Abstract
Sclerotinia sclerotiorum causes a highly destructive disease in oilseed rape (Brassica napus). Oxalic acid (OA) secreted by the pathogen is a key pathogenicity factor. Oxalate oxidase (OXO) can oxidize OA into CO2 and H2O2. In this study, we show that transgenic oilseed rape (sixth generation lines) constitutively expressing wheat (Triticum aestivum) OXO displays considerably increased OXO activity and enhanced resistance to S. sclerotiorum (with up to 90.2 and 88.4% disease reductions compared with the untransformed parent line and a resistant control, respectively). Upon application of exogenous OA, the pH values in transgenic plants were maintained at levels slightly lower than 5.58 measured prior to OA treatment, whereas the pH values in untransformed plants decreased rapidly and were markedly lower than 5.63 measured prior to OA treatment. Following pathogen inoculation, H2O2 levels were higher in transgenic plants than in untransformed plants. These results indicate that the enhanced resistance of the OXO transgenic oilseed rape to Sclerotinia is probably mediated by OA detoxification. We believe that enhancing the OA metabolism of oilseed rape in this way will be an effective strategy for improving resistance to S. sclerotiorum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sclerotinia sclerotiorum is pathogenic to more than 400 plant species (Boland and Hall 1994) and distributes worldwide. On oilseed rape (Brassica napus), it causes rots of leaves, stems and pods, resulting in a tremendous seed yield loss in China. No immune or highly resistant cultivars of oilseed rape have been reported to date, and few genetic sources of resistance to the pathogen are available to breeders (Zhou et al. 1994; Liu et al. 2005). Control of the disease depends heavily on application of fungicides to the crop, but this is expensive and can be ineffective due to the difficulties associated with applying sprays to thick canopies (Luo and Zhou 1994) and a lack of suitable forecasting methods to enable timely application of fungicides. Therefore, transgenic modification of the crop may provide a novel strategy for control of Sclerotinia disease on oilseed rape.
Oxalic acid (OA) secreted by S. sclerotiorum during infection is a key pathogenicity factor of the fungus (Marciano et al. 1983; Godoy et al. 1990; Liu et al. 1998; Cessna et al. 2000). The compound acidifies plant tissue surrounding the site of infection and causes tissue damage (Dutton and Evans 1996). The optimal pH value for many fungal cell wall-degrading enzymes lies in the acidic range and thus acidification by OA may enhance activities of these enzymes (Lumsden 1979; Marciano et al. 1983). Additionally, OA is a strong chelator of divalent cations and sequestration of calcium may weaken cell walls (Bateman and Beer 1965). Phenolics have important roles in plant defence (Métraux and Raskin 1993) and there are reports that OA can inhibit the activities of 0-diphenol oxidase (Ferrar and Walker 1993) and polyphenol oxidase (Marciano et al. 1983; Liu et al. 1998) of host plants during fungal infection.
An attractive strategy for engineering resistance to S. sclerotiorum is to express an exogenous protein to degrade OA in oilseed rape. OA is generally catabolised by two pathways, i.e. decarboxylation and oxidation. Decarboxylation is mediated by oxalyl-CoA decarboxylase or oxalate decarboxylase, producing oxalyl-CoA or formic acid, respectively (Kesarwani et al. 2000). Oxidation can be accomplished by oxalate oxidase (OXO, a member of the germin family of proteins) (Chiriboga 1966; Lane et al. 1993). OXO breaks down OA into CO2 and H2O2, the latter being suggested to play pivotal roles in plant defence responses (Lamb and Dixon 1997; Hu et al. 2003). H2O2 at micromolar concentrations in plant tissues is directly toxic to microbes, contributes to structural reinforcement of plant cell walls, triggers salicylic acid (SA) synthesis, and has a central role in the signaling cascades that coordinate various defence responses (Peng and Kuc 1992; Hammond-Kosack and Parker 2003). It has been proposed that, through the production of H2O2, OXO may cause cross-linking of plant cell wall proteins in papillae at the site of infection (Wei et al. 1998) and functions in the plant hypersensitive response (Lane 1994; Zhou et al. 1998a). Therefore, the OXO transgene may endow plants with multiple resistance mechanisms through detoxification of OA and H2O2-mediated activation of defence response genes.
These functions of OXO have attracted considerable attention due to the potential for engineering plant disease resistance. Transgenic soybean (Glycine max) and sunflower (Helianthus annuus) expressing wheat (Triticum aestivum) OXO showed increased resistance to S. sclerotiorum in both laboratory bioassays and field tests (Scelonge et al. 2000; Donaldson et al. 2001; Hu et al. 2003). It has been demonstrated in transformed sunflower that expression of wheat OXO can result in the induction of plant defence proteins (Hu et al. 2003). Transgenic oilseed rape and peanut (Arachis hypogaea) expressing barley (Hordeum vulgare) OXO enhanced the ability to break down exogenously supplied OA (Thompson et al. 1995; Livingstone et al. 2005). However, to our knowledge there has not been a specific report on whether heterogeneously expressed OXO in oilseed rape can enhance resistance to S. sclerotiorum.
