Abstract
The detection of genes having similar expression profiles following the application of different stimuli that trigger bud break may constitute potent tools for the identification of pathways with a central role in dormancy release. We compared the effects of heat shock (HS) and hydrogen cyanamide (HC) and demonstrated that HS leads to earlier and higher bud-break levels. Changes in transcript levels of catalase, alcohol dehydrogenase and pyruvate decarboxylase were induced following both treatments. However, timing and extent of changes in transcript level differed. Changes occurred earlier in HS-treated buds and were more intense in HC-treated buds. The changes in transcript levels after both treatments were temporary. The rapid and short-lasting changes in gene expression following HS treatment correlated with the faster and higher level of bud-break that this treatment exerted. This correlation may propose that the reported molecular events are mechanistically involved in dormancy release. To test the hypothesis that temporary oxidative stress is part of the mechanism inducing dormancy release, we analyzed the effect of HS and HC treatments on the expression of ascorbate peroxidase, glutathione reductase, thioredoxin h, glutathione S-transferase and sucrose synthase genes and found that they were induced by both treatments in a similar pattern. Taken together, these findings propose that similar cellular processes might be triggered by different stimuli that lead to dormancy release, and are consistent with the hypothesis that temporary oxidative stress and respiratory stress might be part of the mechanism that leads to bud break.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In warm-winter regions, prolonged dormancy is a major obstacle for the commercial production of temperate fruits, including grapevine, which is widely distributed in subtropical regions (Shulman et al. 1983; Saure 1985; George et al. 1986; Erez 1987). At present, the control of dormancy release by the use of artificial dormancy-breaking compounds is needed to compensate for lack of natural chilling and is indispensable for maintaining the economic production of table grapes in these regions (Erez 1985, 1987, 1995). However, the currently available compounds are costly and entail a risk of bud damage due to their phytotoxicity (Erez 1987, 1995; Or et al. 1999). An understanding of the biological mechanisms involved in bud-dormancy release is crucial for the manipulation of bud-break timing. Extensive studies have been performed on various physiological aspects of dormancy (Nitsch 1957; Fuchigami et al. 1982; Saure 1985; Dennis 1986; Fuchigami and Nee 1987; Powell 1987; Martin 1991; Lang 1994; Borchert 2000; Arora et al. 2003). However, characterization of the complex network of molecular, biochemical and cellular processes responsible for the regulation and execution of bud-dormancy release is still limited and fragmentary, although progress was made in that direction since the late 1990s, in which studies at the molecular and cellular levels were initiated. These studies suggest involvement of regulation of gene expression, signaling mechanisms, cell to cell communication and cell cycle in dormancy release (Arora et al. 2003; Pang et al. 2007; Rohde and Bhalerao 2007). Few large-scale transcription profiling studies, using EST collections and microarrays, may shed light on additional candidate proteins and pathways that might be involved in the regulation and execution of bud dormancy release such as Lea proteins, GTP-binding proteins, flavonoid biosynthesis and auxin signaling (Pacey-Miller et al. 2003; Horvath et al. 2005, 2006; Keilin et al. 2007; Mazzitelli et al. 2007; Ruttink et al. 2007). Further studies with well defined experimental systems are needed for the identification of the core cellular processes that are connected with dormancy release.
Application of hydrogen cyanamide (HC), which is the most useful dormancy-breaking agent for grape (Shulman et al. 1983; Erez 1987, 1995; Henzell et al. 1991), provides a controlled system for the analysis of dormancy release in grape buds, with a relatively uniform response from the bud population. This system is an optimal tool for accurately defining the actual timing of induction of dormancy release, thereby enabling the detection of early changes following this induction (Or et al. 1999, 2000a, b, 2002).
Using this system, we have shown that catalase expression and activity are inhibited in grape buds shortly after HC application (Nir et al. 1986; Shulman et al. 1986; Or et al. 2002). Inhibition of catalase activity has been shown to lead to oxidative stress in several systems (Prasad 1996; Dat et al. 1998; Godon et al. 1998). Based on this, and on the induction of the anaerobic machinery following HC application (Or et al. 2000b), we previously speculated that HC application leads to the development of oxidative stress (Or et al. 2000b).
Other than chilling and HC, stress signals such as high temperature, desiccation and anaerobia (Lavee and May 1997) have been reported to release bud dormancy. However, it is not yet clear whether all of these external stimuli trigger a similar cascade of biochemical and cellular events inside the buds.
A combined study of changes in gene expression induced by different dormancy-breaking stimuli might help determine whether identical or different mechanisms are activated by these dormancy-breaking agents. If similar mechanisms are activated, identification of genes showing similar changes in expression pattern following application of two different dormancy-breaking stimuli could provide a potent tool for the discovery of pathways that are essential for dormancy release, independent of the primary artificial signal inducing the process.
