Abstracts
A unique fiber spinning was found in protoplasts from white birch (Betula platyphylla) leaves under an acidic medium containing high concentration of Ca2+. After expanding from 10 to 100 μm in diameter under the culture condition, the protoplast started secreting a gigantic fiber while moving in a spiral way. Real time video analyses elucidated that the orientation, rate and pattern of the motion were directed due to the inverse force of the fiber spinning. Moreover, observation using several microscopic methods accompanied with histochemical staining and nuclear magnetic resonance (NMR) analysis indicated that the fiber was composed of 400–500 nm wide (1→3)-β-glucan hollow sub-fibrils. This entire phenomenon may be a response against the stress imposed. The observation presented provides an understanding of the unique relationship between fiber spinning and the bottom-up fiber fabrication from nano to micro scales.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Previously, the authors (TK) reported that the physicochemical stress caused by cell expansion and stabilization in developing stages might alter the crystalline structure in the transition from primary to secondary walls using spectroscopic analyses accompanied with microscopic attachments (Kataoka and Kondo 1996, 1998, 1999). The studies pointed out the possibility that stress-induced effects may alter cell wall structure and even the higher suprastructure in higher plants. Therefore, the approach undertaken in the present study was to provide a stress for a higher plant protoplast prepared from the leaves by modifying the condition of the Murashige and Skoog (MS) medium (Murashige and Skoog 1962) and the atmosphere of CO2 content, and thus imposing a stress. These could result in the discovery and patenting (Kondo et al. 2000) of a unique phenomenon that a gigantic fiber was spun from a single site on the surface of protoplasts under an acidic culture condition, in the presence of high concentration of Ca2+ under a 5% CO2 atmosphere.
In this article, we report on a new type of hierarchically built-up fiber fabrication at the nano/micro scales of a biological system using protoplasts. The fiber-secreting phenomenon in our discovery was carefully examined in relation to the effect of culture conditions, including the effect of pH and ion concentration, on regeneration of protoplasts and secretion of fibers. Then, structural characterization of the produced fiber was undertaken. Real time video analysis demonstrated a close relationship between native synthesis of the fiber and the movement of the protoplast. This motion is due to an inverse force derived from spinning of the gigantic fiber by the protoplast. Furthermore, the fiber was indicated in fact to be a bundle of hollow sub-fibrils of (1→3)-β-glucan, which may cause spiral formation of both the movement and fiber. This leads to that the individual sub-fibrils might be secreted from the separated sub-sites in a secreting site on the cell surface before aggregating into a single, larger bundle. The additional goal of this study is to offer an insight of designing bio-based fiber materials as well as a response against the stress imposed in biological systems by understanding creation of a hollow fiber structure having a specific nano/micro-structure through the moving behavior due to fiber spinning of the cell.
Materials and methods
Materials
The plantlet of Betula platyphylla Sukatchev var. japonica Kan No.8 was cultivated under a sterilized condition in Murashige and Skoog (MS) agar medium (Murashige and Skoog 1962) including hormones with 2.5 μM indol-3-butyric acid (IBA) and 0.1 μM α-naphthaleneacetic acid (NAA) for isolation of protoplasts from their leaves. Irradiation of light including the whole range of visible wavelength was 3,000–4,000 lux in strength. The interval between the irradiation and the dark was controlled alternately 16 h’ irradiation and 8 h’ dark at 25°C, respectively.
Isolation of protoplasts
Betula leaves at the young stage (3–4 leaves were enough for the preparation) were employed to isolate protoplasts. The leaves at first floated were left in the 20 ml of 0.6 M mannitol solution containing the 1% of cellulase ONOZUKA R10 (Yakult HONSHA Co. Ltd., Tokyo, Japan) and the 1% of driselase (Kyowa Hakko Kogyo Co. Ltd., Tokyo, Japan) for 18 h without shaking. After the enzyme treatment, they were transferred into 0.6 M mannitol enzyme-free solution, and then dismembered gently with tweezers to relieve the protoplasts. The mannitol solution including protoplasts was filtered with a sheet of nylon mesh with the pore size of 40 μm. Following the second and third washing, the isolated protoplasts were collected as precipitates by centrifugation with a constant rotating rate of 800 rpm (150G) for 3 min. Then, a fresh mannitol solution was added for further centrifugation, and this treatment was repeated twice. Finally, 1 ml of mannitol solution was added to pick up the protoplasts from the solution. They were provided on a counter slide glass to count the cell number and to calculate viability by staining with fluorescein diacetate (FDA).