Unlike sunflower, which possesses very low OXO activity (Hu et al. 2003), oilseed rape has an endogenous ability to metabolize OA. Studies using [14C] OA indicated that oilseed rape plants can metabolize OA into organic acids and carbohydrates, and that resistant lines can endure higher concentrations of OA than susceptible ones (Liu et al. 1998). Whether the increase in the ability of transgenic oilseed rape to degrade OA can enhance resistance of the plant to S. sclerotiorum has not been determined.
To address the above question, here we report that the stably inherited transgenic oilseed rape (sixth generation lines) constitutively expressing wheat OXO exhibits significantly greater OXO activity, and higher resistance to OA and S. sclerotiorum in comparison with the untransformed parental cultivar 84039M and a resistant cultivar Zhongyou 821.
Materials and methods
Plant and fungal materials
A B. napus L. cultivar 84039M with moderate resistance to S. sclerotiorum, was used as the recipient of the transgene and as a control in disease resistance evaluation of transgenic lines. Another B. napus cultivar, Zhongyou 821, with the highest S. sclerotiorum resistance ratings during more than 10 years of testing (China National Rapeseed Variety Regional Trials), was used as a resistance control in the disease resistance evaluation. For plants grown in a plant growth room, the growth conditions were 20 ± 2°C under a 16/8 h photoperiod at a light intensity of 44 μmol m−2 s−1 and 60–90% relative humidity. For plants sown in a glasshouse, the conditions were 14–22°C and natural light. Fresh sclerotia of the fungus S. sclerotiorum, collected from oilseed rape stems in the field in Wuhan, China, were germinated to produce mycelial inoculum on potato dextrose agar.
Vector construction and Agrobacterium-mediated transformation
A cDNA encoding wheat OXO with its native signal peptide sequence was derived from pRPA-BD-OX16 (kindly gifted by Rhone-Poulenc Agrochimie, Lyon, France) and cloned into the multiple cloning site (Pst I/Xho I) of pTΩ4A (Wang et al. 2003), thus generating p4A-OXO. A 1.8-kb EcoR I/Hind III fragment, comprising a CaMV 35S promoter, OXO and a NOS terminator, was excised from p4A-OXO and ligated with pBI121 (Jefferson et al. 1987), which had been cut with EcoR I/Hind III. The resulting OXO-expressing vector, designated pBOXO, was transformed into Agrobacterium tumefaciens strain LBA4404 and used for Agrobacterium-mediated transformation.
Agrobacterium-mediated transformation of B. napus cultivar 84039M was performed according to the protocol described previously (Guo and Wang 1999). Kanamycin-resistant plantlets that rooted well in selective medium were transferred to pots and grown in a glasshouse.
PCR-based screening and Southern-blot analysis
DNA was extracted from leaves of transformed plants and their offspring. PCR-based screening was used to maintain the presence of the transgene until homozygous transgenic lines were obtained. The PCR primers used were 5′-GTCCTGCAGCATGGGGTACTCCAAAAC-3′ and 5′-CCCAAGCTTGAATTCCCGATCTAGTAACATAG-3′, which generated a 1.1-kb product specific to wheat OXO. The integration of the target gene was also confirmed by Southern-blot analysis. Total DNA was extracted from T6 generation transgenic plants using E.Z.N.A® plant DNA Mini kit (Omega Bio-tek Inc., Guangzhou, China), and five micrograms of total DNA were digested with Hind III. As oilseed rape OXO is highly similar to the wheat OXO transgene, we utilized a 32P-labeled probe specific to the 35S promoter to avoid non-specific hybridization. The primers used for the 35S promoter probe were 5′-GCCATCATTGCGATAAAGGA-3′ (forward) and 5′-AAGGATAGTGGGATTGTGCGT-3′ (reverse), which amplified a 485-bp product. DNA electrophoresis, digestion, blotting and hybridization were done following the procedures described by Sambrook and Russell (2001). After hybridization, membrane was scanned using a Cyclone Storage Phosphor Scanner B431200 (Perkin Elmer, Waltham, MA, USA).
OXO activity and H2O2 content assays of the OXO transgenic plants
The activity of OXO in homozygous transgenic plants was measured according to the method described previously (Zhang et al. 1995). Untransformed 84039M was used as a control. Briefly, four grams of young leaves were excised from each plant grown in the growth room and homogenized in 5 ml of distilled water at 4°C. The crude enzyme was precipitated with ammonium sulphate at 70% saturation and dissolved in 1 ml of OXO reaction solution [in a 50 ml solution, 0.286 g succinic acid, 12.6 mg OA, 30 ml absolute ethanol, 4 mg 4-aminoantipyrine, 200 U horseradish peroxidase (Sigma, St. Louis, MO, USA), and 10 μl N, N-dimethylaniline, pH 3.3]. The absorbance of the different samples was measured at 550 nm using a spectrophotometer (DU650, Beckman, Fullerton, CA, USA).
H2O2 was visually detected in leaves of line OX10 (sixth generation) using 3,3-diaminobenzidine (DAB) as a substrate. Twelve hours after inoculation with S. sclerotiorum, leaves were excised and incubated with DAB-HCl (1 mg/ml, pH 3.8) in the growth room for 8 h. After the leaves were cleared in boiling ethanol (96%) for 10 min, H2O2 in the leaves was visualized as red brown precipitate and photographed using a microscope (Leica DMRE, Wetzlar, Germany).