Following this rationale, we present a comparative study of the effects of HC and heat shock (HS), which was selected for comparison based on its similar experimental advantages, on the release of grape-bud dormancy and on the expression patterns of selected genes. Our findings suggest that similar cellular processes might be triggered by HC and HS and support previous hypothesis that temporary oxidative stress and respiratory stress may be parts of the mechanism leading to bud-break.
Materials and methods
Plant material
The experiments were conducted with mature, cordon-trained grapevines (Vitis vinifera cv. Perlette) from commercial vineyards. The detached canes, each with nine buds (positions 4–12), were transferred to the lab for treatment. Canes were cut into single-node cuttings that were randomly mixed and placed in vases, with their bases immersed in water, at 22°C overnight. The following day, cuttings were separated into groups and used for the following treatments.
Chemical induction of dormancy release
Cuttings were sprayed with “Dormex” (SKW, Trostberg, Germany), a commercial formulation containing 49% HC. Control cuttings were sprayed with water (SC). Both Dormex and water were sprayed with the addition of a 0.02% Triton-X100 wetting agent.
Heat-shock induction of dormancy release
HS treatment was applied by immersing single-node cuttings in hot water for different periods of time. Details are given in figure legends. Control buds were immersed in water at RT (DC). Canes were treated as described previously (Or et al. 2000a).
Ten groups of ten single-node cuttings were sampled from each treatment. Cuttings were placed in water vases and forced at 22°C under a 14/10 h light/dark regime. Bud-break rate and level were tracked for 3–4 weeks, with bud break being defined as the stage at which green tissue becomes visible under the bud scales. For gene-expression analyses, buds were sampled from single-node cuttings that were placed under the aforedescribed conditions and sampled at 6, 12, 24, 48 and 96 h after treatment. Buds were immediately frozen in liquid nitrogen and kept at −80°C.
Northern-blot hybridization
Total RNA was isolated from the grape buds, collected as described above, according to Or et al. (2000b). RNA was denatured, fractionated by formaldehyde/agarose gel electrophoresis, and transferred to a nylon membrane (Hybond N+, Amersham) according to Sambrook et al. (1989). Hybridization was preformed with 32P-labeled probes. Probes for alcohol dehydrogenase (ADH), pyruvate decarboxylase (PDC) and catalase (CAT) were amplified from EST clones representing AF195866, AF195868, AF236127, respectively. Ascorbate peroxidase (APX) probe was amplified with primers designed based on TIGR Grape Unigene TC45171. Probes for thioredoxin h (TrH; TC25169), glutathione S-transferase (GST; TC25208), sucrose synthase (SuSy; TC31786), and stilben synthase (StSy; TC35597) were amplified from the corresponding pTripleEx2-based clones in our EST collection, using the insert-screening primers LDF and LDR according to the manufacturer’s instructions (Clontech, Palo Alto, CA, USA).
Hybridization buffer containing 5× SSC and 10× Denhardt solution was prepared as previously described by Sambrook et al. (1989) and hybridization temperature was 55°C. The membranes were washed with 2× SSC/0.1% SDS for 30 min at 60°C, followed by a 30-min wash with 1× SSC/0.1% SDS and a 30-min wash with 0.5× SSC/0.1% SDS, both at 60°C. Additional washes with 0.2× SSC/0.1% SDS and 0.1× SSC/0.1% SDS were carried out according to signal intensity at RT.
Quantitative real-time PCR
Specific primers were designed using the primer Express software, ABI PRISM™ (Applied Biosystems, Foster City, CA, USA). Primers for the endogenous control 18S rRNA were designed based on the nucleotide sequence of the 18S rRNA EST from the TIGR database (http://tigrblast.tigr.org/tgi). Primers for glutathione reductase (GR) were designed based on the conserved region of protein sequences of Vitis vinifera (AAB70837.1), Arabidopsis (NP_191026.1), Nicotiana tabacum (CAA53925.1) and Zea mays (CAA06835.1), using the NCBI database (http://www.ncbi.nlm.nih.gov). The annealing temperature was 60°C for all primer pairs.
Total RNA was isolated from mature buds. A total of 2.5 μg RNA from each sample was treated with RQ DNase (Promega, Madison, WI, USA) and reverse-transcribed using random hexamer primers (Promega). Real-time PCR was carried out using the SYBR green amplification kit (ABgene, Blenheim Road, Epsom, UK) according to manufacturer’s instructions.
Each reaction contained 1 μl cDNA and 300 nM of each primer from the relevant primer pair in a final volume of 20 μl. Quantitation of real-time PCR products was carried out by detection of SYBR green fluorescence on an ABI PRISM7000 detection system (AB Applied Biosystems). Dilution series of cDNA were created for each set of primers using cDNA obtained from mature buds and a standard curve was established for each gene. Duplicates of cDNA were used and each reaction was subjected to melting-point analysis to confirm single amplified products. Two biological repeats were carried out for each gene.
