Abstract
The floral organs are formed from floral meristem with a regular initiation pattern in angiosperm species. Flowers of naked seed rice (nsr) were characterized by the overdeveloped lemma and palea, the transformation of lodicules to palea-/lemma-like organs, the decreased number of stamens and occasionally extra pistils. Some nsr spikelets contained additional floral organs of four whorls and/or abnormal internal florets. The floral primordium of nsr spikelet is differentiated under an irregular pattern and an incomplete determination. And molecular analysis indicated that nsr was a novel homeotic mutation in OsMADS1, suggesting that OsMADS1 played a distinct role in regulating the differentiation pattern of floral primordium and in conferring the determination of flower meristem. The gain-of-function of OsMADS1 transgenic lines presented the transformation of outer glumes to lemma-/palea-like organs and no changes in length of lemma and palea, but loss-of-function of OsMADS1 transgenic lines displayed the overdeveloped lemma and palea. Both findings revealed that OsMADS1 played a role in specifying lemma and palea and acted as a repressor of overdevelopment of lemma and palea. Moreover, it was indicated that OsMADS1 upregulated the transcript level of AP3 homologue OsMADS16, using real-time PCR analysis on gain- and loss-of-function of OsMADS1 transgenic lines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Based on the genetic and molecular analyses on specific floral mutants of Arabidopsis and Antirrhinum, the classic ABC model was proposed (Coen and Meyerowitz 1991; Weigel and Meyerowitz 1994). According to the model, A genes function for sepals, and C genes activity specify the identity of carpel. A genes determine the formation of petal with B genes, while C genes specify stamens identity with B genes. The example of the homeotic genes includes the A gene APETALA1 (AP1), the B gene APETALA3 (AP3), PISTILLATA (PI) and the C gene AGAMOUS (AG) in Arabidopsis. In Antirrhinum, SQUAMOSA (SQUA) provides the function of A homeotic gene, DEFICIENS (DEF) and GLOBOSA (GLO) are members of B homeotic genes, and PLENA (PLE) is identified as C homeotic gene (Thomas 2001). Except AP2, ABC homeotic genes are members of MADS-box genes family, which encode MADS-box proteins with MADS, I, K and C domains in plants. The classic ABC model appears generally applicable to distantly related dicotyledon, while it has been extended by the identifications of D genes FBP7 and FBP11 and E genes SEPALLATA1/2/3 (Angenent and Colombo 1996; Pelaz et al. 2000). Ectopic expression of PI-AP3-SEP3 or PI-AP3-AP1 is sufficient to transform leaves into petaloid organs and that of PI-AP3-SEP3-AG converts cauline leaves into staminoid organs (Honma and Goto 2001). Furthermore, the quartet model of floral organs identity has been proposed that complexes of MADS-box proteins bind to the specific genes, respectively, and determine the identity of floral organs of four whorls (Theissen and Saedler 2001).
In monocot rice, MADS-box genes involved in floral development have been isolated and studied. OsMADS15 shares similar sequence and expression pattern with AP1 and is a putative ortholog and functional equivalent of AP1 (Kyozuka et al. 2000). OsMADS4 is PI/GLO paralog and flowers of its transgenic lines exhibit conversion of lodicules into palea-/lemma-like structures (Kang et al. 1998). OsMADS16 is a member of the AP3 family and OsMADS16 does not form a homodimer, but the protein interacts with OsMADS4, OsMADS6 and OsMADS8 (Lee et al. 2003; Xiao et al. 2003). OsMADS3 is highly homologous to AG and PLE in sequence and expression patterns, and loss-of-function of OsMADS3 shows the changes in the stamens and pistil (Kang et al. 1998). OsMADS1 belongs to the AP1/AGL9 sub-group and OsMADS1 protein interacts with the OsMADS14 and OsMADS15 (Lim et al. 2000). The functions of floral organ identity genes appear to be broadly conserved between dicot and monocot plants.