Culture condition
The obtained protoplasts were cultured in the MS liquid medium with 1/2 strength of the standard including hormones 1 μM of NAA and 1 μM of N-(2-chloro-4-pyridyl)-N′-phenylurea (4PU), and then 200 mM CaCl2 was added to the culture medium under an acidic condition adjusted at pH 3.5. Following the cell densities were adjusted to ca. 5 × 104 cells/ml in the culture well (2,500 cells/well), they were incubated at 28°C in the dark without shaking. The protoplasts in the culture plate were observed under the inversed light microscope (CK40, OLYMPUS Co.). It should be noted that all data obtained by observation were averaged with more than a few hundreds samples.
Real time video analysis
Light microscopic images were acquired with 10× objective lenses, coupled with a 1.0× optivar lens using a Leica light microscope with an cooled CCD camera (C-5810, Hamamatsu Photonics Co., Japan), attached with a 1.0× camera lens (HR 100-CMT) from Diagnostic, Instruments Inc. Frames were captured, digitized, saved, and processed using Image Pro Plus software v.4.1 (Media Cybernetics) at an acquisition rate of 1 frame/min. Samples observed were protoplasts in 96 well culture plates at room temperature. The focus of the camera was set at the site on the surface secreting a fiber, so that both cell and the synthesized fiber could be observed. Using Adobe Premier v6.0, each captured frame was sequenced to produce a movie at the rate of 30 fps.
Confocal UV-laser scanning microscopy
Protoplasts producing a gigantic callose fiber were picked up with a pustule pipette from the culture plate and placed them on the hole of a special glass plate. The fiber was stained with aniline blue fluorochrome, whereas the cell was stained with FDA. 3-D successive images across the cross section with changing the focus on the secreting site of the cell were individually captured using a confocal UV-laser scanning microscope (FV 500-UV; Olympus Co.) with a UV-Ar laser source. The images were acquired with 20× objective lenses, attached with a 2.0× camera lens from Olympus Co. UV wave lengths for excitation were 351 and 488 nm, respectively, and the corresponding detected wave lengths for emission were 380–470 nm and 505 nm. The scan speed across the cross section was 4.33 s/image and the step size was 0.625 μm. The scanning z height was 29.375 μm/48 images. The obtained images were de-convoluted to build the 3D-image. The 3D image was also tilted at 45° of the initial position to expose the secreting site on the surface of the cell.
Histochemical staining of the fiber
Fibers secreted directly from protoplasts into the medium under the above stressed condition were picked up by a micromanipulator, and then put into a Petri dish to be washed with sterilized distilled water and 50 mM EDTA (Ethylenediaminetetraacetic acid) solution to remove the medium and the excess Ca2+ ions. The fibers stained with 0.01% aniline blue fluorochrome were provided for observation using fluorescent microscopy.
Chemical identification of the fiber component using nuclear magnetic resonance analysis
The spun fibers from a protoplast as well as curdlan powder (liner (1→3)-β-glucan) from Alcaligenes faecalis (purchased by Sigma Co.) as a reference sample were prepared as follows. The fiber was collected by a micromanipulator from the culture plate and washed with same manner as described above. The washed fibers were put into a small tube and added into D2O to solvent-exchange from H2O to D2O before dissolved in 1.5% NaOD/D2O solution. The curdlan powder was directly dissolved in 1.5% NaOD/D2O solution. Both samples were centrifuged (15,000 rpm, 10 min) and the supernatants were employed to the NMR analysis.