Transcript levels were estimated using a standard curve for each gene, and these levels were normalized against the amount of 18S rRNA transcript level in each sample, establishing a relative expression (RE) value.
Results
Comparison of bud-break rates and levels following HS and HC treatments
The identification of genes with similar expression profiles following the application of different dormancy-release signals could be a potent tool for the characterization of pathways with a central role in dormancy release. To set up such a comparative system, we first demonstrated the effect of HS on bud-break in our single-node-cuttings system and compared it to the effect of HC application.
Detailed studies carried out over several years in the Jordan valley, Israel (31° latitude) have shown that at the beginning of September, cv. Perlette buds are mostly non-dormant. Thereafter, endodormancy develops, reaching a maximum level at the beginning of November. This endodormancy is overcome at the beginning of January (Or et al. 2000a, b, 2002). We therefore performed controlled induction of dormancy release in mid-December, based on a dormancy curve (data not shown). At this stage, the bud population is still dormant, but it is close to the transition point from the endodormancy stage, in which bud growth is repressed by physiological factors inside the bud, to the ecodormancy stage, in which bud break is avoided because of environmental factors that do not support growth (Lang 1987).
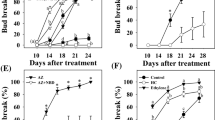
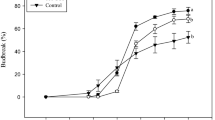
Both application of 5% “Dormex” and incubation for 1 h in a 50°C water bath resulted in increased bud-break rates compared to respective controls (Fig. 1). Bud break of HS-treated buds started as early as 10 days after treatment and reached 100% at 17 days. Bud break of HC-treated buds was first evident after 11 days and reached 88% at 21 days after treatment. Bud-break level of HS-treated buds was consistently higher compared to that of HC-treated buds at all time points. Both SC and DC controls showed significantly lower levels of bud break at all time points up to 21 days after treatment.
Influence of HS and HC treatment on dormancy release. Single-node cuttings from canes collected during endodormancy were divided into four experimental groups, each including ten subgroups of ten nodes each. The first group (HC) was sprayed with 5% Dormex (49% HC); the second group (HS) was immersed in 50°C water for 1 h; the third group (SC) was sprayed with water and the last group (DC) was immersed in RT water for 1 h. The treated cuttings were placed in water and transferred to a growth chamber at 22°C under 14/10 h light/dark. Bud break (%) was calculated at several time points during the forcing period. Values are averages of ten replications, consisting of ten buds each ±SD
In addition to the differences in the rate and level of bud break, HC and HS treatment differed in their effect on shoot appearance. Whereas the shoots developing from buds following heat treatment had a compact appearance and green leaves, similar to controls, those developed from HC-treated buds had an etiolated appearance, with longer nodes and smaller leaves that often carried necrotic edges (Fig. 2).
Effect of HS and HC treatment on shoot morphology. See Fig. 1 for experimental details
Comparison of expression profiles of ADH, PDC and CAT genes in buds following HS and HC treatments
The similar effects of HS and HC on bud break suggest similar release mechanisms. To investigate this hypothesis, we analyzed the effect of HS treatment on the expression profiles of ADH, PDC and CAT whose expression is markedly influenced by HC, as we previously reported (Or et al. 2000b, 2002).
Northern analysis showed that similar to the effect of the HC treatment, HS leads to a reduction in CAT transcript level and an increase in ADH and PDC transcript levels (Fig. 3). The changes in all transcript levels after both HS and HC were temporary and partial recovery was detected after 1–2 days. Whereas the pattern of the changes was similar for both treatments, the timing differed.
Effect of controlled bud-dormancy release by HC and HS on transcript level of alcohol dehydrogenase (ADH), pyruvate decarboxylase (PDC) and catalase (CAT). Equal amounts of total RNA (15 μg) originated from HC-treated (H), HS-treated (S) and control buds (C) were sampled at several time points after treatment. Northern blots were probed with radiolabeled PCR products amplified from clones representing ADH (AF195866), PDC (AF195868) and CAT (AF236127) genes. The ethidium bromide-stained gel is presented as a reference. Data are representative of four biological replications
Transcript levels of ADH and PDC were greater than in the control as early as 6 h after HS treatment, peaked at 12 h and decreased 1 day after treatment. CAT expression level showed the opposite expression profile following HS treatment, with some reduction at 6 h, its lowest level at 12 h, and by 24 h, its transcript had increased to a level exceeding that of the control. The effect of HC treatment on gene expression from all three genes was evident 1 day after treatment, when CAT transcript was hardly detectable and ADH and PDC levels were first induced. The lowest level of CAT and the highest levels of ADH and PDC were evident 2 days after HC treatment and partial recovery appeared after 4 days. Overall, HS treatment led to earlier changes than HC treatment.