Detection of leaf pH values in transgenic plants
The leaf pH value of line OX10 (sixth generation) was analysed using a pH micro-sensor equipped with a modified polyaniline (PA) electrode (Zou et al. 2007). Plants were grown in the glasshouse up to the four-true-leaf stage. The fabrication and optimization procedure of the pH micro-sensor was similar to the method described previously (Wan et al. 1997; Zhang et al. 2002). The surfaces of leaves were lightly rubbed with a cylindrical eraser so that they would retain drops of OA solution. One hundred microlitres of OA (10 mM) was applied onto the surface of leaves of transgenic and control plants. The Ag/AgCl reference electrode was inserted into the main vein 1 cm away from the petiole, and the PA working electrode into the lamina 1 cm away from the OA drop. The experiment was repeated three times (three different plants) and for each plant, three leaves were measured.
Resistance of transgenic plants to OA
The OXO transgenic lines (sixth generation) were assessed for their resistance to OA using detached leaf assays. Untransformed 84039M and Zhongyou 821 were used as controls. Plants were grown in the glasshouse up to the three-true-leaf stage. Detached leaves were excised and arranged on wet paper towels placed in boxes. In each of four replicates, eight leaves, one for each concentration of OA, were used for each plant line. Two small areas (7 mm in diameter) on each side of the main vein of each leaf were lightly rubbed with a cylindrical eraser so that they would retain drops of OA. Twenty microlitres of OA (0, 5, 10, 20, 40, 100 or 200 mM) was applied onto the rubbed area. Thereafter, the boxes were covered with transparent polyethylene bags and placed in the growth room at 20 ± 2°C with a 16/8 h photoperiod at an intensity of 44 μmol m−2 s−1. Every 12 h, symptoms, including the sizes of brown lesions and yellowing area surrounding the lesions, were measured in length and width.
Disease resistance evaluation
Sclerotinia resistance of the transgenic lines (sixth generation) was assessed using two methods: detached leaf inoculation tests and field disease nursery tests (Liu et al. 2005). Untransformed 84039M and Zhongyou 821 were used as controls. For the detached leaf inoculation tests, plants were grown in the glasshouse up to the four-true leaf stage. Mycelia of S. sclerotiorum were cultured on potato dextrose agar. Agar discs were excised from the edges of growing colonies and upended onto detached leaves. Each of four replicates included eight leaves from each transgenic line. All leaves were labelled and randomly arranged on wet gauze in containers that were collectively covered with transparent polyethylene bags. The leaves in the containers were incubated at 20 ± 2°C in a dark room. Twenty hours after inoculation and at intervals thereafter, lesion sizes were measured in length and width.
The field disease nursery tests were arranged as a randomized block design with two blocks and a plot size of 1.0 × 2.2 m (16–20 plants per plot). Plots were covered with nylon net to prevent pollen drift. S. sclerotiorum inoculum in the disease nursery was maintained by growing oilseed rape consecutively for four seasons and placing two sclerotia in each row before sowing. Crop management followed the standard agronomic practice, but without any pesticide application. Seven days before harvest, disease severity was assessed on a 0–4 scale [0, no lesions; 1, percentage stem circumference with lesions (PS) <25 or percentage branches with lesions (PB) <30; 2, 25 ≤ PS < 50 or 30 ≤ PB < 60; 3, 50 ≤ PS < 75 or PB ≥ 60; 4, PS ≥ 75 or almost all branches with lesions]. Disease index was calculated by 100Σ(I n I )/(N k), where I is a disease severity score on the 0–4 scale, n I is number of plants with each score, N is total number of plants assessed and k is the highest score (here it is 4) (Liu et al. 2005).
Statistical analysis
Variance analysis for all the data relating to OXO activity, pH value, brown lesion size, yellowing area, Sclerotinia lesion size and disease index was done using the SAS program (SAS Institute Inc.).
Results
Generation of the stably inherited OXO transgenic oilseed rape lines
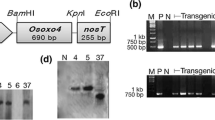
The binary vector pBOXO (see “Materials and methods”; Fig. 1a) was constructed and used for transformation of 84039M. A total of 13 independent OXO transgenic plants were obtained. PCR-based screening and, from the third generation and onwards, Sclerotinia disease resistance selection were used to maintain the presence of the OXO transgene and associated resistance. As a consequence, five independent OXO transgenic lines, including OX1, OX6, OX9, OX10 and OX14, were selected and selfed to the stably inherited generations. The transgenic nature for each of them was confirmed by PCR and Southern-blot analysis (Fig. 1b, c). In the second generation, lines OX1, OX9, OX10 and OX14 segregated in a 3:1 ratio for the transgene as determined by PCR detection. For line OX6, the ratio was 29:15. From the fourth generation, these lines were no longer segregating for the transgene and thus were considered homozygous. Southern-blot analysis indicated these transgenic lines had a single copy of the transgene.
