Abstract
Epiphylly, occurring in a somaclonal variant (EMB-2) of the interspecific hybrid Helianthus annuus × H. tuberosus, was used to investigate molecular and cyto-physiological mechanisms that underlie cellular fate change. EMB-2 plants are characterized by profuse proliferation of shoot- and embryo-like structures on some leaves. We addressed the putative relationship between cytokinins and knox genes in EMB-2 plants. A class I knox gene, HtKNOT1, was isolated from H. tuberosus. A high level of HtKNOT1 transcripts was detected in EMB-2 epiphyllous leaves compared to non-epiphyllous (NEP) ones. In addition, epiphylly was related to a localized increases in zeatin and N-glycosylated cytokinins. As ectopic morphogenesis proceeded, HtKNOT1 transcripts and zeatin co-localized and showed different patterns in ectopic shoot compared with embryo-like structures, consistent with the differential role of both cytokinin and knox genes in the two morphogenetic events. Notably, a massive shoot/embryo regeneration was induced in EMB-2 NEP leaves by in vitro zeatin treatment. These results clearly indicate that localized cytokinin accumulation and ectopic expression of HtKNOT1 are closely linked in the epiphylly of EMB-2 plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Higher plants exhibit an enormous capacity for regeneration (D’Amato 1985). This totipotency is exemplified by processes of vegetative propagation in vivo (Steeves and Sussex 1989) as well as in the ability of plant cells to develop into a complete and fertile plants by in vitro somatic embryogenesis and/or adventitious organogenesis (Halperin 1986). For example, structures such as embryos, shoots, leaves, and inflorescences can form on leaves. This phenomenon is epiphylly, a mechanism of propagation for several species (Dickinson 1978; Steeves and Sussex 1989).
Regeneration demonstrates the flexibility of plant cells that can re-acquire embryogenic or meristematic competence, following either developmental or environmental signals. Although widely studied, the physiological and genetic factors that underlie shifts in cell fate, leading to adventitious morphogenesis, are not yet fully understood. Work mainly focused on cell lineages or mutants exhibiting high embryogenic/organogenic potential, in Arabidopsis thaliana have begun to resolve some genetic aspects of the regeneration processes (Mordhorst et al. 1998; Sugiyama 2000). An indeterminate cell state is necessary to express organogenic and embryogenic competence (Halperin 1986). Several gene “markers” of these processes have been identified (Ma et al. 1994; Teo et al. 2001; Hjortswang et al. 2002; Zhang et al. 2002; Daimon et al. 2003). In particular, a family of knotted1-like homeobox genes (knox) are important components that maintain cells in an indeterminate state (Sinha et al. 1993; Reiser et al. 2000; Hake et al. 2004).
Based on comparative analysis of the conserved motifs of encoded proteins, the knox family has been subdivided in two classes, class I and class II (Reiser et al. 2000). The class I genes are mainly expressed in the shoot apical meristem (SAM) and they may play a role in the establishment and maintenance of meristematic activity. However, their early repression is necessary for leaf primordium initiation (Reiser et al. 2000). In dicots as well as in monocots, knox gain of function induces considerable alteration in leaf morphology and plant architecture (Hareven et al. 1996; Tamaoki et al. 1997; Frugis et al. 2001). Moreover, ectopic expression of class I knox genes can restore morphogenetic potential in differentiated cells (Sentoku et al. 2000; Gallois et al. 2002). Such a phenotype was also mimicked by the mutation of genes that negatively regulate the knotted1-like genes (Byrne et al. 2000; Daimon et al. 2003). Hence, substantial evidence exists to link these homeobox genes to the acquisition of meristematic competence that is such an important feature of regenerative responses both in vivo and in vitro.
Naturally occurring morphogenetic competence in epiphylly is well documented in many species (Dickinson 1978; Steeves and Sussex 1989) and in some interspecific hybrids of Nicotiana, Lycopersicon (Bayer 1982) and Helianthus (Fambrini et al. 2000). Paradoxically, epiphylly has been under exploited to address molecular mechanisms that regulate changes in cellular fate. The work reported here has focused on the epiphyllous (EP) response of plants (EMB-2) regenerated in vitro from the interspecific hybrid H. annuus × H. tuberosus (Fambrini et al. 2000). EMB-2 plants are characterized by the occurrence of numerous shoots and embryo-like structures on the adaxial surface of leaves both in vivo and in vitro conditions, devoid of exogenous hormones. These structures are localized mainly in the proximal half of the oldest leaves (Fambrini et al. 2000), which raises interesting questions regarding the morphogenetic competence expressed by different parts of the leaf and different leaves on the plant. Notably, the importance of cytokinins in promoting shoot-like structure in vitro and in vivo has been widely reported (Halperin 1986; Estruch et al. 1991). Moreover, a relationship has been found between the level and translocation of hormones and the expression of knox genes (Tamaoki et al. 1997; Rupp et al. 1999; Hewelt et al. 2000; Sakamoto et al. 2001; Frugis et al. 2001; Hamant et al. 2002; Hay et al. 2002; Rosin et al. 2003).
In the present study, we demonstrate that the ectopic expression of HtKNOT1, a class I knox gene isolated from H. tuberosus, and the level of endogenous cytokinins are closely linked in the epiphylly exhibited by EMB-2 plants.
Materials and methods
Plant material and growth conditions
An EP plant, denominated EMB-2, was regenerated by a somatic embryo induced in vitro from a leaf explant of the tetraploid (2n=4x=68) interspecific hybrids H. annuus × H. tuberosus (A-2) (Fambrini et al. 2000). In order to obtain clones under sterile conditions, both the A-2 [non-epiphyllous genotype (NEP)] and the EMB-2 plants (R1 generation) were multiplied by single-node cutting on solidified (8 g l−1 Bactoagar, Oxoid, Basingstoke, UK) MS basal medium (Murashige and Skoog 1962) without growth regulators in 150- ml Erlenmeyer flasks. The cultures were incubated under a regime of 25±1°C and a 16-h daily photoperiod in a growth chamber. Irradiance intensity during the light period was 30 μmol photons m−2 s−1, and it was provided by cool-light fluorescent lamps (Philips TLD 36W/33; Philips, Eindhoven, The Netherlands). Moreover, H. tuberosus (accession S. Pietro 1457, provided by the Department of Crop Plant Biology, University of Pisa, Pisa, Italy) plants were grown in growth chamber into pots containing a mixture of vermiculite, peat and soil at 22±1°C under a 16-h daily photoperiod using fluorescent tubes (Sylvania Day light F36W/56) with 200 μmol photons m−2 s−1.
Regeneration from leaf explants of EMB-2 and A-2 plants
Explants from young leaves of EMB-2 and A-2 plants were used as starting material for in vitro experiments. Leaves of the EMB-2 clone were selected under a reflecting stereoscope, before the in vitro culture, to exclude the presence of EP structures (NEP leaves). Leaves were placed in Petri dishes (100×15 mm2) on basal medium without growth regulators (MS) and on MS medium supplemented with 1 mg l−1 zeatin (ZEA). The media contained 30 g l−1 sucrose and was solidified with 8 g l−1 Bactoagar. Cultures were incubated in a growth chamber under the same conditions as described above. Experimental data were taken every day after placing leaves on the test media. Regeneration frequencies were presented as the percentage of explants producing adventitious structures, from three independent experiments with five to six replications (Petri dishes), each with 8–10 explants.