Effect of HC and HS treatment on the expression profile of members of the ascorbate cycle
Based on the peroxide-scavenging activity of CAT and the roles of ADH and PDC during respiratory stress, we previously speculated that HC application leads to the development of temporary oxidative stress (Or et al. 2000b, 2002). The current results may justify similar hypothesis in the case of HS treatment. To further support it, we analyzed the effects of both HC and HS on the expression profiles of ascorbate peroxidase (APX) and glutathione reductase (GR) genes, two central players in the antioxidant defense system.
Northern analysis (Fig. 4a) indicated a sharp increase in APX transcript level which was highest at 12 h after HS treatment. HC treatment led to induction that was first evident at 12 h as well, but transcript level reached its highest level at 48 h and maintained this peak level to 96 h. Signals were more intense in response to the HC treatment.
Effect of controlled bud-dormancy release by HC and HS on transcript level of ascorbate peroxidase (APX) and glutathione reductase (GR). a The APX probe was amplified from an EST clone representing TC45171. b Expression levels of glutathione reductase (GR) were analyzed by quantitative real-time PCR using primers designed as described in “Materials and methods”. Expression values were determined based on a standard curve for the GR gene. Levels were normalized against the amount of ribosomal 18S RNA transcript in each sample. For experimental details see Fig. 3 and “Materials and methods”
Real-time PCR analysis (Fig. 4b) showed that GR gene expression is also induced following both treatments. The expression profile for GR following HS treatment was identical to that of APX. In response to HC treatment, GR transcript level increased after 12 h and maintained a similar level from then on.
Effect of HS and HC treatments on the expression pattern of genes related to the antioxidant defense system
We recently generated a large-scale grape-bud EST collection, based on four bud libraries from different developmental stages. Computational expression profiling of this collection exposed genes/functions whose expression is altered in response to HC treatment (Keilin et al. 2007), including several genes known to be involved in the antioxidant defense system. Among them were glutathione S transferase (GST), stilbene synthase (StSy) and thioredoxin h (TrH). Here we compared the expression profiles of GST, StSy and TrH after both HC and HS treatments. Transcription of GST and TrH was induced by both HS and HC (Fig. 5a). Induction was evident 6 h after both treatments, exhibited its highest level for HS-treated buds at 12 h (TrH) and 48 h (GST) after which it decreased. Following HC treatment, transcripts reached their highest level at 48 h and decreased at 96 h. Signals were more intense in response to HC treatment. In contrast, StSy expression was induced only following HC treatment and its highest transcript level was reached at 24 h (Fig. 5b).
Effect of controlled bud-dormancy release by HC and HS on transcript level of glutathione S-transferase (GST), thioredoxin h (TrH), stilbene synthase (StSy) and sucrose synthase (SuSy). a Probes for SuSy, GST, and TrH were amplified from clones representing TC31786 (692 bp), TC25208 (753 bp) and TC25169 (900 bp), respectively. b Probe for StSy was amplified from a clone representing TC35597 (800 bp). For experimental details see Fig. 3
Effect of HS and HC treatments on carbon metabolism/respiration
Induction of ADH and PDC is indicative of respiratory stress. In plant tissues exposed to low oxygen, these fermentation-related enzymes are induced in parallel with a small group of glycolysis-related enzymes, including SuSy, which has recently been shown to be required for survival under low oxygen conditions (Subbaiah and Sachs 2003; Koch 2004). SuSy expression was induced following both treatments (Fig. 5a). Increased transcript level was evident 12 h after both treatments and presented a pattern similar to that described for TrH and GST.
Effect of temperature and incubation period on bud-break rate and total bud break
We examined whether the observed positive effect of HS on bud break would be retained following modifications in HS application. A matrix of incubation temperature and duration was created and examined. From Fig. 6a, it can be concluded that under long incubation (2 h) at 45°C and 50°C, bud break is similar to that following 3% HC treatment. Higher temperature had a negative effect. Shorter incubation (1 h) allowed optimal bud break at 55°C as well (data not shown).
Influence of HS temperature (a) and duration (b) on dormancy release. One group was sprayed with 3% Dormex (HC) and another with water (Control). The other groups were incubated in water baths at different temperatures for 2 h (a) or for different periods of time at 70°C (b). For experimental details, see Fig. 1 and “Materials and methods”
Based on our results, it appears that long incubation periods at lower temperatures can be replaced by very short incubations of 10–30 s at 70°C (Fig. 6b), which lead to a bud-break rate similar to that achieved by HC treatment. A longer incubation of 1 min (Fig. 6b), as well as a 10-s incubation at higher temperatures (80°C and above; data not shown), had a negative effect on bud break.
Discussion
The stress-related characteristics of natural and artificial signals such as chilling, heat and HC suggest similar dormancy-release mechanisms. To inquire whether such similarity exists, we compared expression patterns of selected genes following both HS and HC treatments.