Transformation of oilseed rape with a gene encoding wheat oxalate oxidase (OXO). a Schematic diagram of T-DNA of the binary vector pBOXO. RB, right border; P nos , nopaline synthase promoter; npt II, neomycin phosphotransferase (II) coding region; T nos , nopaline synthase terminator; E, enhancer; P 35s , cauliflower mosaic virus 35S promoter; Ω, the lead sequence of tobacco mosaic virus; germin, wheat OXO cDNA; LB, left border. The sites of a 485-bp probe used for Southern blot and a 1.1-kb fragment used for PCR template are indicated. b PCR analysis of the OXO transgenic plants using genomic DNA as the template. Lane 1, positive control (pBOXO); lane 2, untransformed 84039M (CK1); lanes 3–7, the transgenic plants tested: OX14, OX10, OX9, OX6 and OX1. c Southern-blot analysis of the OXO transgenic plants. Lane 1, DNA marker (unit, bp); lane 2, untransformed 84039M (CK1); lanes 3–7, the transgenic plants tested: OX14, OX10, OX9, OX6 and OX1. Genomic DNA from all transgenic and CK1 plants was digested by Hind III
In terms of agronomic characters (within lines and between lines), all five homozygous transgenic lines were uniform and comparable to untransformed 84039M.
Transgenic plants exhibit significantly increased OXO activity and elevated H2O2 levels
To determine whether the OXO transgene was functionally expressed in transformed plants, OXO activity in leaf extracts was measured using a spectrophotometric assay and expressed as OD550. The five homozygous transgenic lines OX1, OX6, OX9, OX10 and OX14, exhibited significantly (P < 0.05) greater OXO activities than did untransformed 84039M (Fig. 2a). Lines OX1 and OX14 had similar OD550 values (0.2433 and 0.2845, respectively) and were twice that of 84039M. The OD550 value of OX10 was 0.4512, three times greater than that of 84039M (0.1229). This result was confirmed by histochemical detection of H2O2 in leaves of OX10 (sixth generation) infected with S. sclerotiorum. Upon staining with DAB, compared with 84039M, the leaves (especially the veins) of the transgenic plants displayed stronger red-brown colouration in the transverse sections 12 h after inoculation with S. sclerotiorum, indicating higher levels of H2O2 accumulation in this transgenic line (Fig. 2b).
Expression and functional analysis of the wheat oxalate oxidase (OXO) transgene in oilseed rape. a OXO activities in the transgenic plants. The activity was measured using a spectrophotometric assay and expressed as OD550. CK1 is 84039M, an untransformed control; OX1, OX6, OX9, OX10 and OX14 were the transgenic plants tested. The data were obtained from three independent experiments and expressed as mean ± SD. b Histochemical detection of H2O2 levels in line OX10. Leaves were inoculated with S. sclerotiorum and stained with DAB, and observed from transverse sections. The white arrow indicates the red-brown colouration caused by H2O2 precipitate in the leaf veins. The short scale bar stands for 12.5 μm in length
Transgenic OXO ameliorates acidification of leaf tissue by OA
It is important to investigate whether the OXO transgenic plants can degrade OA and thus prevent pH values from decreasing after exogenous OA application or Sclerotinia infection. Here, we used a modified, steady PA electrode (Zou et al. 2007) for in situ and real-time detection of leaf pH values. The experiments were carried out on leaves of the homozygous line OX10 (sixth generation) and untransformed 84039M. Following OA application to the leaf surface, the pH values in both transgenic and untransformed plants decreased immediately (Fig. 3). However, the rate of decrease was greater in the untransformed plants than in the transgenic plants. By 110 min after OA application, the pH values were significantly (P < 0.05) higher in the transgenic plants than in 84039M plants. By 392 min after OA application, the pH values in the transgenic plants remained at 4.9, whereas the pH values in the untransformed plants had dropped to 3.2 (Fig. 3). Therefore, the transgenic line displayed a considerable ability to protect the transgenic plants from OA-induced acidification.
In-situ and real-time detection of the pH values in the wheat oxalate oxidase (OXO) transgenic oilseed rape lines. Following application of exogenous oxalic acid (OA), the plants of line OX10 and untransformed 84039M (CK1) were analysed using a pH micro-sensor as described in “Materials and methods”. The data were obtained from three independent experiments (three different plants and for each plant, three leaves were measured) and expressed as mean ± SD
The OXO transgenic oilseed rape shows enhanced resistance to OA
The OXO transgenic lines (sixth generation) were evaluated for their resistance to OA by detached leaf assays. Two types of symptoms caused by exogenous OA were observed: brown lesions and yellowing areas surrounding the brown lesions (Fig. 4). The symptom severity of all the lines tested increased with the concentrations of OA, but the non-transgenic controls were more sensitive to OA than the transgenic lines in terms of both brown lesion size and yellowing area (Figs. 4, 5). Eighty-four hours after OA treatment, brown lesion sizes of the transgenic lines and two controls showed slow increases with the concentrations of OA in the range of from 0 to 40 mM and rapid increases at more than 40 mM OA (Fig. 5a). At the same time point, the changes in yellowing areas of the transgenic lines were very similar to those in brown lesion sizes over the concentrations of OA (Fig. 5b); however, changes in yellowing areas of two controls were different and they started to rapidly increase at very low concentrations of OA and kept the increase over the concentrations of OA (Fig. 5b). At 200 mM OA, all of the transgenic lines tested, OX1, OX6, OX9, OX10 and OX14, exhibited significantly (P < 0.05) smaller lesions than 84039M and Zhongyou 821 84 h after OA treatment (Fig. 5a). At the same concentration of OA, all of these transgenic lines exhibited significantly (P < 0.05) smaller yellowing areas than 84039M, and OX9 and OX14 were significantly (P < 0.05) smaller in yellowing areas than Zhongyou 821 (Fig. 5b). Eighty-four hours after OA treatment, differences in yellowing area were greater than those in brown lesion size between transgenic lines and the controls (Fig. 5a, b). The correlation between yellowing area (mean over seven concentrations of OA) and brown lesion size (mean over seven concentrations of OA) of five transgenic lines and two controls was highly significant (r = 0.933, P < 0.01).