Extraction, purification and analysis of cytokinins
Leaves of control (A-2) and EP (EMB-2) plants were collected from different individuals (n=3). For EMB-2 plants, basal EP leaves (EP) were also separated from apical NEP ones. For all the samples the proximal halves of leaf (about 300 mg fresh weight) were dissected and frozen in liquid nitrogen. Samples were extracted overnight in Bieleski’s solvent. Before centrifugation at 24,000g for 15 min at 4°C, deuterated standards for cytokinins (Apex International) were added and then the extract was purified using a combination of solid-phase and immunoaffinity purification, as described by Redig et al. (1996). Quantitative analysis of different cytokinins was performed as previously described by Dewitte et al. (1999), using capillary column switching on a fully automated workstation (Famos, LC Packings, Amsterdam, The Netherlands) coupled to a liquid chromatography setup, consisting of an HPLC pump (model 325S, Kontron Instrument, Milan, Italy), an in-line UV detector (model 322, Kontron), and a triple-quadruple MS (Quattro II, Micromass UK Ltd., Cheshire, UK). The results were calculated according to the principles of isotope dilution and expressed in pmol g−1 fresh weight. For the calculation of detection limits and errors, fresh weight and recovery was taken into account. The data were treated using analysis of variance procedures and means (± SE) were separated by Tukey’s test (P=0.05).
RNA isolation
The total RNA was obtained from plant tissues with a single-step preparation, as previously described by Chomczynski and Sacchi (1987). Vegetative shoot apices (HTVS), blades (HTLB) and veins (HTLV) of leaves (5-cm long), petiole (HTP) and stem internodes (HTST) were harvested from 30-day-old plants of H. tuberosus grown in vivo. Vegetative shoot apices (VS) and leaves (L) of the A-2 interspecific hybrid (NEP clone), EP and NEP leaves of EMB-2 (EP clone) were harvested from plants grown in vitro.
Cloning of H. tuberosus knotted1-like gene (HtKNOT1)
The total RNA (5 μg) from VS of H. tuberosus was used, with the Superscript preamplification kit (Invitrogen S.R.L., Life Technologies, Milan, Italy), to produce the first-strand cDNA in conditions recommended by manufacturer. Reverse transcription was carried out with Superscript II retrotranscriptase in the presence of the adapter primer (AP, 5′-GGCCACGCGTCGACTAGTACTTTTTTTTTTTTTTTTT-3′) provided in the kit. The cDNA was employed in a PCR with the following degenerate primers: P-1, 5′-CCDGMDYTRGAYCARTTCATGG-3′ (forward) and P-4, 5′-ATRAACCARTTGTTKATYTGYTTC-3′ (reverse). These primers were selected in a region with highest conservation among members of the class I knox genes: KNAT1 and KNAT2 from Arabidopsis (Lincoln et al. 1994), SBH1 from soybean (Ma et al. 1994) and LET6 from tomato (Chen et al. 1997). PCRs were performed in 50 μl of 1x buffer (Applied Biosystems, Applera Italia, Monza, Italy) containing 0.1 mM dNTPs, 1 μM of each primer, 2 mM MgCl2, 2 U AmpliTaq DNA polymerase (Applied Biosystems) and 2 μl of single-strand cDNA. Amplifications were carried out with Gene Amp® PCR System 2700 (Applied Biosystems) thermocycler. After an initial 4 min/94°C denaturation step, 40 cycles were run each with 30 s of denaturation at 94°C, followed by 30 -s annealing at 55°C and 30- s extension at 72°C. Final extension was at 72°C for 7 min. The PCR reaction yielded a product of about 450 bp, which was cloned into the pDrive Cloning Vector (Qiagen) and sequenced (HtK1). The RACE (rapid amplification of cDNA ends) approach was used to isolate the 5′ and 3′ ends of the HtKNOT1 cDNA. All reactions were performed with kits according to the manufacturer’s instructions (Invitrogen). The 5′ end of the HtKNOT1 cDNA was amplified using first-strand cDNAs performed with Superscript II retrotranscriptase in the presence of the HtKNOT1-specific primer P4 K (5′-ATGAACCAATTGTTTATCTGCTTC-3′). The terminal deoxynucleotidyl transferase (TdT) was used to add, at the 5′ end of cDNA, a poly-C tail that allowed the coupling of the 5′ RACE abridged anchor primer (5′-GGCCACGCGTCGACTAGTACGGGIIGGGIIGGGIIG-3′). After C-tailing, PCR amplification was performed with the HtKNOT1-specific primer K5R (5′-GAGAAAGTGATTGCCTTGAAC-3′) and anchor primers provided in the kit. The 3′ end of the HtKNOT1 cDNA was amplified using first-strand cDNAs made with the AP primer. Each PCR was done with the HtKNOT1-specific primer K5 (5′-GTTCAAGGCAATCACTTTCTC-3′), and the abridged universal amplification primer (AUAP, 5′-GGCCACGCGTCGACTAGTAC-3′) provided in the kit. Specific cDNAs from 3′ and 5′ RACE were subcloned into the pDrive Cloning Vector (Qiagen) and sequenced; the overlapping regions with the first clone (HtK1) were confirmed. To isolate full-length HtKNOT1 cDNA, sequence information from the 5′ and 3′ RACE products was used to design the following primers: 5′ primer (Kstart), 5′-TAGTTCCATTTAAAAAGTTGC-3′ (located 29 nucleotides upstream the ATG); and 3′ primer (Kend), 5′-CGTGTTTCTTGATTAGGTATTTAT-3′ (located 258 nucleotides downstream from the translational stop codon). The primers were used for RT-PCR with the Pfu-DNA polymerase with proof-reading function (Promega). PCRs were performed in 50 μl of 1x buffer (Promega) containing 0.1 mM dNTPs, 1 μM of each primer, 2 mM MgCl2, 2.5 U Pfu DNA polymerase and 2 μl of single-strand cDNA. After an initial 4 min/95°C denaturation step, 35 cycles were run each with 30 s of denaturation at 94°C, followed by 30 s annealing at 55°C and 2 min extension at 72°C. Final extension was at 72°C for 7 min. A product of 1,378 bp were cloned into the pDrive Cloning Vector (Qiagen) and sequenced. A clone, named HtK3, containing the entire sequence was selected.
Amino acid sequence analysis
Database researches were carried out with the Blast program (BLAST 2.0a) at the National Center for Biotechnology Information (NCBI) (Altschul et al. 1997). A data set including the HtKNOT1 and other 23 aminoacidic sequences of knox genes was constructed. Homeodomain (HD) regions of ATBELL1 (BELL group) from A. thaliana and metazoan sequences, DMEXD, MMPBX1, HSPR1 (the EXD group), and CEW05 (the SIX2 group) were included (Bharathan et al. 1999). The sequences were aligned by ClustalW (Thompson et al. 1994), and manually adjusted. According to Bharathan et al. (1999), regions corresponding respectively to a putative helix–loop–helix (HLH), amphipatic helix (H3), ELK domain and HD were used in the following steps, but Linker regions (L1 and L2) were excluded (Bürglin 1997). Phylogenetic analysis was performed using programs from the PHYLIP group, PHYLogeny Inference Package, Version 3.6 (Felsenstein 1985). As support for the trees was obtained, a bootstrap analysis, with 100 replicates, was performed by SEQBOOT. The search for most-parsimonious trees was done by PROTPARS and strict consensus trees were obtained by CONSENSE.