HS led to an earlier and higher level of bud break and eliminated the negative effects that often arise following HC treatment. Similar to the HC treatment, HS led to a reduction in CAT transcript level and an increase in ADH and PDC transcript levels. The changes in transcript levels after both treatments were temporary and recovery was detected after 1–2 days. These data are consistent with the hypothesis that HS and HC trigger similar cellular processes that may lead to the induction of bud break. It also supports our previous hypothesis regarding the transient nature of the response to the HC signal (Or et al. 2000b) and suggests a similar response following HS treatment. While the pattern of changes was similar, timing and extent of changes in transcript level differed. Changes occurred earlier in HS-treated buds and were more intense in HC-treated buds. The rapid and short-lived changes in gene expression following HS positively correlated with the faster and higher bud break that this treatment exerts. This association between molecular and horticultural findings supports the hypothesis that dormancy release is governed, at least in part, by the products of the affected genes, in a process that is controlled at the level of gene expression.
The similar effect of the two different dormancy-breaking signals on the expression of CAT, PDC and ADH genes was in accordance with the previously raised hypothesis that temporary oxidative stress and respiratory stress are part of the mechanism leading to grape bud break (Or et al. 2000b). To further study this assumption, we compared the effects of HS and HC treatments on the function of the antioxidant defense system. Expression from APX and GR genes, coding for the main players in the ascorbate–glutathione cycle (Buchanan et al. 2000), was induced following both treatments. As with the former analyzed genes, the response following HS treatment was earlier and shorter. The induction of APX and GR may testify on the development of oxidative stress within the bud during induction of dormancy release. Although lacking physiological characterization of the buds dormancy status at the sampling time points, preliminary data presented by Pacey-Miller et al. (2003) support this assumption. In that work, induction of GR and glutathione peroxidase transcript levels was recorded 3–4 weeks before natural bud burst. Based on our experience with induced dormancy release of dormant grape buds, it takes about 4 weeks under spring field conditions from controlled induction of dormancy release (by HC or HS) to actual bud break (Or et al. 1999, 2000a and unpublished data). Therefore, the induction reported by Pacey-Miller et al. (2003) probably represents events occurring during the dormancy-release stage.
In accordance with these results at the transcriptional level, Wang et al. (1991a, b) reported on a temporary enhancement of activities of APX, ascorbate free radical reductase (AFR), and dehydroascorbate reductase (DHAR), as well as steady increase in GR activity in response to application of thidiazuron that induces apple bud break. However, based on their preliminary finding that thidiazuron diminished free radical formation several days after thidiazuron application (Wang and Faust 1988), the authors’ interpretation was that decrease in free radical level is required for the termination of dormancy and the induction of bud break.
To further support the assumption that temporary oxidative stress is part of the mechanism leading to grape bud break, we compared the expression profiles of GST, TrH and StSy, known to be induced by oxidative stress and involved in the antioxidant defense system (Keilin et al. 2007), after both HC and HS treatments. In accordance with the results described above, both treatments led to increase in GST and TrH transcript levels.
When comparing HS and HC treatments, we also expect the presence of treatment-unique effects that may stem from quantitative or qualitative differences between the HS and HC signals. One such example observed here was the difference in the response of a StSy gene, which was induced by HC but not by HS. This may be the result of the stronger oxidative stress developed following HC treatment. Analyses of other members from the same gene family are needed to further explore this issue.
Altogether, the above described findings are consistent with the concept that temporary oxidative stress may be a part of the mechanism leading to bud break following both HC and HS. The putative involvement of oxidative stress in signal transduction is now widely accepted (Conrath et al. 1997; Foyer et al. 1997; Anderson et al. 2001) and the induction of calcium signaling in plant cells by oxidative stress was recently demonstrated (Price et al. 1994; Pei et al. 2000; Foreman et al. 2003; Rentel and Knight 2004). Interestingly, we recently detected HC-induced expression of a Ca2+-ATPase, calmodulin, calmodulin-binding protein and a calcium-dependent protein kinase (CDPK) in grape buds. We also showed that both LaCl3 and EGTA blocked the inducing effect of HC on bud break, and their inhibitory effects were removed by supplying exogenous Ca2+. Additionally, we detected a protein that presented strong and Ca2+-dependent phosphorylation only in HC-treated buds, and presented evidence for a potential role of CDPK in the phosphorylation of this protein (Pang et al. 2007). These data suggest that calcium signaling is involved in the mechanism of bud-dormancy release. To investigate the hypothesis that oxidative stress directly induces calcium signaling cascade in grape buds, further study will be needed.