In angiosperm species, the particular number of floral organs is formed with the precise pattern. In dicotyledon Arabidopsis, the abnormal number of floral organs in flowers is resulted from some causes, such as the mutations in ABC homeotic genes (Coen and Meyerowitz 1991), the changes of floral meristem size in clavata and wiggum (Clark et al. 1993; Running et al. 1998), the alteration of organ spacing in perianthia (Running and Meyerowitz 1996), or the change in floral meristem determination in wuschel (Laux et al. 1996). In monocot rice, several mutations associated with floral organ number have been identified. superwoman1 is a homeotic mutant of OsMADS16 and displays the conversion of stamens and lodicules into carpels and palea-/lemma-like organs, respectively (Nagasawa et al. 2002). drooping leaf (dl) exhibits the complete homeotic transformation of gynoecium to stamens, and DL is a member of the YABBY gene family and regulates carpel specification in rice (Yamaguchi et al. 2004). floral organ number1 (fon1) exhibits the enlargement of the floral meristem and contains the increased number of all floral organs, and FON1 is orthologous to Arabidopsis CLAVATA1, which encodes a leucine-rich repeat receptor kinase (Suzaki et al. 2004). Two missense mutations at MADS domain of OsMADS1 generate lhs1 mutant, in which all four floral whorls are affected (Jeon et al. 2000).
nsr mutant was derived from the hybrid progeny between indica rice and Triticum aestivum wheat, and the morphological and physiological characters and cytological mechanism of low fertility were studied briefly in nsr (Tang et al. 1981; Wu et al. 2004). nsr shows normal karyotype and 12 bivalents during meiosis, and is allelic to lhs1 (Khush and Librojo 1985). According to data published, the morphology of nsr is different from lhs1 in abnormal panicle, internal florets, variation of stamens and abnormality of pistils. In this experiment, the elucidation on the morphogenesis and molecular basis of nsr mutant was conducted. The results showed that the variation of nsr spikelets was caused by the irregular differentiation pattern and incomplete determination of floral primordium. And nsr was a novel homeotic mutation in OsMADS1, which played a role in specifying lemma and palea, acted as a repressor of overdevelopment of lemma and palea and regulated transcript level of AP3 homologue OsMADS16.
Materials and methods
Plant materials
Naked seed rice was a natural mutant derived from the hybrid progeny between indica rice (Tieguai 1) and Triticum aestivum wheat (Kangxiu 1). The F1 cross was made between nsr mutant and Zhenongda 104 (japonica). The mapping population of 1160 F2 mutant plants was used in this experiment. Tiefuai 1 (indica) was selected as a control plant for morphogenesis and sequence analyses. Nipponbare (japonica) was applied in transgenic experiments.
Microscopic observation
After heading, spikelets were selected randomly and floral organs were investigated under a light microscopy. For scanning electron microscopy (SEM), young panicles at differentiation stages were fixed in fixative solution of 2.5% glutaric dialdehyde and washed with a sodium phosphate buffer (0.1 M, pH 7.2). Then the samples were fixed in 1% osmic acid, dehydrated with an ethanol series, incubated in an ethanol–isoamyl acetate (1:1[v/v]) and isoamyl acetate, in turn. The samples were dried, mounted and coated with gold. The mounted specimens were observed with a scanning electronic microscope (model KYKY-1000B) at an accelerating voltage of 15 kV.
Genetic analysis on nsr mutant
At heading stage, genomic DNA was extracted from F2 mutant plants and used for genetic mapping by microsatellite markers. The total RNA from young panicles was isolated and the cDNA molecules were synthesized with oligo-dT15 primers and a first-strand cDNA synthesis kit (SuperScriptsII; Invitrogen). The coding sequences of OsMADS1 cDNA were amplified by RT-PCR with a set of primers, 5′-TGCAAAGGGGATAGAGTAGTAGAGA-3′ and 5′-GGGGAGAAGGTCGTAAGAGA-3′. The amplified fragments were sequenced by MegaBACE 1000 DNA Analysis System.