All spectra were recorded with 700 MHz NMR spectrometer (JNM-ECA700, JEOL). For 1H NMR a 45° observe pulse was used to record 256 transients of 16,384 data points. The recorded spectrum was modified with DANTE presaturation method (Zuiderweg et al. 1986) to remove signals of water. The 13C DEPT135 (Distortionless Enhancement by Polarization Transfer) NMR spectrum of the fiber was recorded by collecting 200k scans of 32468 data points with an acquisition time (AQ) of 0.59 s and relaxation delay of 2 s. All signals in the spectrum were modified into same direction during analysis. 13C NMR spectrum of curdlan was recorded with single pulse decoupled for NaOD/D2O.
Structural characterization of the fibers spun from protoplasts
Preparation of fiber samples for AFM and TEM observations
The collected fibers spun from protoplasts were washed in the same manner as described above. For AFM observation, the fiber specimens were provided without further preparation.
For TEM observations, the fibers were embedded in a resin and then sectioned with less than 100 nm in thickness for preparation of sample specimens: the fibers were fixed with 2.5% glutaraldehyde and then washed with 50 mM Piperazine-N,N′-bis(2-ethane sulfonic acid) (PIPES) buffer adjusted at pH 6.9. They were dehydrated with an ethanol series of 30, 50, 70, 90 and 100% of aqueous ethanol, prior to the embedding in a LR White resin. After the sample specimens were treated at 50°C for 24 h to allow the resin to be polymerized, the obtained embedded samples were sectioned using an ultramicrotome (LKB Co.).
The samples on the capper 3.0 mm TEM grids were then subjected by an immuno-gold labeling method; they were incubated at 30°C for 2 h in a 100 μl blocking solution including a 5% normal serum, 0.8% BSA, 0.1% gelatin and 2 mM NaN3. After the incubation, they were treated with 100 μl of the blocking solution including the callose ((1→3)-β-glucan) antibody as a primary antibody (Meikle et al. 1991) at 30°C for 2 h or at 4°C over night. After washed with the serum-free blocking solution as the washing solution, the sections were incubated at 30°C for 2 h in 100 μl of the same blocking solution including a secondary antibody, mouse anti-rabbit IgG, labeled with 20 nm in diameter of gold particle. Then, the sections were washed with the washing solution and PBS (Phosphate buffer saline, pH 7.4). After fixed with 2% glutaraldehyde in PBS, the sections were washed with PBS and then with sterilized, distilled water.
Before observation by TEM, all sample specimens above treated were stained with both 2% aqueous uranyl acetate and Reynold’s lead citrate, and finally air-dried.
Atomic force microscopic analysis
The fibers prepared for AFM observation as described above were put on a mica substrate, and then dried in the air. AFM images of the fiber were acquired on a NanoScope IIIa (Digital Instruments) at room temperature, being controlled in the tapping mode with a scan rate 0.5 Hz to observe 5 × 5 μm2 areas. The AFM tip employed was an etched-silicon tip with a nominal radius from 5 to 15 nm, and a spring (force) constant of 42 N m−1, which was shaped like a polygon-based pyramid with a height of about 10–15 μm and a cone angle of 35°. The scanning was carried out in both directions of the fiber axis and perpendicular to the axis. The width and height of the fiber aggregates were measured using a cross-sectional line profile analysis. The apparent width data (D) were supposed to include geometrical enhancement (E) depending on the radius (R) of the AFM tip (Hanley et al. 1992). In this observation, as height of the aggregates was larger than radius of the tip, the following correction equation was employed (Kondo et al. 2002). The equation takes the tip cone angle (A) into account in the form,
where E geometrical enhancement on the real width value (w), W apparent width observed in AFM, H height of the object observed in AFM, and R the AFM tip radius. In this study we employed the R-value as 10 nm in radius.
As it was difficult in determining the size of specific patterns such as a distance between two parallel lines in the surface morphology of the AFM image, the width of the fiber was obtained mathematically in the following: the second derivative of the calibrated cross section image was employed to determine width of the individual sub-fibril. The second derivative was performed using Microcal Origin 6.0J software (Origin Lab Co.). The tops of the second derivative peaks correspond groove lines in the surface. Therefore, the distance between neighboring peaks are supposed to be width of the individual sub-fibrils.