SuSy upregulation that was recorded following both HC and HS is also evident in response to cold shock, water deprivation, anoxia and salt stress, and in several cases has been shown to be mediated by calcium (Marana et al. 1990; Subbaiah and Sachs 2003; Baud et al. 2004; Gu et al. 2004). The induction of SuSy, PDC and ADH following application of HC and HS implies that these signals affect the tissue in a way that mimics anoxic conditions and hints at the mitochondria as a potential sensing center for the signals. Interestingly, TrH was recently proven to reside in the mitochondria and regulate activity of the alternative oxidase (Gelhaye et al. 2004). Further support for temporary induction of glycolysis in response to bud break stimuli was supplied by Wang et al. (1991b), which documented temporary increase in activities of the glycolytic enzymes glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and pyruvate kinase (PK) following application of thidiazuron to apple buds (Wang et al. 1991b).
Under the light of these molecular findings as well as the slower but more intensive response of the bud to HC, we raise here the possible existence of a primary module in the cascade. The extent of effect of HC on this module may be responsible for the delay in response, which may be attributed to a primary period of metabolic arrest. The degree of such arrest may determine the intensity of the antioxidative machinery response at a second stage. Also lacking direct evidence to support this hypothesis at the moment, we suggest the oxidative phosphorylation process as a candidate for such primary module. Temporary inhibition of this process may lead to a respiratory stress, induction of glycolysis as a feedback effect, production of ROS and induction of the antioxidative machinery (Buchanan et al. 2000). Primary oxidative stress may then be amplified by inactivation of catalase at the second stage. Inhibition of Cat activity under oxidative stress was recently attributed to prior accumulation of salicylic acid under such conditions (Shim et al. 2003). The delayed reduction in CAT expression in response to HC, as compared to HS, may support this scenario. The production of ROS may then serve to induce a signaling cascade. Accordingly, a recent study (Perez and Lira 2005) reconfirmed the inhibitory effect of HC and chilling on CAT activity and the rise in peroxide level in grape bud, which was reported by our group in the past (Nir et al. 1986; Nir and Lavee 1993). This study demonstrated a transient rise in H2O2 levels that preceded the release of bud from endodormancy, and recorded earlier peak of H2O2 following HC application, which was correlated with earlier onset of bud break, compared to control (Perez and Lira 2005). These findings led the authors to support the hypothesis regarding the involvement of temporary oxidative stress in the mechanism leading to grape bud break and the possible participation of H2O2 as a signal molecule in the release of endodormancy in grape buds (Or et al. 2000b; Perez and Lira 2005).
The first attempt to use microarrays for a large scale characterization of changes in gene expression of woody bud during dormancy release was reported very recently by Mazzitelli et al. (2007). Endodormancy release of raspberry lateral buds, which was stimulated by controlled chilling, was accompanied by induction of APX, GST and GR genes, among other changes detected based on the sequencing of selected, most significantly differentiated genes. Mazzitelli et al. (2007) also presented findings supporting enhanced import of sugars that support the data documented in the current study. Reduction in the level of dehydrin transcript and induction of members of the ubiquitination machinery, calmodulin––regulated ion channel, ATPase, GTP-binding proteins and SNF-related kinase also resemble changes that we detected during grape bud dormancy release (Or et al. 2000b; Keilin et al. 2007; Pang et al. 2007). These similarities may suggest similar cellular processes during dormancy release in woody buds from different species specious in response to different stimuli-natural and artificial.
Abbreviations
- ADH:
-
Alcohol dehydrogenase
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- CDPK:
-
Calcium-dependent protein kinase
- EST:
-
Expressed sequence tag
- GR:
-
Glutathione reductase
- GST:
-
Glutathione S-transferase
- HC:
-
Hydrogen cyanamide
- HS:
-
Heat shock
- PDC:
-
Pyruvate decarboxylase
- RE:
-
Relative expression
- ROS:
-
Reactive oxygen species
- SDS:
-
Sodium dodecyl sulfate
- StSy:
-
Stilben synthase
- SuSy:
-
Sucrose synthase
- TrH:
-
Thioredoxin h
References
Anderson JV, Chao WS, Horvath DP (2001) A current review on the regulation of dormancy in vegetative buds. Weed Sci 49:581–589