Detached leaf assays for resistance to oxalic acid (OA) in the wheat oxalate oxidase (OXO) transgenic oilseed rape lines. Twenty microlitres of OA of eight different concentrations were applied to leaves of plants. Brown lesion and yellowing area surrounding the lesion were statistically analysed 84 h after OA treatment (see Fig. 5). Detached leaves tested were photographed 96 h after OA treatment and just one of four replicates is presented in the figure. CK1 (cv. 84039M) was used as an untransformed control and CK2 (cv. Zhongyou 821) as a resistant control; OX1, OX6, OX9, OX10 and OX14 are the transgenic lines tested
Sensitivity of the wheat oxalate oxidase (OXO) transgenic lines of oilseed rape to oxalic acid (OA) in detached leaf assays. Twenty microlitres of OA of eight different concentrations were applied to leaves of plants. Brown lesion size (a) and yellowing area surrounding the lesion (b) were measured as length by width 84 h after OA treatment and expressed as means for four replicates of each OA concentration. CK1 (cv. 84039M) was used as an untransformed control and CK2 (cv. Zhongyou 821) as a resistant control; OX1, OX6, OX9, OX10 and OX14 are the transgenic lines tested. The vertical bar indicates standard error of difference (SED; SED is 0.14 for brown lesion sizes and 2.18 for yellowing areas) between means
Enhanced resistance of transgenic oilseed rape lines to S. sclerotiorum
The OXO transgenic lines (sixth generation) were evaluated for resistance to S. sclerotiorum by detached leaf inoculation tests and field disease nursery tests. In the detached leaf inoculation, all five transgenic lines, OX1, OX6, OX9, OX10 and OX14, had significantly (P < 0.05) smaller lesion sizes than did untransformed 84039M 76 h after inoculation although no line was considered to be immune (Fig. 6; Table 1). Lesion sizes of lines OX10, OX14, OX6, OX1 and OX9 were reduced by 44.4, 42.8, 38.3, 35.0, 34.6 and 34.2%, respectively, compared with that of 84039M (Table 1). There were no significant (P = 0.05) differences between the transgenic lines or between each of them and Zhongyou 821 (Table 1).
Detached leaf inoculation tests for resistance to S. sclerotiorum in the wheat oxalate oxidase (OXO) transgenic oilseed rape lines. Detached leaves were inoculated with mycelial plug of S. sclerotiorum, and photographed 20 h after inoculation. Lesion sizes were statistically analysed 76 h after inoculation (see Table 1). CK1 (cv. 84039M) was used as an untransformed control and CK2 (cv. Zhongyou 821) as a resistant control. OX1, OX6, OX9, OX10 and OX14 are the transgenic lines tested. The white scale bars stands for 0.8 cm in length
In the nursery tests, disease indices of all five transgenic lines tested were significantly (P < 0.05) smaller than those of untransformed 84039M and Zhongyou 821 (Table 1). Disease indices of Zhongyou 821 and 84039M were 57.7 and 68.5%, respectively (Table 1). Disease indices of the transgenic lines tested, including OX14, OX1, OX10, OX6 and OX9, were no more than 20%, a reduction of 88.4, 84.5, 83.4, 80.2 and 65.3% when compared with that of Zhongyou 821, and of 90.2, 87.0, 86.0, 83.4 and 70.8% when compared with that of 84039M (Table 1). Thus all the transgenic lines tested exhibited increased resistance to S. sclerotiorum. These transgenic lines tested can be divided into two resistance groups: one included OX14, OX10 and OX1 with smaller indices (6.7, 8.9, and 9.6%, respectively) and another included OX6 and OX9 with greater indices (11.4 and 20.0%, respectively). There were significant (P < 0.05) disease resistance differences between these two groups (Table 1). The correlation between lesion size and disease index of five transgenic lines and two controls was significant (r = 0.803, P < 0.05).
Relationships between OXO activity and resistance to OA and S. sclerotiorum
In the OXO transgenic plants, OXO activity increased significantly (Fig. 2), resulting in elevation of H2O2 through OA degradation. OA degradation prevented pH decrease and tissue acidification. There was a significant correlation between OXO activity and resistance of the transgenic lines to OA. The correlation coefficients were −0.817 (P < 0.05) for OXO activity and yellowing area (mean over seven concentrations of OA and at 84 h after OA treatment) and −0.770 (P < 0.1) for OXO activity and brown lesion size (mean over seven concentrations of OA and at 84 h after OA treatment). Furthermore, OXO activity was highly correlated with Sclerotinia lesion size in the detached leaf inoculation tests (r = −0.783, P < 0.1) and with disease index in the nursery tests (r = −0.677) although the latter correlation was statistically not significant.