Confocal sectioning fluorescent microscope observation
Confocal sectioning microscopy was employed as a tool for in situ observation of the phenomenon, because of using a weaker light source of Hg lump. Protoplasts producing a gigantic callose fiber were picked up with a pustule pipette from the culture plate, and placed them on a special glass plate with the same thickness as a cover glass. Samples were incubated by both aniline blue fluorochrome and FDA, which specifically stained a (1→3)-β-glucan and living cells, respectively. After staining, the samples were observed by a sectioning microscope with fluorescent attachments using a Hg lump (Delta vision 4.0, Seki Technotron co.); the sectioning step across the cell in observation was 0.2 μm each using ×60 oil lens, and two kinds of emission wave lengths, 457 and 528 nm were detected due to aniline blue fluorochrome and FDA, respectively. 3-D successive images across the cross section with changing the focus on the secreting site of the protoplast were individually captured and then de-convoluted.
Transmission electron microscopic analysis
The sample specimens were observed with a transmission electron microscope (JEM-2000EX; JEOL Co. Ltd) operated at accelerating voltage of 100 kV with a beam current less than 5 μA.
Results
Secretion of a gigantic fiber from a birch protoplast directly into the medium
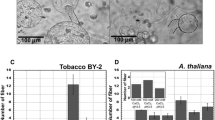
The initial culture condition employed in our study was chosen on the basis of its inducing effect of cell division for protoplasts with hormones (Wakita et al. 1996), and then modified. Under an acidic culture condition developed here using saturated amount of Ca2+ under a 5% CO2 atmosphere, protoplasts from birch leaves expanded from its original 10 μm to size of 100 μm 2 months before each of them started spinning a gigantic fiber-like substance (Fig. 1a).
Images of a light microscope and a confocal UV-laser scanning microscope. Note a an expanded protoplast (arrow head) secreting a gigantic fiber (arrow) after about 2 months culture. b The fiber was stained with aniline blue fluorochrome, whereas the cell was stained with FDA. UV excitation: 351 and 488 nm, detected emission: 380–470 nm (351 nm ex) and 505 nm < (488 ex). Scan speed: 4.33 s/image, step size: 0.625 μm and z height: 29.375 μm/48 images
Instead, the cell division was not significantly seen in this modified condition. Confocal microscopy with a UV-laser source showed ca.30 μm wide coiled fiber spinning out from a single site on the cell surface (Fig. 1b). The staining of the protoplast with FDA typically indicated presence of an active plasma membrane of living cells, confirming that the protoplast was alive. The vacuole enlarged greatly during the cell expansion. Detailed changes in the organelle during the fiber secretion will be reported in a future paper.
The entire phenomenon was dependent on concentrations and types of the ions added into the culture medium. Effect of pH for the fiber production under a 5% CO2 atmosphere is shown in terms of an average number of fiber-secreting cells and colonies due to aggregation of the divided cells per 96 culture wells (Fig. 2), depending on types of the ions used. The symbol, †, in Fig. 2 showed that protoplasts could regenerate in the initial medium including hormones to accelerate cell divisions under an acidic condition without addition of ions. The ions, Na+ and K+, did not affect the fiber secretion across the entire pH range examined, but both ions affected colony formation under acidic conditions less than 100 mM of each concentration. On the contrary, higher concentrations of both ions inhibited the colony formation. Adding Mg2+ enhanced neither fiber secretion nor colony formation (data not shown). However, addition of Ca2+ into the medium induced the fiber production in the concentration range of 100–300 mM across the entire pH range instead of cell division, as indicated by arrowheads in Fig. 2. In particular, the Ca2+ concentration of 200 mM was the most influential within the range to enhance the fiber secretion in the protoplasts. Interestingly, the phenomenon was strongly influenced by the most acidic condition at pH 3.5 of the examined. The atmosphere of 5% CO2 could easily maintain and control the acidic condition. Therefore, we settled on a culture condition for the fiber spinning as the 1/2 MS medium at 28°C with 200 mM Ca2+ at pH 3.5 under a 5% CO2 atmosphere.
