare L.). J Exp Bot 51:1843–1849
Lynch DV, Sridhara S, Thompson JE (1985) Lipoxygenase generated hydroperoxides account for the non-physiological features of ethylene formation from 1-aminocyclopropane-1-carboxylic acid by microsomal membranes of carnation. Planta 164:121–125
Machabee S, Saini HS (1991) Differences in the requirement for endogenous ethylene germination of dormant and non-dormant seeds of Chenopodium album L. J Plant Physiol 138:97–101
Mathooko FM, Kubo Y, Inaba A, Nakamura R (1993) Partial characterization of 1-aminocyclopropane-1-carboxylate oxidase from excised mesocarp tissue of winter squash fruit. Sci Rep Fac Agric Okayama Univ 82:49–59
Matilla AJ (2000) Ethylene in seed formation and germination. Seed Sci Res 10:111–126
Matilla AJ, Matilla-Vazquez MA (2008) Involvement of ethylene in seed physiology. Plant Sci 175:87–97
Nonogaki H, Chen F, Bradford KJ (2007) Mechanisms and genes involved in germination sensu stricto. In: Bradford KJ, Nonogaki H (eds) Seed development dormancy and germination. Blackwell, Oxford, pp 264–304
Oracz K, El-Maarouf Bouteau H, Farrant JM, Cooper K, Belghazi M, Job C, Job D, Corbineau F, Bailly C (2007) ROS production and protein oxidation as a novel mechanism for seed dormancy alleviation. Plant J 50:452–465
Oracz K, El-Maarouf Bouteau H, Bogatek R, Corbineau F, Bailly C (2008) Release of sunflower seed dormancy by cyanide: cross-talk with ethylene signaling pathway. J Exp Bot 59:2241–2251
Oracz K, El-Maarouf Bouteau H, Kranner I, Bogatek R, Corbineau F, Bailly C (2009) The mechanisms involved in seed dormancy alleviation by hydrogen cyanide unravel the role of reactive oxygen species as key factors of cellular signaling during germination. Plant Physiol 150:494–505
Peck SC, Kende H (1995) Sequential induction of ethylene biosynthetic enzymes by indole-3-acetic acid in etiolated peas. Plant Mol Biol 28:293–301
Petruzzelli L, Coraggio I, Leubner-Metzger G (2000) Ethylene promotes ethylene biosynthesis during pea seed germination by positive feedback regulation of 1-aminocyclopropane-1-carboxylic acid oxidase. Planta 211:144–149
Pierik R, Sasidharan R, Voesenek LACJ (2007) Growth control by ethylene: adjusting phenotypes to the environment. J Plant Growth Regul 26:188–200
Puga-Hermida MI, Gallardo M, Rodrigez-Gacio MC, Matilla AJ (2006) Polyamine contents, ethylene synthesis and BrACO2 expression during turnip germination. Biol Plant 50:574–580
Sarath G, Bethke PC, Jones R, Baird LM, Hou G, Mitchell RB (2006) Nitric oxide accelerates seed germination in warm season grasses. Planta 223:1154–1164
Satoh S, Esashi Y (1984) Identification and content of 1-malonylaminocyclopropanecarboxylic acid in germinating cocklebur seeds. Plant Cell Physiol 25:583–587
Sergiev I, Alexieva V, Karanov E (1997) Effect of spermine, atrazine and combination between them on some endogenous protective systems and stress markers in plants. Compt Rend Acad Bulg Sci 51:121–124
Siegień I, Bogatek R (2006) Cyanide action in plants—from toxic to regulatory. Acta Physiol Plant 28:483–497
Simontacchi M, Jasid S, Puntarulo S (2004) Nitric oxide generation during early germination of sorghum seeds. Plant Sci 167:839–847
Sisler EC, Serek M (2003) Compounds interacting with the ethylene receptor in plants. Plant Biol 5:473–480
Yip WK, Dong JG, Yang SF (1991) Purification and characterization of 1-aminocyclopropane-1-carboxylate synthase from apple fruits. Plant Physiol 95:251–257
Zagórski S, Lewak ST (1985) Germination of lettuce seeds promoted by red light, gibberellin or cyanide is differently affected by far red illumination and temperature. Acta Physiol Plant 7:65–70
Acknowledgments
This work was supported by grant N N303 0905 34 founded by the Ministry of Science and Higher Education, Poland.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gniazdowska, A., Krasuska, U. & Bogatek, R. Dormancy removal in apple embryos by nitric oxide or cyanide involves modifications in ethylene biosynthetic pathway. Planta 232, 1397–1407 (2010). https://doi.org/10.1007/s00425-010-1262-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-010-1262-2