Arora R, Rowland LJ, Tanino K (2003) Induction and release of bud dormancy in woody perrenials: ascience comes of age. HortScience 38:911–921
Baud S, Vaultier MN, Rochat C (2004) Structure and expression of the sucrose synthase multigene family in Arabidopsis. J Exp Bot 396:397–409
Borchert R (2000) Organismic and envrionmental controls of bud growth in tropical trees. In: Viemont JD, Crabbe J (eds) Dormancy in plants. From whole plant behaviour to cellular control. CABI Publishing, New York, pp 87–108
Buchanan BB, Gruissem W, Jones RL (2000) Biochemistry and molecular biology of plants. American Society of Plant Physiologists, Rockville, pp 1189–1191
Conrath U, Silva H, Klessig DF (1997) Protein dephosphorylation mediates salicylic acid-induced expression of Pr-1 genes in tobacco. Plant J 11:747–757
Dat JF, Lopez-Delgado H, Foyer CH, Scott IM (1998) Parallel changes in H2O2 and catalase during thermotolerance induced by salicylic acid or heat acclimation in mustard seedlings. Plant Physiol 116:1351–1357
Dennis FG (1986) Two methods of studying rest: temperature alteration and genetic analysis. HortScience 22:820–824
Erez A (1985) Defoliation of deciduous fruit trees with magnesium chlorate and cyanide. HortScience 20:452–453
Erez A (1987) Chemical control of bud break. HortScience 22:1240–1243
Erez A (1995) Means to compensate for insufficient chilling to improve bloom and leafing. Acta Hortic 395:81–95
Foreman J, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Torresk MA, Linstead P, Costa S, Bronlee C, Jonesk JDG, Davies JM, Dolan L (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422:442–446
Foyer CH, Lopes-Delgado H, Dat JF, Scott IM (1997) Hydrogen peroxide and glutathione-associated mechanisms of acclimatory stress tolerance and signaling. Physiol Plant 100:1554–1561
Fuchigami LH, Nee CC (1987) Degree growth stage model and rest-breaking mechanisms in temprate woody perennials. HortScience 22:836–844
Fuchigami LH, Weiser CJ, Kobayashi K,Timmis R, Gusta LV (1982) A degree growth stage (oGS) model and cold acclimation in temperate woody plants. In: Li PH, Sakai A (eds) Plant cold hardiness and freezing stress, vol 2. Academic Press, New York, pp 93–116
Gelhaye E, Rouhier N, Gerard J, Jolivet Y, Gualberto J, Navrot N, Ohlsson PI, Wingsle G, Hirasawa M, Knaff DB, Wang H, Dizengremel P, Meyer Y, Jacquot JP (2004) A specific form of thioredoxin h occurs in plant mitochondria and regulates the alternative oxidase. Proc Natl Acad Sci USA 101:14545–14550
George AP, Nissen RJ, Baker JA (1986) Low chill peach and nectarine cultivars. Qld Agric J 112:27–33
Godon C, Lagniel G, Lee J, Buhler J, Kieffer S, Perrot M, Boucherie H, Toledano MB, Labarre J (1998) The H2O2 stimulon in Saccharomyces cerevisiae. J Biol Chem 273:22480–22489
Gu R, Fonseca S, Puskas LG, Hackler L Jr, Zvara A, Dudits D, Pais MS (2004) Transcript identification and profiling during salt stress and recovery of Populus euphratica. Tree Physiol 24:265–276
Henzell RF, Briscoe MR, Gravett I (1991) Improving kiwifruit vine productivity with plant growth regulators. Acta Hortic 297:345–350
Horvath DP, Anderson JV, Soto-Suarez M, Chao WS (2006) Transcriptome analysis of leafy spurge (Euphorbia esula) crown buds during shifts in well-defined phases of dormancy. Weed Sci 54:821–827
Horvath DP, Soto-Suarez M, Chao WS, Jia Y, Anderson JV (2005) Transcriptome analysis of paradormancy release in root buds of leafy spurge (Euphorbia esula) crown buds during shifts in well-defined phases of dormancy. Weed Sci 53:795–801
Keilin T, Pang X, Venkateswari J, Halaly T, Crane O, Keren A, Ogrodovitch A, Ophir R, Volpin H, Galbraith D, Or E (2007) Digital expression profiling of a grape-bud EST collection leads to new insight into molecular events during grape-bud dormancy release. Plant Sci 173:446–457
Koch K (2004) Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr Opin Plant Biol 7:235–246
Lang GA (1987) Dormancy: a new universal terminology. HortScience 22:817–820
Lang GA (1994) Dormancy-the missing links: molecular studies and integration of regulatory plant and environmental interactions. HortScience 29:1255–1263
Lavee S, May P (1997) Dormancy of grapevine buds-facts and speculation. Aust J Grape Wine Res 3:31–46
Marana C, Garcia-Olmedo F, Carbonero P (1990) Differential expression of two types of sucrose synthase-encoding genes in wheat in response to anaerobiosis, cold shock and light. Gene 88:167–172
Martin GC (1991) Bud dormancy in deciduous fruit trees. In: Steward FC (ed) Plant physiology: a treatise. Vol. X: Growth and development. Academic Press, New York, pp 183–225