The relationship of pH with the average numbers of the fiber and cell bunches due to regeneration of the cells per 96 culture wells (50 μl/well). C cell colony and F fiber. The symbol, †, indicates the formation of cell bunches under an acidic condition without ions. Arrowheads show fiber formation in the modified media including Ca2 +
The secreted fibers were collected using an inversed microscope equipped with a micromanipulation, washed thoroughly, and then used for histochemical staining tests in order to identify fiber components. The results are shown in Fig. 3. Aniline blue fluorochrome was then employed as a specific staining agent for (1→3)-β-glucan, namely callose. Aniline blue fluorochrom stained the fiber intensively (Fig. 3b), showing that fiber was composed mainly of callose.
Light microscopic image and the corresponding fluorescent microscopic image of the spun fiber from the protoplasts with the alternative histochemical staining. Images of a was obtained by light microscopic observation, while the sample specimens for images of was stained by aniline blue fluorochrome. Scale bars = 50 μm
Chemical identification of the fiber component using nuclear magnetic resonance analysis
The 1H NMR spectra for both the spun fiber and curdlan, namely liner (1→3)-β-glucan were obtained as shown in Fig. 4, respectively. The signals appeared in 3.9, 3.6 and 3.4 ppm corresponded with both samples (Fig. 4a, b), which indicated that the two samples should be identical. The wider range of chemical shifts between 4.5 and 4.7 ppm suggested that other carbohydrate-like components.
As the obtained amount of the secreted fibers was too small, the sample was provided for 13C DEPT NMR spectroscopy (Fig. 5a). The major signals in the spectrum of the sample were identical to those appeared in the 13C NMR signals of curdlan when compared between the two spectra of Fig. 5a, b. In particular, the single signal from the fiber of C1 corresponded to curdlan at 103.5 ppm. It indicated that the spun fiber and curdlan were same component of liner (1→3)-β-glucan. Therefore, the above NMR analysis indicated that the main component of the spun fiber from protoplast was (1→3)-β-glucan linkage without branches, namely callose. We should add here that these NMR results were still preliminary, and more detailed NMR investigation will be required.
The behavior of spinning a gigantic callose fiber from a protoplast
In the present study, we also focus on the movement of a single protoplast that spun a gigantic fiber for a comparison with the bacterial system. When expanded, active protoplasts were induced to spin a callose fiber after 2 months incubation. Then, they started moving in a spiral way that parallels the inverse direction of fiber secretion. Direct video imaging of the motion of the cell as it spun a callose fiber clearly showed this behavior in motion. The cell movement was the result of an inverse force imposed by the directed polymerization and spinning of the (1→3)-β-glucan (Fig. 6). The diameter of the cell was ca. 100 μm, whereas the fiber was ca. 30 μm in width.
The directed protoplast motion and fiber secretion can be studied over time and under varied conditions. For example, in Fig. 6, a time-course analysis of spinning a fiber shows that the production proceeds at a constant rate of 0.3 μm/min at room temperature, with a constant fiber size of 30 μm. Midway through this series, the protoplast may “rotate” and continues to secrete its callose fiber, but now in the form of a spiral.
Formation of a gigantic bundle of callose hollow sub-fibrils
The measurements given above indicate that the callose fiber spun from the protoplast was much larger than microbial nanofibers spun from terminal complexes (TCs), which are enzyme complexes as linear arrangements of the cellulose synthesizing enzymes on the plasma membrane. As the nano-hierarchical structure of the microbial cellulose fiber was determined from the arrangements of the enzymes, it was important to understand the nano/micro hierarchical structure of the callose fiber in this study.
The surface image of the callose fiber obtained by AFM exhibited groove lines parallel to the fiber axis as shown in Fig. 7, which indicates presence of an individual sub-fibrils structure. Even in the light microscopic images of Figs. 3 and 6, the bundle formation with parallel lines can be seen. These observations indicate that the gigantic callose fiber may be a bundle of sub-fibrils.