In further analysis, we found that increased resistance to OA was significantly (P < 0.05 or 0.01) and positively correlated with enhanced resistance to S. sclerotiorum. The correlation coefficients were 0.949 (P < 0.01) between yellowing area and Sclerotinia lesion size, 0.818 (P < 0.05) between brown lesion size and Sclerotinia lesion size, 0.914 (P < 0.01) between yellowing area and disease index, and 0.877 (P < 0.01) between brown lesion size and disease index. These values indicated a close association between OXO activity and resistance to OA and to S. sclerotiorum.
Discussion
Genetic transformation with a gene encoding OXO is an effective strategy for improvement of resistance to S. sclerotiorum in oilseed rape. Previously, transgenic oilseed rape expressing barley OXO showed it has a role in OA degradation (Thompson et al. 1995); however, no laboratory or field assessments of resistance to S. sclerotiorum have been reported to date. In this study, laboratory bioassays and field disease nursery tests showed clearly that the stably inherited OXO transgenic oilseed rape lines (sixth generation) exhibit significantly increased resistance to S. sclerotiorum. In the field disease nursery tests, the disease indices of OX14, OX10 or OX1 were less than one seventh of that of the untransformed 84039M (Table 1), a moderately resistant cultivar which served as a recipient for the transgene, and no more than one sixth of that of Zhongyou 821, one of the most Sclerotinia-resistant cultivars of oilseed rape available (Liu et al. 2005). The improvement in resistance achieved using transgenic biotechnology was in this study greater during a period of 4 years (six generations) than that achieved by a single plant or population selection (Zhou et al. 1994) or recurrent selection (Zhou et al. 1998b) during a period of 7 or 9 years. Genetic gains of improvement for Sclerotinia disease resistance have been slow and small using traditional breeding practices during the past 40 years. Over the last two decades, more than 6,000 Brassica oilseed rape accessions (some from other countries) in the China Rapeseed Germplasm Pool have been evaluated for their resistance to S. sclerotiorum and some resistant lines have been selected (Zhou et al. 1994). However, none of these resistant lines exhibited greater resistance to S. sclerotiorum than OX1, OX10 or OX14. Among these resistant lines selected, ZhongRS083 had the highest resistance to S. sclerotiorum. In the field nursery tests, the disease index of ZhongRS083 was reduced by less than 75% when compared with that of the resistant control Zhongyou 821, whereas those of OX1, OX10 or OX14 were reduced by more than 83%.
The enhanced resistance of the OXO transgenic lines to S. sclerotiorum can be attributed to the significantly increased resistance to OA. The increased resistance to OA is likely due to the increased capability for OA degradation in the OXO transgenic lines. OA degradation is valuable to protect infected tissues from acidification and damage caused by excess OA. We employed a novel method to show in situ and in real-time that the OXO transgenic plants can immediately degrade OA and rapidly prevent pH values from decreasing upon exogenous OA application. The increased resistance to S. sclerotiorum is likely to result from one or more of the following effects of constitutively expressed OXO in the transgenic lines: preventing plant tissue acidification, inhibiting the activity of fungal cell wall-hydrolytic enzymes (Lumsden 1979; Noyes and Hancock 1981; Godoy et al. 1990), reducing chelation of divalent cations by OA (which may serve to weaken cell walls) (Bateman and Beer 1965), enhancing plant defence by counteracting the OA-mediated suppression of the oxidative burst (Cessna et al. 2000), and impeding OA inhibition of other plant oxidases like 0-diphenol oxidase (Ferrar and Walker 1993) and polyphenol oxidase (Marciano et al. 1983; Liu et al. 1998).
Oilseed rape can tolerate OA to a quite high level as it has an endogenous ability to metabolize OA (Liu et al. 1998; Liu et al. 2005). Such ability varies with variety and this variation is consistent with known varietal resistances to S. sclerotiorum. 84039M has a lower ability to metabolize OA than the resistant control Zhongyou 821 (Liu et al. 1998). Interestingly, after 84039M was transformed with wheat OXO, its ability to metabolize OA was increased, and as a consequence, its resistance to OA became significantly greater than that of Zhongyou 821 (Figs. 4, 5). These results suggest that enhancing the OA metabolism in this way will be an effective strategy for improving resistance to S. sclerotiorum in oilseed rape.
OXO-generated H2O2 may also play an important role in contributing to the enhanced resistance of the OXO transgenic plants to S. sclerotiorum. Wheat and barley germins have been proposed to possess both OXO and superoxide dismutase activities, leading to production of the defence-inducing molecule H2O2 (Lane et al. 1993; Kotsira and Clonis 1997; Woo et al. 2000). There is increasing evidence showing that OXO is involved in disease defence responses associated with H2O2 (Lane 1994; Wei et al. 1998; Zhou et al. 1998a; Dunwell et al. 2000). In our case, the OXO transgenic oilseed rape exhibited obviously elevated levels of H2O2 (Fig. 2b), which may contribute to the enhanced resistance of the transgenic lines to S. sclerotiorum.
In this study, we noted two features that distinguish line OX6 from the other OXO transgenic lines. One is that lesion development in line OX6 was slightly quicker than in the other transgenic lines from 84–96 h after OA treatment and OX6 was more sensitive to OA at high concentrations of OA (40–200 mM). Another is that the transgene segregation in OX6 detected by PCR did not have a typical 3:1 ratio although Southern-blot analysis indicated a single copy of the 35S promoter present in OX6. The OXO transgene was expressed in OX6 at the RNA level (data not presented) and functioned at the protein level. The reasons for the exceptions need to be studied. Whether there is an association between the two phenomena remains unknown.