Construction of DIG-RNA probe
A clone K245, containing a region (244 bp), spanning from the HtKNOT1-specific primers, P1S: 5′-CAGCACTTGATCAGTTCATG-3′, and Kint: 5′-GATCCTCAAGCTGAAGATCG-3′, was linearized with appropriate restriction enzymes and used as a template to synthesize digoxigenin-labelled RNA sense and antisense probes, according to the DIG-RNA labeling Kit protocol (Roche Diagnostics GmbH, Mannheim, Germany). The P1S and Kint were designed to exclude from the probe both the ELK and the homeobox domains.
Relative RT-PCR analyses
Extraction of total RNA and reverse transcription were carried out as described above for the method of cDNA cloning. First-strand cDNAs derived from 5 μg of total RNA from each sample were used for each PCR amplification. PCRs were performed using gene-specific primers from cDNA of HtKNOT1 (K5, 5′-GTTCAAGGCAATCACTTTCTT-3′ and Kint) and β-actin (ACTINA 5, 5′-GATTCCGTTGCCCA/TGAGGTC/T-3′ and ACTINA 3, 5′-TC/TTCTGGA/TGGA/TGCAACCACC-3′). Primers were designed to yield 132- bp and 243-bp fragments for HtKNOT1 and β-actin, respectively. PCRs were carried out for 32 cycles and were performed in 50 μl of 1x buffer (Applied Biosystems) containing 0.1 mM dNTPs, 0.5 μM of each primer (1 primer pair for β-actin and 1 primer pair for HtKNOT1), 2 mM MgCl2, 2 U AmpliTaq DNA polymerase (Applied Biosystems) and 2 μl of single-stranded cDNA. Amplifications were carried out according to the following temperature profile: 95°C for 4 min for denaturation, then 94°C for 30 s, 55°C for 30 s, 72°C for 25 s and a final extension of 7 min at 72°C.
HtKNOT1-primers were designed externally to an intron of 1,318 bp (data not shown). The relative amounts of each PCR product were readily quantified by direct scanning of ethidium-stained 2% TAE-agarose gels with a UVP Image Store 5000 (Ultra Violet Product Ltd, Cambridge, England) densitometer equipped with the UVP GelBase-GelBlot TM Windows Software. To normalize for equal amounts of total RNA and efficiency of cDNA synthesis from various tissue samples, the intensities of each band were normalized with the intensity of the β-actin product within the sample investigated. An arbitrary value of 100% was assigned to the β-actin product. The relative expression levels of the HtKNOT1 transcripts were expressed as percentage with respect to the β-actin product. The data are means (± SD) of three independent experiments. The data, after arcsin transformation, were treated using analysis of variance procedures and means were separated by Tukey’s test (P=0.05).
Zeatin immunocytolabelling
Leaves of control (A-2) and EP clone (EMB-2) were collected from different plantlets (n=3). For EMB-2 plants, basal EP leaves were separated from apical NEP-ones. For all the samples the proximal half of leaf was cut into different portions (sectors) starting from the petiole. The leaf portions were pre-embedded, cut using the vibratome (Leica VT1000E, Bensheim, Germany) and incubated with primary antibody against zeatin according to the procedure described by Dewitte et al. (1999). Colloidal gold (<1 nm)-labelled secondary antibodies (1:40, Aurion, Wageningen, The Netherlands) were used. The antibodies allowed us to detect specifically only the free cytokinin base since this fixation procedure abrogates the ability to detect conjugate zeatin in tissues.
In situ hybridization
Excised tissues were fixed, dehydrated, embedded in paraffin, cut into 8-μm sections and hybridized (55°C) to a digoxigenin-labelled antisense K245 RNA probe as previously described (Construction of DIG-RNA probe) by Cañas et al. (1994). For immunological detection, the slides were incubated in buffer 1 (1% [w/v] blocking solution, 100 mM Tris, pH 7.5 and 150 mM NaCl) for 45 min and then equilibrated with buffer 2 (100 mM Tris, pH 7.5 and 150 mM NaCl, 1% [w/v] bovine serum albumin, and 0.3% [w/v] Triton X-100) for 45 min. Tissue sections were then incubated with anti-digoxygenin-alkaline-phosphatase conjugate diluted 1:100 in buffer 2 in a humidified box for 2 h and then washed four times for 10 min in buffer 2. The tissue sections were equilibrated in buffer 3 (100 mM Tris, pH 9.5 and 100 mM NaCl and 50 mM MgCl2) for 20 min and then incubated in 3.2 μg ml−1 5-bromo-4-chloro-3-indolyl-phosphate: 6.6 μg ml−1 nitroblue tetrazolium salt in buffer 3 in a humidified box overnight. Accumulation of HtKNOT1 transcript is visualized as a violet/brown stain. After stopping the reaction with TE (50 mM Tris–HCl, pH 8.0, 5 mM EDTA), sections were mounted with 50% glycerol in TE.
Accession number
The sequence data of the H. tuberosus HtKNOT1 cDNA have been submitted to the DDBJ/EMBL/GenBank databases under accession No. AJ519674.
Results
Phenotypic expression of epiphylly in the EMB-2 clone
The variant clone EMB-2 derived by in vitro tissue culture of the interspecific hybrid H. annuus × H. tuberosus shows an unusual pattern of development in that it produces, both in vitro and in vivo, EP embryo- and shoot-like structures (Fig. 1a, b) (Fambrini et al. 2000). More precisely, in addition to leaves that expanded normally, some leaves of EMB-2 plants exhibit knobs and curling or a prominent proliferation of ectopic structures. Generally, these ectopic structures are borne on the most basal leaves. Sometimes they fill the leaf adaxial surface (Fig. 1c) but usually they are arranged in clusters along veins on the proximal half of the leaf (Fig. 1d), especially at the junction between the enlarged petiole and lamina (Fig. 1e). Isolated EP structures are also sporadically observed near the leaf tip. The EMB-2 plants were infertile; they were propagated in vitro by single-node cutting and in vivo by tubers that displayed typical EP structures (Fig. 1f).
a–f Development of EP structures in EMB-2 plants of the interspecific hybrid H. annuus × H. tuberosus grown in vitro (a–e) or in vivo (f). a Embryogenic structures (arrows). b Shoot-like structures (arrows). c Leaf adaxial side filled with ectopic somatic embryos. d Ectopic structures (arrow) developed along leaf midvein. e EP structures at the junction between enlarged petiole (arrow) and lamina. In d and e the leaves were partly cleared according to Candela et al. (1999). f Ectopic embryos (arrows) on a leaf developed from a shoot borne on a tuber recovered from an in vivo grown plant. Bars, 2.5 mm (a, b), 2.0 mm (c), 1.5 mm (d), 3.0 mm (e), 2.7 mm (f)
Endogenous levels of cytokinins are altered in EP leaves of EMB-2 plants
Given the clear link between cytokinins and organogenesis shown for other plants in vitro, the level of endogenous cytokinins was measured in EP and NEP leaves of EMB-2 plants. Comparisons were then made with a control NEP genotype (A-2). Since morphogenetic structures preferentially formed in the proximal half of EP leaves, all measurements were confined to this region of the leaves. Total cytokinin content did not significantly differ in EMB-2 compared to A-2 plants as evidenced in Fig. 2a where values estimated in EP and NEP leaves are plotted together. However, a clear difference in cytokinin levels was observed between different leaves of EMB-2 plants; cytokinin content was 1.8-fold higher in EP leaves compared with NEP ones (Fig. 2b). Compared with NEP leaves, zeatin-N-glycosylated (ZNG) was 3.2-fold higher, isopentenyl adenine glycosylated (iPG) was 2.4-fold higher and zeatin was 1.8-fold higher in EP leaves (Fig. 2c). No significant differences were detected in the levels of other cytokinins. These results indicate that total level of cytokinins per se is not necessarily an indicator of EP competence.