Confocal sectioning fluorescent microscopy has an advantage that the living, weak and flexible sample can be significantly protected by structural damages from the incident light, because it requires only a normal Hg light as a source for the fluorescent excitation, when compared with confocal UV-laser scanning microscopes. The sectioning microscopy enabled in situ observation of the sectioned images of both the cell and the secreted fiber. Upper images in Fig. 8a, b show the sectioning fluorescent microscopic images of the callose fiber parallel to the oblique (a) and the longitudinal (b) directions, respectively.
Sectioning microscopic and TEM images of a bundle of callose hollow sub-fibrils with the callose immuno-gold labeling. Note a, c and b, d indicate oblique and longitudinal sections, respectively. In upper images (a, b), the callose fiber was stained by aniline blue fluorochrome having a high specificity for staining with (1→3)-β-glucan. Bottoms (c, d) are TEM images with immuno gold labeling of callose fiber
The both images revealed that the fiber was in fact a bundle of individual straight tubes with the empty inside, i.e. a hollow with an average width of a few hundreds nanometers for smaller one and a few micrometers for larger one. This was also proved by a TEM observation of the ultra thin sections of the fiber with immuno gold-labeled callose antibody as shown in the bottom images of Fig. 8c, d. The gold particles followed the callose frames for the hollow sub-fibrils in each sectioned image. In the oblique section (c), the secondary antibodies with gold colloids for the initial (1→3)-β-glucan antibodies were concentrated on the elliptic frames, whereas they were also concentrated on the column frames in the longitudinal section (d). Therefore, the secreted and gigantic callose fiber proved to be a large bundle of sub-fibrils with a cylindrical shape. It is noted that the individual hollow sub-fibrils in the medium, not dried, had an average width of 600–800 nm according to in situ observation using the confocal sectioning fluorescent microscopy.
These results indicate that the callose fiber was a gigantic bundle of sub-fibrils, which may be still hierarchically built from callose molecules through a certain nano scaled structure.
Secretion site of the protoplast
Some circles clearly appeared on the secretion site of the protoplast surface (Fig. 9). Each sub-fibril seemed to be secreted from the corresponding single circle and bundled (Fig. 9b). The site was located only on the certain point on the protoplast surface. The structure of the spun fiber supported Fig. 7.
Discussion
It is well known that Ca2+ induces secretion of callose as reported by Kauss et al. (1987, 1996), Fink et al. (1987) and Waldmann et al. (1988). The callose is a substance produced at the interface of a plasma membrane in response to wounding, pathogen infection and chemical and mechanical damage. In the present study, formation of a gigantic fiber after synthesis of callose was also induced by Ca2+ presence in the medium. It has been reported that in situ biosynthesis of callose fibrils is directed by arrays of cortical microtubules at many loci on the membrane sheet (Hirai et al. 1998). However, our study shows secretion of a gigantic callose fiber from a single protoplast directly into the medium, which is a very unique and unusual phenomenon. To our knowledge, there are no earlier reports of individual protoplasts secreting or spinning out, and the callose fiber secretion in our system is an interesting phenomenon particularly in relation to following aspects: (1) A fiber was spun only from one site on the surface of each protoplast, (2) the fiber was massive and a gigantic callose bundle as compared with other polysaccharide fibers reported in previous studies (Brown et al. 1976), and (3) the fiber was hierarchically composed of hollow sub-fibrils.
The behavior of this fiber production could be compared with that of Acetobacter xylinum (=Gluconacetobacter xylinus), which secretes a crystallized cellulose nanofiber directly into the culture medium. The crystallized cellulose nanofibers with 40–60 nm in width are formed and spun from fixed enzyme complexes arranged in a row parallel to the long axis of the cell. The arrangement and position of the enzyme complexes determines the native hierarchical aggregation of the polymer chains into the final crystalline product, a ribbon of cellulose microfibrils with the average microfibril size of 3.5 nm. The gigantic callose fiber with 30 μm in width in our study is much larger than the microbial cellulose fiber. Therefore, taking the microbial cellulose case into accounts, formation of the callose fiber may also include a specific aggregation system of the synthesizing enzymes on the plasma membrane, but in this case these enzyme complexes might be much larger.