In summary, we demonstrate that expressing a gene encoding wheat OXO in oilseed rape can efficiently improve resistance to S. sclerotiorum although oilseed rape has considerably high endogenous OA metabolism activity. The efficacy of constitutively expressed OXO in enhancing the Sclerotinia resistance may be a consequence of enhanced OA detoxification and H2O2 production. Future work is necessary to develop a novel oilseed rape cultivar highly resistant to S. sclerotiorum by hybridizing OX1, OX10 or OX14 with some commercially available cultivars having superior agronomical traits such as Zhongshuang 10, and to further investigate the role of H2O2 in contributing to enhanced resistance of the OXO transgenic plants to S. sclerotiorum.
Abbreviations
- DAB:
-
3,3-Diaminobenzidine
- OA:
-
Oxalic acid
- OXO:
-
Oxalate oxidase
- PA:
-
Polyaniline
- PB:
-
Percentage branches with lesions
- PS:
-
Percentage stem circumference with lesions
References
Bateman DF, Beer SV (1965) Simultaneous production and synergistic action of oxalic acid and polygalacturonase during pathogenesis by Sclerotiorum rolfsii. Phytopathology 55:204–211
Boland GJ, Hall R (1994) Index of plant hosts of Sclerotinia sclerotiorum. Can J Plant Pathol 16:93–108
Cessna SG, Sears VE, Dickman MB, Low PS (2000) Oxalic acid, a pathogenicity factor for Sclerotinia sclerotiorum, suppresses the oxidative burst of the host plant. Plant Cell 12:2191–2200
Chiriboga J (1966) Purification and properties of oxalic acid oxidase. Arch Biochem Biophys 116:516–523
Donaldson PA, Anderson T, Lane BG, Davidson AL, Simmonds DH (2001) Soybean plants expressing an active oligomeric oxalate oxidase from the wheat gf-2.8 (germin) gene are resistant to the oxalate-secreting pathogen Sclerotinia sclerotiorum. Physiol Mol Plant Pathol 59:297–307
Dunwell JM, Khuri S, Gane PJ (2000) Microbial relatives of the seed storage proteins of higher plants: conservation of structure and diversification of function during evolution of the cupin superfamily. Microbiol Mol Biol Rev 64:153–179
Dutton MV, Evans CS (1996) Oxalate production by fungi: its role in pathogenicity and ecology in the soil environment. Can J Microbiol 42:881–895
Ferrar PH, Walker JRL (1993) O-Diphenol oxidase inhibition: an additional role of oxalic acid in the phytopathogenic arsenal of Sclerotinia sclerotiorum and Sclerotium rolfsii. Physiol Mol Plant Pathol 43:415–442
Godoy G, Steadman JR, Dickman MB, Dam R (1990) Use of mutants to demonstrate the role of oxalic acid in pathogenicity of Sclerotinia sclerotiorum on Phaseolus vulgaris. Physiol Mol Plant Pathol 37:179–191
Guo X, Wang H (1999) Transformation of CMS restorers in Brassica napus L. using Agrobacterium-mediated methods. Chin J Oil Crop Sci 21:1–3
Hammond-Kosack KE, Parker JE (2003) Deciphering plant-pathogen communication: fresh perspectives for molecular resistance breeding. Curr Opin Biotechnol 14:177–193
Hu X, Bidney DL, Yalpani N, Duvick JP, Crasta O, Folkerts O, Lu G (2003) Overexpression of a gene encoding hydrogen peroxide-generating oxalate oxidase evokes defense responses in sunflower. Plant Physiol 133:170–181
Jefferson RA, Kavangh TA, Bevan MW (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Kesarwani M, Azam M, Natarajan K, Mehta A, Datta A (2000) Oxalate decarboxylase from Collybia velutipes-Molecular cloning and its overexpression to confer resistance to fungal infection in transgenic tobacco and tomato. J Biol Chem 275:7230–7238
Kotsira VP, Clonis YD (1997) Oxalate oxidase from barley roots: purification to homogeneity and study of some molecular, catalytic, and binding properties. Arch Biochem Biophys 340:239–249
Lamb C, Dixon RA (1997) The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol 48:251–275
Lane BG (1994) Oxalate, germin, and the extracellular matrix of higher plants. FASEB J 8:294–301
Lane BG, Dunwell JM, Ray JA, Schmitt MR, Cuming AC (1993) Germin, a protein marker of early plant development, is an oxalate oxidase. J Biol Chem 268:12239–12242
Liu S, Pan J, Zhou B (1998) Uptake, metabolisms of oxalate by and mechanism of resistance to Sclerotinia disease in oilseed rape. Acta Phytopathol Sinica 28:33–37
Liu S, Wang H, Zhang J, Fitt BDL, Xu Z, Evans N, Liu Y, Yang W, Guo X (2005) In vitro mutation and selection of doubled-haploid Brassica napus lines with improved resistance to Sclerotinia sclerotiorum. Plant Cell Rep 24:133–144
Livingstone DM, Hampton JL, Phipps PM, Grabau EA (2005) Enhancing resistance to Sclerotinia minor in peanut by expressing a barley oxalate oxidase gene. Plant Physiol 137:1354–1362