a–c Cytokinins’ content (pmol g−1 FW) in EP (EMB-2 EP), NEP (EMB-2 NEP) and A-2 control leaves. DHZ, dihydrozeatin; DHZR, dihydrozeatin riboside; iP, isopentenyladenine; DHZNG, dihydrozeatin-N-glucoside; iPA, isopentenyladenosine; iPG, isopentenyladenine-N-glucoside; ZR, zeatin riboside; ZNG, zeatin-N-glucoside. Values followed by the same letter are not significantly different at the 0.05 probability level according to Tukey’s test
Ectopic shoot-like structures and increased zeatin levels co-localize in EP leaves
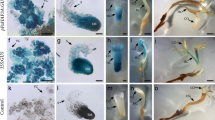
We wanted to test the idea that it is localized, tissue-specific increases in, as opposed to, total cytokinin levels that are important indicators of EP competence. Hence, the spatial distribution of cytokinins was examined through immunocytolocalization of zeatin in both EP and NEP leaves of EMB-2, as well in control leaves (A-2). In cross sections of EP leaves, a strong immunostaining was detected wherever there ectopic structures formed, as well as at the level of the vascular bundles (Fig. 3a, b, f, g, i). In addition, a strong immunoreaction marked adaxial epidermal cells, which in some case undergo periclinal division (Fig. 3c), as well as clusters of epidermal cells (Fig. 3d, e, h), from which morphogenetic structures were likely to originate. An uninterrupted immunoreaction was often observed to link the vascular bundles to these cell clusters (Fig. 3d, e). As morphogenetic structures developed, a strong immunostaining was clearly detected at the level of the whole shoot-like structures (Fig. 3f, g). EP embryo-like structures, at different developmental stages, were also observed on the adaxial surface of EP leaves and zeatin localization exhibited a distinct pattern, immunostaining being confined to a few scattered cells (Fig. 3i, j). In contrast, a weak immunostaining was detected in NEP (Fig. 3k) and in A-2 (Fig. 3l) leaves; an immunoreaction was not observed in EP leaves processed without primary antibody (Fig. 3m).
a–m Zeatin immunolocalization on cross sections of EMB-2 and A-2 control plants. a–j EMB-2 EP leaves showing shoot (a–g) and embryo-like (h–j) structures. k EMB-2 NEP leaves. l A-2 control leaves. m EMB-2 EP leaves treated in absence of primary antibody. a Section at the level of petiole where arrows indicate a cluster of ectopic structures and arrow heads indicate vascular bundles. b–g Sections at the distal level of proximal portion. Arrow indicates: developing structures (b–f), periclinal division of immunoreactive epidermal cells (c), cluster of immunoreactive cells and continuity of immunoreaction between vascular bundle and epidermal cells (d, e), nuclear localization of zeatin immunoreaction (g). h Cluster of embryogenic cells. i, j Advanced globular embryos where arrow indicates globular embryo and arrow heads indicate scattered reactive cells along epidermis and inside embryo. k, l Arrows indicate immunostained vascular bundles. Bars, 120 μm (a), 100 μm (b), 70 μm (c), 25 μm (d, e), 50 μm (f), 40 μm (g), 20 μm (h, j), 35 μm (i), 45 μm (k), 50 μm (l), 45 μm (m)
Zeatin treatment enhances adventitious morphogenesis in EMB-2 clone
To further investigate the relationship between zeatin and epiphylly of the EMB-2 clone, adventitious morphogenesis was monitored in explants of NEP leaves cultured on either MS basal medium (without growth regulators) or zeatin enriched medium (ZEA medium). Leaves collected from A-2 plants and cultured under the same conditions were used as a control.
After 3 days of culture, organized white globular structures and buds were initiated on the adaxial surface of some EMB-2 leaves cultured on both media (Fig. 4a). However, a significant higher frequency of regeneration was obtained on ZEA medium compared to MS medium. After 14 days the regeneration frequency of EMB-2 explants increased to 74.8% and 48.7% in ZEA and MS medium, respectively (Fig. 4a). In addition, EMB-2 explants on ZEA medium showed a higher number of adventitious structures compared to MS medium (Fig. 4b, c). By contrast, regeneration from A-2 leaves was never detected (Fig. 4a).
Effects of in vitro culture on regeneration of A-2 and EMB-2 leaves. a Time course of the regeneration frequency (%) in A-2 and EMB-2 leaves cultured in vitro on basal medium without hormones (MS) or on MS medium supplemented with 1 mg l−1 of zeatin (ZEA); the data are means (± SD) of three independent experiments. b Adventitious morphogenetic structures observed in EMB-2 leaves cultured on MS medium. c ZEA-dependent increase of morphogenetic structures in EMB-2 explants in vitro. Bars, 1.2 mm (b), 2.0 mm (c)
Isolation of HtKNOT1, a class I knox gene
In order to provide a functional link between ectopic morphogenesis and a cytokinin signal, it was necessary to clone a knox gene that in other species is expressed as a prerequisite for meristematic competence. Hence, with the aim of isolating Helianthus knotted1-like cDNAs, H. tuberosus was chosen as one of the parental species of the EMB-2 clone. Degenerate primers were used to amplify cDNAs derived from VS of H. tuberosus. A product of the expected size of 450 bp was purified, cloned, and sequenced. This H. tuberosus fragment showed a high degree of sequence similarity to the conserved positions of the consensus sequences created from knox class I genes (data not shown). The reconstructed full-length cDNA sequence (1,398 bp), obtained from 3′ and 5′ RACE and confirmed from the sequence of the clone HtK3 (1,378 bp), contained a 1,089 bp CDS, 54-nucleotides of 5′-untranslated region (UTR), and 255-nucleotides of 3′-UTR.
The predicted protein displayed 362 amino acids with a calculated molecular mass of 40.2 kDa (Fig. 5a). A BLAST search against the protein database of the National Center for Biotechnology Information (NCBI) indicated that the encoded protein shared high sequence identity with members of the class I knox subfamily. Thus the gene product was designated as H. tuberosus KNOTTED1 (HtKNOT1).