The fiber was found to have a hierarchical structure composed of hollow sub-fibrils made of (1→3)-β-glucan chains, as already described. As shown in the schematic image of Fig. 10, the fiber was composed of (1→3)-β-glucan hollow sub-fibrils as building blocks ranging from a few hundreds nanometers to a few micrometers.
The TEM image in Fig. 10 shows a sub-fibril of the secreted fiber negatively stained with uranyl acetate. Though the secreted fiber included water and the medium, the entire fiber shape was not totally altered from the initial one after washed in the preparation process for the observation. The area surrounded by the broken line in the image exhibited a still established sub-fibril with a width of 400 nm. The image also indicated presence of a certain twisting towards the fiber direction with the smaller fibrillar substances in a single sub-fibril. In the case of in vitro synthesis of callose with an isolated membrane fraction including a callose synthase, a short callose fibrillar structure with a few 10 nm in width was found to be formed (Him et al. 2001; Pelosi et al. 2003; Colombani et al. 2004) but such a structure was not observed in a long and large fiber of this study. A curdlan molecule, which is a linear type of a (1→3)-β-glucan without branches produced by Alcaligenes faecalis, is known to form a triple helical structure (Deslandes et al. 1980). Therefore, it can be considered as a standard example to be compared with the callose structure in our study. The spun fiber from a protoplast was a large bundle of callose sub-fibrils as described above, and now it is indicated by TEM that the single sub-fibril was also an oriented assembly of smaller fibrillar substances that might be engaged by a triple helical structure of (1→3)-β-glucan, as illustrated in Fig. 10.
How is the gigantic bundle of hollow sub-fibers formed? Is there a callose-synthesizing enzyme complexes in the plasma membrane of the protoplast similar to that located on the surface of A. xylinum cells? The sectioning microscopic images of Fig. 11 showed the secreting site of the gigantic fiber.
Sectioning microscopic images for secreting sites of the individual hollow sub-fibrils. Note Arrows showed appearance of small circle cavities on the protoplast surface (a, b) in sectioning micrographs staining with aniline blue fluorochrome. b was the enlarged image of * part in the a. The scale bars = 15 μm (a, b)
Sectioning images across the cross section at the fiber-secretion site acquired by a sectioning microscope provide some clues, as shown in Fig. 11a, b. There appear to be cavities on the surface of the plasma membrane that secrete a sub-fibril (Fig. 11). The diameter of the cavities varied from 0.9 to 2.6 μm, which corresponds to that of the individual hollow tubes in the secreted fiber. The individual cavities may be located on the plasma membrane surface as a fractal structure in a circular arrangement. Moreover, this indicates that the individual sub-fibrils might be spun from the cavities on the cell surface before aggregating into a larger bundle. The more detailed investigation is required in this aspect. The question is further extended to whether the sub-fibrils are secreted simultaneously, which may have a relation to a spiral movement of protoplasts and also to the bundle formation.