Mazzitelli L, Hancock RD, Haupt S, Walker PG, Pont SDA, McNicol J, Cardle L, Morris J, Viola R, Brennan R, Hedley PE, Taylor MA (2007) Co-ordinated gene expression during phases of dormancy release in raspberry (Rubus idaeus L.) buds. J Exp Bot 58:1035–1045
Nir G, Lavee S (1993) Metabolic changes during cyanaimide induced dormancy release in grapevines. Acta Hortic 329:271–274
Nir G, Shulman Y, Fanberstein L, Lavee S (1986) Changes in the activity of catalase (EC 1.11.1.6) in relation to the dormancy of grapevine (Vitis vinifera L.) buds. Plant Physiol 81:1140–1142
Nitsch JP (1957) Photoperiodism in woody plants. Proc Am Soc Hort Sci 70:526–527
Or E, Nir G, Vilozny I (1999) Timing of hydrogen cyanamide application to grapevine buds. Vitis 38:1–6
Or E, Belausov E, Popilevsky I, Ben-Tal Y (2000a) Changes in endogenous ABA level in relation to the dormancy cycle in grapevine grown in hot climate. J Hortic Sci Biotechnol 75:190–194
Or E, Vilozny I, Eyal Y, Ogradovitch A (2000b) The transduction of the signal for grape bud dormancy breaking induced by hydrogen cyanamide may involve the SNF-like protein kinase GDBRPK. Plant Mol Biol 43:483–494
Or E, Vilozny I, Fennell A, Eyal Y, Ogrodovitch A (2002) Dormancy in grape buds: isolation and characterization of catalase cDNA and analysis of its expression following chemical induction of bud dormancy release. Plant Sci 162:121–130
Pacey-Miller T, Scott K, Ablett E, Tingey S, Ching A, Henry R (2003) Genes associated with the end of dormancy in grapes. Funct Integr Genomics 3:144–152
Pang X, Halaly T, Crane O, Keilin T, Keren A, Ogrodovitch A, Galbraith D, Or E (2007) Involvement of calcium signaling in dormancy release of grape buds. J Exp Bot 58:3249–3202
Pei ZM, Murata Y, Benning G (2000) Calcium channels activatd by hydrogen peroxide mediate abscisic acid signaling in guard cells. Nature 406:731–734
Perez FJ, Lira W (2005) Possible role of catalase in post-dormancy bud break in grapevines. J Plant Physiol 162:301–308
Powell LE (1987) Hormonal aspects of bud and seed dormancy in temperate-zone woody plant. HortScience 22:845–850
Prasad TK (1996) Mechanisms of chilling-induced oxidative stress injury and tolerance in developing maize seedling: changes in antioxidant system, oxidation of proteins and lipids, and protease activities. Plant J 10:1017–1026
Price AH, Taylor A, Ripley SJ, Griffiths A, Trewavas AJ, Knight MR (1994) Oxidative signals in tobacco increase cytosolic calcium. Plant Cell 6:1301–1310
Rentel MC, Knight MR (2004) Oxidative stress-induced calcium signaling in Arabidopsis. Plant Physiol 135:1471–1479
Rohde A, Bhalerao RP (2007) Plant dormancy in the perennial context. Trends Plant Sci 12:218–223
Ruttink T, Arend M, Morreel K, Storme V, Rombauts S, Fromm J, Bhalerao RP, Boerjan W, Rohde A (2007) A molecular timetable for apical bud formation and dormancy induction in poplar. Plant Cell 19:2370–2390
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning. A laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Saure M (1985) Dormancy release in deciduous fruit trees. Hortic Rev 7:239–300
Shim I, Momose Y, Yamamoto A, Kim D, Usui K (2003) Inhibition of catalase activity by oxidative stress and its relationships to salicylic acid accumulation in plants. Plant Growth Regul 39:285–92
Shulman Y, Nir G, Fanberstein L, Lavee S (1983) The effect of cyanamide on the release from dormancy of grapevine buds. Sci Hortic 19:97–104
Shulman Y, Nir G, Lavee S (1986) Oxidative processes in bud dormancy and the use of hydrogen cyanamide in breaking dormancy. Acta Hortic 179:141–145
Subbaiah CC, Sachs MM (2003) Molecular and cellular adaptations of maize to flooding stress. Ann Bot 91:119–127
Wang SY, Faust M (1988) Metabolic activities during dormancy and blooming of deciduous fruit trees. Isr J Bot 37:227–243
Wang SY, Jiao HJ, Faust M (1991a) Changes in ascorbate, glutathione, and related enzyme activities during thidiazuron-induced bud break of apple. Physiol Plant 82:231–236
Wang SY, Jiao HJ, Faust M (1991b) Changes in metabolic enzyme activities during thidiazuron-induced lateral budbreak of apple. HortScience 82:231–236
Acknowledgments
This research was supported by Research Grant Award No. IS-3340-02 from BARD, The United States-Israel Binational Agricultural Research and Development Fund.
Author information
Authors and Affiliations
Corresponding author
Additional information
T. Halaly and X. Pang contributed equally to the paper.
Rights and permissions
About this article
Cite this article
Halaly, T., Pang, X., Batikoff, T. et al. Similar mechanisms might be triggered by alternative external stimuli that induce dormancy release in grape buds. Planta 228, 79–88 (2008). https://doi.org/10.1007/s00425-008-0720-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-008-0720-6