Sequence analysis of HtKNOT1 gene of H. tuberosus. a Deduced amino acid sequence of the HtKNOT1 gene. The asparagine stretch in N-terminus are dotted underlined, the helix–loop–helix region (HLH) is underlined, the anphipatic helix (H3) is shaded while the complete MEINOX domain is delimited by opposed arrows. The GSE domain is bold, the ELK region is double underlined and the homeodomain (HD) is represented with dashed underline. b Diagrammatic representation of the organization of coding region of HtKNOT1 gene and deduced amino acid sequence of the HD of the HtKNOT1 gene. The Pro-Tyr-Pro (PYP) sequence is double underlined and the placement of the helix–turn–helix motif is shown above the sequence. The helices of HD are depicted as in Vollbrecht et al. (1991). In the HD, the highly conserved amino acid residues in the loop are bolded. Conserved position for the SBH1 subclass is shaded. The HD of HtKNOT1 and selected class I plant homeobox proteins were aligned. The deduced amino acid sequences used for the comparison and their corresponding accession numbers are: H. annuus HAKN1 (AY096802) and HAKN2 (AY096803), Nicotiana tabacum NTH15 (T01735), Glycine max SBH1 (L13663), Lycopersicon esculentum LET6 (AF000141) and LETKN1 (U32247), Arabidopsis thaliana KNAT1 (U14174) and KNAT2 (U14175), Zea mays ZMLG3 (AF100455), KN1 (CAA43065) and RS1 (T03946), Hordeum vulgare HVKNOX3 (X83518), Oryza sativa OSH1 (D16507), and Picea abies HBK1 (AF063248). Asterisks indicate conserved amino acid residues among these homeodomains. M, methionine
To compare the H. tuberosus gene with knox genes from other species, a phylogenetic analysis employing protein sequences from several monocots and dicots together with sequences of metazoan HD proteins was constructed. All the analyses yielded results largely consistent with a previously published dendrogram (Bharathan et al. 1999). The KNOX proteins are well supported as monophyletic and there is strong confirmation for both KNOX class I and class II clades (100 bootstrap values). Based on our phylogenetic analysis (Fig. 6) and the detailed comparison of the HDs of 15 class I knox genes from various species (Fig. 5b), HtKNOT1 can be assigned as a class I of KNOX HD protein. Furthermore, our results also indicated that HtKNOT1 along with HAKN1, NTH15, LET6, SBH1 and STM, among others, constitute a distinguishable subgroup (SBH1 subclass) of the class I knox genes.
Bootstrap consensus tree based on maximum parsimony, generated using Protpars program (Phylip package 3.572 version). The bootstrap replicates were 100 (values are given at the nodes); the deduced amino acid sequence of the HtKNOT1 of H. tuberosus was compared to representative sequences of the knox homeobox genes. Homeodomain regions of ATBELL1 from A. thaliana and metazoan sequences DMEXD, MMPBX1, HSPR1 were included. The amino acid sequence CEW05 from C. elegans was used as outgroup (Bharathan et al. 1999). The GenBank accession numbers of the sequences are indicated in brackets
HtKNOT1 is highly expressed in shoot meristems and stem internodes of H. tuberosus and in EP leaves of the EMB-2 clone
Relative levels of HtKNOT1 transcripts in vegetative organs of in vivo grown plants of H. tuberosus were estimated by using RT-PCR. Expression of HtKNOT1 was detected in vegetative shoot apices and stem internodes, while leaves (blades and veins) and petioles did not accumulate any detectable HtKNOT1 transcripts (Fig. 7).
Expression analysis of HtKNOT1. Amplification of HtKNOT1 and β-actin transcripts performed using 5 μg of total RNA isolated from vegetative shoot apices (HTVS), blades (HTLB), veins (HTLV) and petioles (HTP) of 5-cm-long leaves, stem internodes (HTST) of H. tuberosus; the PCR products were resolved on a TAE 2.0% agarose gel. M, marker
In EMB-2 plants, epiphylly is manifested by in vivo grown plants but the development of ectopic structures is far more evident in in vitro conditions (Fambrini et al. 2000). Thus, the expression of HtKNOT1 in interspecific hybrids was examined in in vitro grown plants. Firstly, we examined shoot meristems for the presence of HtKNOT1 transcripts by using in situ hybridization. Sections of VS from H. annuus × H. tuberosus plants (A-2) were hybridized to an antisense HtKNOT1-specific probe (K245). In longitudinal sections, HtKNOT1 transcripts were strongly detected in the meristematic dome (Fig. 8a, b). Weak presence of transcripts was detected also in incipient leaf primordia (Fig. 8b) and young leaves (Fig. 8a). No transcription was detected with the sense RNA probe (Fig. 8c).
a–e Expression analysis of HtKNOT1. a Distribution of HtKNOT1 transcripts in vegetative shoot apex of in vitro grown plantlets of the interspecific hybrid H. annuus × H. tuberosus (A-2); longitudinal section through the vegetative shoot apex hybridized with an antisense probe (K245) labelled with digoxigenin-UTP; the transcript-specific hybridization signal is visible as purple staining; arrow indicates the shoot apical meristem. b Magnification of a; arrows indicate the leaf primordia. c Control experiment performed with dig-labelled HtKNOT1 sense probe. d Amplification of HtKNOT1 and β-actin transcripts performed using 5 μg of total RNA isolated from vegetative shoot apex (VS) and leaves (L) of control A-2 plants, from NEP and EP leaves of the EMB-2 clone; the PCR products were resolved on a TAE 2.0% agarose gel. e The relative expression levels of the HtKNOT1 transcripts, expressed as percentage with respect to β-actin product levels, were estimated by measuring the ethidium bromide staining of the PCR products resolved by gel electrophoresis in d; the data are means (± SD) of three independent experiments; values followed by the same letter are not significantly different at the 0.05 probability level according to Tukey’s test. M, marker. Bars, 250 μm (a), 70 μm (b, c)
Relative RT-PCR showed that HtKNOT1 was highly expressed in vegetative shoots of A-2 plants (Fig. 8d, e). A comparatively low levels of HtKNOT1 transcripts was detected in both leaves of A-2 and NEP leaves of EMB-2 plants (Fig. 8d, e). By contrast, the HtKNOT1 expression in EP leaves was significantly higher with respect to NEP ones.
In EP leaves of the EMB-2 clone, HtKNOT1 expression precedes the ectopic morphogenetic processes
To determine the spatial pattern of HtKNOT1 expression during the initiation and development of EP structures, in situ hybridization with digoxigenin-labelled RNA probes was performed (Fig. 9).
a–h Localization of HtKNOT1 transcripts (b–h) on cross sections of EP leaves of EMB-2 plants. a Morphological features of EP leaves showing sectors with (A) or without (B) ectopic structures. b–c Cross sections at the level of the B sector: arrows (b) indicate scattered labelled cells along leaf lamina. d–i Cross sections at the level of the A sector: initial stage of ectopic structures (d), ectopic shoot-like structures (e, f), early globular embryo (g), advanced globular embryo (h). i Control experiment performed with dig-labelled HtKNOT1 sense probe. Bars, 3.0 mm (a), 18 μm (b), 20 μm (c), 35 μm (d, e), 40 μm (f), 75 μm (g, i), 45 μm (h)
It was evident that in the absence of morphogenetic structures (Fig. 9a), HtKNOT1 transcripts were confined to discrete zones along the leaf lamina (Fig. 9b) at the level of the palisade cell layer (Fig. 9c). Thereafter, transcripts accumulated in developing morphogenetic structures, but the signal did not spread to epidermal cells (Fig. 9d). However, as development of shoot-like structures proceeded, transcript signal was also spread throughout epidermal cells (Fig. 9e, f). Afterwards, the level of HtKNOT1 transcripts decreased and mainly accumulated in the external cell layers of ectopic structures (Fig. 9e, f). In contrast, in the case of putative embryo-like structures, HtKNOT1 transcripts were strictly confined to the basal portion of the ectopic structure (Fig. 9g), whereas in the advanced globular embryos, the signal was restricted to a few scattered cells inside the structure (Fig. 9h). A clear signal was also observed at the level of vascular bundles (Fig. 9h). HtKNOT1 transcript accumulation was not detected in either A-2 or NEP expanded leaves (data not shown). No transcription was detected with the HtKNOT1 sense RNA probe (Fig. 9i).