Lumsden RD (1979) Histology and physiology of pathogenesis in plant disease caused by Sclerotinia species. Phytopathology 69:890–896
Luo K, Zhou B (1994) Disease management in oilseed rape. Chemical Industry Press, Beijing, pp 15–25
Marciano P, Lenna PD, Magro P (1983) Oxalic acid, cell wall-degrading enzymes and pH in pathogenesis and their significance in the virulence of two Sclerotinia sclerotiorum isolates on sunflower. Physiol Plant Pathol 22:339–345
Métraux JP, Raskin I (1993) Role of phenolics in plant disease resistance. In: Chet I (ed) Biotechnology of plant disease control. Wiley-Liss, New York, pp 191–209
Noyes RD, Hancock JG (1981) Role of oxalic acid in the Sclerotinia wilt of sunflower. Physiol Plant Pathol 18:123–132
Peng M, Kuc J (1992) Peroxidase-generated hydrogen peroxide as a source of antifungal activity in vitro and on tobacco leaf disks. Phytopathology 82:696–699
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, New York
Scelonge C, Wang L, Bidney D, Lu G, Hastings C, Cole G, Mancl M, D’Hautefeuille J-L, Sosa-Dominguez G, Coughlan S (2000) Transgenic Sclerotinia resistance in sunflower (Helianthus annuus L.). In: The proceedings of 15th international sunflower conference, Toulouse, France, June 2000, pp K66–71
Thompson C, Dunwell JM, Johnstone CE, Lay V, Schmitt M, Watson H, Nisbet G (1995) Degradation of oxalic acid by transgenic oilseed rape plants expressing oxalate oxidase. Euphytica 85:169–172
Wan QJ, Zhang XJ, Zhang CG, Zhou XR (1997) Investigations on carbon fiber pH ultramicrosensor modified by polyaniline film and its application to the in vivo detection on Brassica stigmata. Chem J (Chin) 18:226–228
Wang ZX, Liu YH, Sun JS, Jia SR (2003) Cloning of glucose oxidase gene and expression in tobacco plants. Prog Nat Sci 13:248–252
Wei YD, Zhang Z, Anderson CH, Schmelzer E, Gregerson PL, Collinge DB, Smedegaard-Peterson V, Thordal-Christensen H (1998) An epidermis/papilla-specific oxalate oxidase-like protein in the defense response of barley attacked by the powdery mildew fungus. Plant Mol Biol 36:101–112
Woo EJ, Dunwell JM, Goodenough PW, Marvier AC, Pickersgill RW (2000) Germin is a manganese containing homohexamer with oxalate oxidase and superoxide dismutase activities. Nat Struct Biol 7:1036–1040
Zhang Z, Collinge DB, Thordal-Christensen H (1995) Germin-like oxalate oxidase, an H2O2-producing enzyme, accumulates in barley attacked by the powdery mildew fungus. Plant J 8:139–145
Zhang XJ, Ogorevc B, Wang J (2002) Solid-state pH nanoelectrode based on polyaniline thin film electrodeposited onto ion-beam etched carbon fiber. Anal Chim Acta 452:1–10
Zhou L, Yu Q, Liu S, Zhou B (1994) Resistance evaluation of rapeseed germplasm against Sclerotinia disease. Chin J Oil Crop Sci 17(Suppl):69–72
Zhou F, Zhang Z, Gregersen PL, Mikkelsen JD, de Neergaard E, Collinge DB, Thordal-Christensen H (1998a) Molecular characterization of the oxalate oxidase involved in the response of barley to the powdery mildew fungus. Plant Physiol 117:33–41
Zhou Y, Lin Z, Dong J (1998b) Recurrence selection in Brassica napus IV: efficiency of selection for resistance to Sclerotinia sclerotiorum. J Hua zhong Agric Univ 17:312–316
Zou Q, Liu S, Dong X, Bi Y, Cao Y, Xu Q, Zhao Y, Chen H (2007) In vivo measurements of changes in pH triggered by oxalic acid in leaf tissue of transgenic oilseed rape. Phytochem Anal 18:341–346
Acknowledgments
This work was supported by the Hi-Tech Research and Development Program of China (2006AA10A112 and JY04-B-01), National Natural Science Foundation of China (30671344). We thank Rhone-Poulenc Agrochimie (Lyon, France) for the kind gift of the cDNA encoding wheat oxalate oxidase. We are grateful to Zhixing Wang (Institute of Biotechnology, CAAS, Beijing, China) for kindly providing the pTΩ4A vector. We thank Yuandi Zhao and Qiuju Zou (Huazhong University of Science and Technology, Wuhan, China) for the in situ and real-time pH value measurement.
Author information
Authors and Affiliations
Corresponding author
Additional information
Xiangbai Dong and Ruiqin Ji contributed equally to this paper.
Rights and permissions
About this article
Cite this article
Dong, X., Ji, R., Guo, X. et al. Expressing a gene encoding wheat oxalate oxidase enhances resistance to Sclerotinia sclerotiorum in oilseed rape (Brassica napus). Planta 228, 331–340 (2008). https://doi.org/10.1007/s00425-008-0740-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-008-0740-2