Abbreviations
- NMR:
-
Nuclear magnetic resonance
- IBA:
-
Indol-3-butyric acid
- NAA:
-
α-Naphthaleneacetic acid
- FDA:
-
Fluorescein diacetate
References
Brown RM Jr, Willison JHM, Richardson CL (1976) Cellulose biosynthesis in Acetobacter xylinum: visualization of the site of synthesis and direct measurement of the in vivo process. Proc Nat Acad Sci USA 73(12):4565–4569
Colombani A, Djerbi S, Bessueille L, Blomqvist K, Ohlsson A, Berglund T, Teeri TT, Bulone V (2004) In vitro synthesis of (1→3)-β-D-glucan (callose) and cellulose by detergent extracts of membranes from cell suspension cultures of hybrid aspen. Cellulose 11:313–327
Deslandes Y, Marchessault RH, Sarko A (1980) Triple-Helical structure of(1→3)-β-D-Glucan. Macromolecules 13:1466–1471
Fink J, Jeblick W, Blaschek W, Kauss H (1987) Calcium ions and polyamines activate the plasma membrane-located 1,3-β-glucan synthase. Planta 171:130–135
Hanley SJ, Giasson J, Revol J-F, Gray DG (1992) Atomic force microscopy of cellulose microfibrils: comparison with transmission electron microscopy. Polymer 33:4639–4642
Him JLK, Pelosi L, Chanzy H, Putaux J-L, Bulone V (2001) Biosynthesis of (1→3)-β-D-glucan (callose) by detergent extracts of microsomal fraction from Arabidopsis thaliana. Eur J Biochem 268:4628–4638
Hirai N, Sonobe S, Hayashi T (1998) In situ synthesis of β-glucan microfibrils on tobacco plasma membrane sheets. Proc Natl Acad Sci USA 95:15102–15106
Kataoka Y, Kondo T (1996) Changing cellulose crystalline structure in forming wood cell walls. Macromolecules 29:6356–6358
Kataoka Y, Kondo T (1998) FT-IR microscopic analysis of changing cellulose crystalline structure during wood cell wall formation. Macromolecules 31:760–764
Kataoka Y, Kondo T (1999) Quantitative analysis for the cellulose Iα crystalline phase in developing wood cell walls. Int J Biol Macromol 24:37–41
Kauss H (1987) Some aspects of calcium-dependent regulation in plant metabolism. Annu Rev Plant Physiol 38:47–72
Kauss H (1996) Callose synthesis. In: Smallwood M, Knox JP, Bowles DJ (eds) Membranes: specialized functions in plants. BIOS Scientific Publishers, Oxford, pp 77–92
Kondo T, Nojiri M, Hishikawa Y, Togawa E, Romanovicz D, Brown RM Jr (2002) Biodirected epitaxial nanodeposition of polymers on oriented macromolecular templates. Proc Natl Acad Sci USA 99:14008–14013
Kondo T, Magoshi J, Abe H, Sasamoto H (2000) Method for producing of non cellulosic callose fibers from protoplasts and the callose fiber. Japan Patent No.3936522
Meikle PJ, Bonig I, Hoogenraad NJ, Clarke AE, Stone AB (1991) The location of (1→3)-β-glucans in the walls of pollen tubes of Nicotiana alata using a (1→3)-β-glucan-specific monoclonal antibody. Planta 185:1–8
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Pelosi L, Imai T, Chanzy H, Heux L, Buhler E, Bulone V (2003) Structural and Morphological diversity of (1→3)-D-Glucans synthesized in Vitro by enzymes from Saprolegnia monoïca. Comparison with a corresponding in vitro product from blackberry (Rubus fruticosus). Biochemistry 42:6264–6274
Wakita Y, Sasamoto H, Yokota S, Yoshizawa N (1996) Plantlet regeneration from mesophyll protoplasts of Betula platyphylla var. japonica. Plant Cell Reports 16:50–53
Waldmann T, Jeblick W, Kauss H (1988) Induced net Ca2+ uptake and callose biosynthesis in suspension-cultured plant cells. Planta 173:88–95
Zuiderweg ERP, Hallenga K, Olejniczak ET (1986) Improvement of 2D NOE spectra of biomacromolecules in H2O solution by coherent suppression of the solvent resonance. J Magn Reson 70:336–343
Acknowledgments
This research was supported partly by a Grant-in-Aid for Scientific Research (No. 14360101), Japan Society for the Promotion of Science (JSPS). The authors are grateful to Dr. Adya P. Singh at ENSIS (The Joint Forces of CSIRO & SION) for the editing of the text, and to Dr. Kazuaki Shikii for the NMR measurement (Nissan Chemical Industries, Ltd.). SEKI TECHNOTRON CORP. is also acknowledged by the assistance for observation using sectioning microscopy.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00425-008-0769-2
Rights and permissions
About this article
Cite this article
Seyama, T., Kimura, S., Sasamoto, H. et al. Spinning of a gigantic bundle of hollow fibrils by a spirally moving higher plant protoplast. Planta 227, 1187–1197 (2008). https://doi.org/10.1007/s00425-008-0689-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-008-0689-1