Notably, before ectopic structures became evident, HtKNOT1 expression and zeatin signal in EP leaves were localized in distinct histological domains from which ectopic shoots/embryos would arise (mesophyll tissue vs. epidermis) (compare Fig. 9b, c with Fig. 3e, h, respectively). However, as morphogenetic processes occurred, the accumulation of HtKNOT1 transcripts and zeatin clearly overlapped (compare Fig. 9e, h with Fig. 3g, i, respectively).
Discussion
Isolated through a PCR approach from vegetative shoot cDNA of H. tuberosus, HtKNOT1 is a homeobox gene belonging to the knox gene family. Several lines of evidence presented here support the notion that HtKNOT1 is phylogenetically related to the class I knox genes. First, sequence analyses revealed that HtKNOT1 is similar to this class of genes, especially in the regions of encoding functional domains of KNOX proteins. Second, HtKNOT1 is expressed in apical meristems and is down-regulated in leaves of H. tuberosus as usually reported for class I knox genes (Hake et al. 2004).
Phylogenetic analyses of the homeodomains from angiosperm class I knox genes showed a high degree of sequence conservation throughout plant evolution, demonstrating that dicot and monocot genes occur in this family without very clear demarcations by subfamilies, in spite of evolutionary distances between some genes (Bharathan et al. 1999; Reiser et al. 2000). However, the results presented here clearly demonstrated that HtKNOT1 is clustered within a group comprising only dicot genes such as HAKN1, STM, SBH1, NTH15, MTKNOX, and LET6. The homology of this cluster of genes is well supported by sequence data but their relationship based on expression studies is ambiguous (Ma et al. 1994; Long et al. 1996; Chen et al. 1997; Tamaoki et al. 1997; Bharathan et al. 1999; Tioni et al. 2003).
In particular, STM and NTH15, as most of class I knox genes, are typically expressed in the SAM and subtending stem and excluded from lateral organs (Long et al. 1996; Tamaoki et al. 1997). By contrast, LET6 transcripts were detected in leaf primordia of tomato (Parnis et al. 1997). Transcripts of the HAKN1 gene, for which HtKNOT1 showed the highest identity, were accumulated in several organs of H. annuus, including petioles, leaves, and leaf homologues (Tioni et al. 2003). HtKNOT1 expression was not widespread as that of HAKN1. In fact, HtKNOT1 mRNAs was detected in vegetative shoot apices and stem internodes of H. tuberosus, but not in petioles and leaves. However, a low level of HtKNOT1 transcripts was detected in leaf primordia and developing leaves of the interspecific hybrid H. annuus × H. tuberosus grown in vitro. These findings indicate that, in some species, the transcriptional down-regulation of class I knox genes is not a prerequisite for lateral organ initiation (Parnis et al. 1997; Bellaoui et al. 2001; Müller et al. 2001; Chen et al. 2003).
Our data showed that HtKNOT1 is strongly expressed in EP leaves of EMB-2 plants as compared to NEP ones. Note that contrasting expression of the mutant phenotype within a single plant (i.e. NEP and EP leaves) is a somewhat common trait of the class I knox mutants. For example, in both knotted1 of mais (Freeling and Hake 1985) and hooded of barley (Müller et al. 1995), the mutant phenotype, also in relation to genetic background, is strongly associated with ectopic gene expression but only in some leaves and awns, respectively. Thus, it is likely that the differential HtKNOT1 expression in EP leaves compared with lack of expression in NEP leaves is also regulated by other factors that are expressed in a spatially or temporally differential pattern during leaf development.
Using in situ hybridization we demonstrated that in EP leaves localized HtKNOT1 overexpression precedes the development of ectopic structures. Indeed, before epiphylly becomes evident, transcript presence was selectively detected at the level of mesophyll and vascular bundles whereas epidermal cells never exhibited HtKNOT1 expression. Notably, in leaves of Arabidopsis overexpressing KN1 under the control of tissue specific promoters, the gene product was shown to move from the inner layers to epidermis, but not in the opposite direction (Kim et al. 2003). However, when KN1 was expressed in the epidermis, the plants did not exhibit ectopic morphogenetic structures but transgenic plants overexpressing KN1 in the mesophyll cells produced ectopic shoots on the adaxial leaf side (Kim et al. 2003). These results fully agree with the ectopic HtKNOT1 expression that we detected in the mesophyll cells of EMB-2 EP leaves.
An intriguing feature of the EMB-2 variant is that it produces not only ectopic shoots but also ectopic embryo-like structures, which have been never described either in transgenic or mutant plants with altered expression of class I knox genes (Lincoln et al. 1994; Sentoku et al. 2000; Gallois et al. 2002). However, members of a knox gene family mark the embryogenic potential of cell lines of Abies alba, being a key factor for in vitro development of somatic embryos (Hjortswang et al. 2002). Thus, to our knowledge, the epiphylly of EMB-2 represents a unique example of an ectopic embryogenic process related to misexpression of a knox gene. In this context, it is important to recall that the EMB-2 clone is derived from a single somatic embryo induced in an enriched cytokinin medium (Fambrini et al. 2000), which is an unusual condition to restore embryogenic competence in determinate cells (Halperin 1986).
The pattern of HtKNOT1 expression was clearly different in embryo- compared to shoot-like structures. In somatic embryos, HtKNOT1 transcripts were confined to the basal region and became scattered in a few embryogenic cells as development proceeded. Similarly, in somatic embryos of maize at the globular stage, KN1 was expressed in a small group of cells (Zhang et al. 2002), but these cells were clearly located in a more specific region (e.g. initial stage of SAM organization) than those of EMB-2 EP embryos. Perhaps in the latter, some cells can themselves become new embryogenic growth centres rather than participate in the organization of a functional SAM. The abnormal cellular proliferation, which frequently characterizes the development of ectopic EMB-2 embryos, strongly supports this suggestion (Fambrini et al. 2000). In contrast, shoot-like structures were heavily labelled at the beginning of their formation, while in more developed structures, HtKNOT1 transcripts were progressively confined to the external layers, which perhaps represent the histological domains of SAM establishment. An analogous pattern of expression was observed for BROSTM, a class I knox gene of Brassica oleracea, during the development of adventitious shoots induced in vitro from stem segments (Teo et al. 2001).
Due to the above-mentioned characteristics and well-documented relationship between knox genes and cytokinin signalling (Rupp et al. 1999; Hewelt et al. 2000; Frugis et al. 2001; Hamant et al. 2002), the level and location of this hormone class in EMB-2 leaves was also investigated. Clear differences in cytokinin levels were observed between NEP and EP leaves. However, our discovery that EMB-2 plants are not cytokinin overproducers is not surprising. In fact, although EMB-2 is characterized by EP shoots, like ipt overexpressing plants (Estruch et al. 1991), other features of cytokinin overproduction were not observed (i.e. reduced internode length, reduced leaf surface, retarded leaf senescence). Indeed, our results indicate that epiphylly of EMB-2 plants is tightly related to a localized increase of cytokinins (ZNG, iPG and zeatin) in EP leaves as compared to NEP ones. Zeatin-induced stimulation of shoot and embryo development on NEP leaves cultured in vitro further supports this link between cytokinin availability and adventitious morphogenesis in EMB-2 plants. However, EP structures were not formed in zeatin-treated A-2 leaves suggesting that an intrinsic cell competence to regeneration is required. It is likely that in EMB-2 plants such competence is switched by specific inductors (i.e. cytokinins and knox misexpression).
The involvement of cytokinins in ectopic morphogenetic events of EMB-2 plants is strengthened by immunolocalization results. The presence of active zeatin, revealed by immunostaining, marks the development of adventitious shoot-like structures, from the first division of epidermal cells to SAM establishment (data not shown). However, in the ectopic embryogenesis, the scattered distribution of zeatin, from the initial cluster of embryogenic cells until the globular stage, suggests that cytokinin action is limited to the initial switch of epidermal cell fate. Accordingly, in vitro somatic embryo development does not occur in the presence of a continuous/high cytokinin treatment (Halperin 1986).
A characteristic of zeatin distribution in EP leaves was the massive localization at the level of vascular bundles, a feature that was also observed in lettuce plants overexpressing KNAT1 (Frugis et al. 2001). In particular, ectopic shoot-like structures formed wherever zeatin was found in vascular bundles through to the epidermis. These distinct histological traits and the preferential accumulation of cytokinins in EP leaves strongly suggest that EP leaves could acts as a sink for cytokinins that could be related to the conspicuous presence of N-glycosylated forms. Although the precise role of each cytokinin is not yet fully understood, N-glucoside conjugates are usually regarded as irreversible inactive forms (Mok and Mok 2001). Hence, the excessive amount of cytokinins loaded in EP leaves could be inactivated through the formation of N-glucosyl conjugate. Clearly, hormonal activities are a function of not only their endogenous concentrations but also on their interactions with other growth regulators. For example, in addition to the association between class I knox genes and cytokinins, several other studies have shown that knox expression is also linked to other hormonal activities, including gibberellic acid (Sakamoto et al. 2001; Hay et al. 2002), ethylene (Hamant et al. 2002) and polar auxin transport (PAT) (Tsiantis et al. 1999; Scanlon 2003). In general, ectopic KNOX accumulation is correlated with a defective PAT, which in turn could influence cytokinin level and/or translocation (Mok and Mok 2001).
In conclusion, we have isolated a HtKNOT1, a homeobox gene which belongs to the knox gene family and both sequence analyses and expression pattern support the notion that HtKNOT1 is phylogenetically related to the class I knox genes. In EP leaves of the EMB-2 clone, the ectopic proliferation of shoot- and embryo-like structures appears to be associated with both a local accumulation of zeatin and HtKNOT1 misexpression. On this basis we propose that both events are intimately linked to the epiphylly of EMB-2 plants.
Abbreviations
- EP:
-
Epiphyllous leaves
- HtKNOT1 :
-
Helianthus tuberosus knotted1-like gene
- NEP:
-
Non-epiphyllous leaves
- SAM:
-
Shoot apical meristem
References
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programmes. Nucleic Acids Res 25:3389–3402
Bayer MH (1982) Genetic tumors: physiological aspects of tumor formation in interspecific hybrids. In: Kahl G, Schell JS (eds) Molecular biology of plant tumors. Academic Press, New York, pp 33–67
Bellaoui M, Pidkowich MS, Samach A, Kushalappa K, Kohalmi S, Modrusan Z, Crosby WL, Haughn GW (2001) The Arabidopsis BELL1 and KNOX TALE homeodomain proteins interact through a domain conserved between plants and animals. Plant Cell 13:2455–2470
Bharathan G, Janssen BJ, Kellog EA, Sinha NR (1999) Phylogenetic relationship and evolution of the KNOTTED class of plant homeodomain proteins. Mol Biol Evol 16:553–563
Bürglin TR (1997) Analysis of TALE superclass homeobox genes (MEIS, PBC, KNOX, Iroquois, TGIF) reveals a novel domain conserved between plants and animals. Nucleic Acids Res 25:4173–4180
Byrne ME, Barley R, Curtis M, Arroyo JM, Dunham M, Hudson A, Martienssen RA (2000) Asymmetric leaves1 mediates leaf patterning and stem cell function in Arabidopsis. Nature 408:967–971
Cañas LA, Busscher M, Angenent GC, Beltrán JP, van Tunen AJ (1994) Nuclear localization of petunia MADS box protein FBP1. Plant J 6:597–604
Candela H, Martinez-Laborda A, Micol JL (1999) Venation pattern formation in Arabidopsis thaliana vegetative leaves. Dev Biol 205:202–216
Chen JJ, Janssen BJ, Williams A, Sinha N (1997) A gene fusion at a homeobox locus: alterations in leaf shape and implications for morphological evolution. Plant Cell 9:1289–1304
Chen H, Rosin FM, Prat S, Hannapel DJ (2003) Interacting transcription factors from three-amino acid loop extension superclass regulate tuber formation. Plant Physiol 132:1391–1404
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159
Daimon Y, Takabe K, Tasaka M (2003) The CUP-SHAPED COTYLEDON genes promote adventitious shoot formation on calli. Plant Cell Physiol 44:113–121
D’Amato F (1985) Cytogenetics of plant cell and tissue cultures and their regenerates. Crit Rev Plant Sci 3:73–112
Dewitte W, Chiappetta A, Azmi A, Witters E, Strnad M, Rembur J, Noin M, Chriqui D, Van Onckelen H (1999) Dynamics of cytokinins in apical shoot meristem of a day-neutral tobacco during floral transition and flower formation. Plant Physiol 119:111–122
Dickinson TA (1978) Epiphylly in angiosperms. Bot Rev 44:181–232
Estruch JJ, Prinsen E, Van Onckelen H, Schell J, Spena A (1991) Viviparous leaves produced by somatic activation of inactive cytokinin-synthesizing gene. Science 254:1364–1367
Fambrini M, Cionini G, Bianchi R, Pugliesi C (2000) Epiphylly in a variant of Helianthus annuus × H. tuberosus induced by in vitro tissue culture. Int J Plant Sci 161:13–22
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Freeling M, Hake S (1985) Developmental genetics of mutants that specify knotted leaves in maize. Genetics 111:617–634
Frugis G, Giannino D, Mele G, Nicolodi C, Chiappetta A, Bitonti MB, Innocenti AM, Dewitte W, Van Onckelen H, Mariotti D (2001) Overexpression of KNAT1 in lettuce shifts leaf determinate growth to a shoot-like indeterminate growth associated with an accumulation of isopentenyl-type cytokinins. Plant Physiol 126:1370–1380
Gallois JL, Woodward C, Reddy GV, Sablowski R (2002) Combined SHOOT MERISTEMLESS and WUSCHEL trigger ectopic organogenesis in Arabidopsis. Development 129:3207–3217
Hake S, Smith HMS, Holtan H, Magnani E, Mele G, Ramirez J (2004) The role of knox genes in plant development. Annu Rev Cell Dev Biol 20:125–151
Halperin W (1986) Attainment and retention of morphogenetic capacity in vitro. In: Vasil IK (ed) Plant regeneration and genetic variability, vol 3. Academic Press, London, pp 3–47
Hamant O, Nogué F, Belles-Boix E, Jublot D, Grandjean O, Traas J, Pautot V (2002) The KNAT2 homeodomain protein interacts with ethylene and cytokinin signaling. Plant Physiol 130:657–665
Hareven D, Gutfinger T, Parnis A, Eshed Y, Lifschitz E (1996) The making of a compound leaf: genetic manipulation of leaf architecture in tomato. Cell 84:735–744
Hay A, Kaur H, Phillips A, Hedden P, Hake S, Tsiantis M (2002) The gibberellin pathway mediates KNOTTED1-type homeobox function in plants with different body plans. Curr Biol 12:1557–1565
Hewelt A, Prinsen E, Thomas M, Van Onckelen H, Meins FJ (2000) Ectopic expression of maize knotted1 results in the cytokinin-autotrophic growth of cultured tobacco tissues. Planta 210:884–889
Hjortswang HI, Sundås Larsson A, Bharathan G, Bozhkov PV, Von Arnold S, Vahala T (2002) KNOTTED1-like homeobox genes of gymnosperm, Norway spruce, expressed during somatic embryogenesis. Plant Physiol Biochem 40:837–843
Kim JY, Yuan Z, Jackson D (2003) Developmental regulation and significance of KNOX protein trafficking in Arabidopsis. Development 130:4351–4362
Lincoln C, Long J, Yamaguchi J, Serikawa K, Hake S (1994) A knotted1-like homeobox gene in Arabidopsis is expressed in the vegetative meristem and dramatically alters leaf morphology when overexpressed in transgenic plants. Plant Cell 6:1859–1876
Long JA, Moan EI, Medford JI, Barton MK (1996) A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature 378:66–69
Ma H, McMullen MD, Finer JJ (1994) Identification of a homeobox-containing gene with enhanced expression during soybean (Glycine max L.) somatic embryo development. Plant Mol Biol 24:465–473
Mok DWS, Mok MC (2001) Cytokinin metabolism and action. Annu Rev Plant Physiol Plant Mol Biol 52:89–118
Mordhorst AP, Voerman KJ, Hartog MV, Meijer EA, Van Wen J, Koornneef M, De Vries SC (1998) Somatic embryogenesis in Arabidopsis thaliana is facilitated by mutations in genes repressing meristematic cell divisions. Genetics 149:549–563
Müller J, Wang Y, Franzen R, Santi L, Salamini F, Rohde W (2001) In vitro interactions between barley TALE homeodomain protein suggest a role for protein–protein associations in the regulation of Knox gene function. Plant J 27:13–23
Müller KJ, Romano N, Gerstner O, Garcia-Maroto F, Pozzi C, Salamini F, Rohde W (1995) The barley Hooded mutation is caused by a duplication in a homeobox gene intron. Nature 374:727–730
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Parnis A, Cohen O, Gutfinger T, Hareven D, Zamir D, Lifschitz E (1997) The dominant developmental mutant of tomato Mouse-ear and Curl, are associated with distinct modes of abnormal transcriptional regulation of a Knotted gene. Plant Cell 9:2143–2158
Redig P, Schmülling T, Van Onckelen H (1996) Analysis of cytokinin metabolism in ipt transgenic tobacco by liquid chromatography-tandem mass spectrometry. Plant Physiol 112:141–148
Reiser L, Sánchez-Baracaldo P, Hake S (2000) Knots in the family tree: evolutionary relationships and functions of knox homeobox genes. Plant Mol Biol 42:151–166
Rosin FM, Hart Jk, Horner HT, Davies PJ, Hannapel DJ (2003) Overexpression of a Knotted-like homeobox gene of potato alters vegetative development by decreasing gibberellin accumulation. Plant Physiol 132:106–117
Rupp HM, Frank M, Werner T, Strand M, Schmülling T (1999) Increased steady state mRNA levels of the STM and KNAT1 homeobox genes in cytokinin overproducing Arabidopsis thaliana indicate a role for cytokinins in the shoot apical meristem. Plant J 18:557–563
Sakamoto T, Kamiya N, Ueguchi-Tanaka M, Iwahori S, Matsuoka M (2001) KNOX homeodomain protein directly suppresses the expression of a gibberellin biosynthetic gene in the tobacco shoot apical meristem. Genes Dev 15:581–590
Scanlon MJ (2003) The polar auxin transport inhibitor N-1-naphthylphthalamic acid disrupts leaf initiation, KNOX protein regulation, and formation of leaf margins in maize. Plant Physiol 133:597–605
Sentoku N, Sato Y, Matsuoka M (2000) Overexpression of rice OSH genes induces ectopic shoots on leaf sheaths of transgenic rice plants. Dev Biol 220:358–364
Sinha NR, Williams Jr RE, Hake S (1993) Overexpression of the maize homeo box gene, KNOTTED-1, causes a switch from determinate to indeterminate cell fate. Genes Dev 7:787–795
Steeves TA, Sussex IA (1989) Patterns in plant development, 2nd edn. Cambridge University Press, Cambridge
Sugiyama M (2000) Genetic analysis of plant morphogenesis in vitro. Int Rev Cytol 196:67–84
Tamaoki M, Kusaba S, Kano-Murakami Y, Matsuoka M (1997) Ectopic expression of a tobacco homeobox gene, NTH15, dramatically alters leaf morphology and hormone levels in transgenic tobacco. Plant Cell Physiol 38:917–927
Teo WL, Kumar P, Goh CJ, Swarup S (2001) The expression of Brostm, a KNOTTED1-like gene, marks the cell type and timing of in vitro shoot induction in Brassica oleracea. Plant Mol Biol 46:567–580
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTALW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Tioni MF, Gonzalez DH, Chan RL (2003) Knotted1-like genes are strongly expressed in differentiated cell types in sunflower. J Exp Bot 54:681–690
Tsiantis M, Brown MIN, Skibinski G, Langdale JA (1999) Disruptions of auxin transport is associated with aberrant leaf development in maize. Plant Physiol 121:1163–1168
Vollbrecht E, Veit B, Sinha N, Hake S (1991) The developmental gene Knotted-1 is a member of a maize homeobox gene family. Nature 350:241–243
Zhang S, Wong L, Meng L, Lemaux PG (2002) Similarity of expression patterns of knotted1 and ZmLEC1 during somatic and zygotic embryogenesis in maize (Zea mays L.). Planta 215:191–194
Acknowledgements
This work was supported by grants from PRIN 2004 and from Scuola Normale Superiore to M. Salvini.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chiappetta, A., Michelotti, V., Fambrini, M. et al. Zeatin accumulation and misexpression of a class I knox gene are intimately linked in the epiphyllous response of the interspecific hybrid EMB-2 (Helianthus annuus × H. tuberosus). Planta 223, 917–931 (2006). https://doi.org/10.1007/s00425-005-0150-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-005-0150-7