Construction of binary vector
Binary vector pCAMBIA1301 was modified for ectopic expression. The cauliflower mosaic virus 35S promoter (35S) was cloned in as a HindIII-BamHI fragment in the region of multi-cloning site. Subsequently, the nopaline synthase terminator was cloned as a SacI–EcoRI fragment downstream of the 35S promoter.
For construction of overexpressing OsMADS1, the coding sequence of OsMADS1 cDNA was isolated with specific primer sets. PCR products were cloned into pUCm-T. The sense orientation of the cDNA was verified by EcoRV and SalI digestion and was inserted into the SmaI and SalI sites of the modified pCAMBIA1301. The recombinant was called 35S-OsMADS1.
To make the construct for expressing dsRNA in plant cells, we first applied a bridge vector pBS-in, in which a 605-bp intron fragment was placed between two multi-cloning sites. The 351 bp-length fragment of coding sequence of OsMADS1 was amplified by RT-PCR from wild-type rice with primer sets, 5′-GAGCAGCTTGAGAACCAGATAGA-3′ and 5′-TCATTGCTCAGATGGTCCATGTAG-3′. PCR products were cloned into pUCm-T. The subcloned products were placed upstream and downstream of the intron fragment in opposite directions, in turn. The constructed bridge vector was inserted into the KpnI and SacI sites of the modified pCAMBIA1301. The resulting RNAi construct was denoted as 35S-dsRNAiOsMADS1.
Rice transformation
The Agrobacterium strains EHA105 harboring 35S-OsMADS1 or 35S-dsRNAiOsMADS1 plasmid were used to transform rice calli induced from the mature embryos of Nipponbare, respectively, according to the methods by Hiei et al. (1994). Co-cultivation was for 2–3 days in the dark at 25°C and the co-cultivated calli were transferred onto an NCH medium containing 50 mg l−1 hygromycin and 50 mg l−1 cefotaxime in the light at 28°C until actively proliferating calli developed. The actively growing calli were transferred onto a regeneration MS medium supplemented with 0.05 mg l−1 NAA, 3 mg l−1 kinetin, 1% sorbitol, 0.8% phytagar for 2–4 weeks. A light/dark cycle of 16/8 h was provided during the regeneration. The regenerated plantlets were grown in a greenhouse.
Real-time PCR
For quantitative real-time PCR experiments, iCycler iQ™ Real-Time PCR Detection System (DIO-RAD, USA) was used. For PCR reactions, a mastermix of reaction components was prepared, according to Instruction of iQ SYBR Green Supermix (DIO-RAD, USA). The following primers were used for real-time PCR experiments: OsMADS1 forward primer, 5′-CTACATGGACCATCTGAGCAATGA-3′, and reverse primer, 5′-AAGAGAGCACGCACGTACTTAG-3′; RAP1B forward primer, 5′-GCGAAAGGATAGAGGATGTACCAG-3′, and reverse primer, 5′-GCAACCGCAAGATGACAATAG-3′; OsMADS4 forward primer, 5′-AGCACAAGATGTTGGCTTTTAGGG-3′, and reverse primer, 5′-CATCTAGCAGCGCATGAGG-3′; OsMADS16 forward primer, 5′-TCAAGGACATCAACCGCAACCTG-3′, and reverse primer, 5′-ATGATACTTCCTGTGGCGAACCTC-3′; OsMADS3 forward primer, 5′-AACGCAAACAGTAGGACCATAGTG-3′, and reverse primer, 5′-CCCCTCTCATTCTCAACAACC-3′; OsMADS13 forward primer, 5′-GCGATAATGTGAGCAACCTGT-3′, and reverse primer, 5′-TTCTGAGGTCCATGTTGTCGTTCT-3′; OsMADS15 forward primer, 5′-TCTTCCACCACAAAATATCTGCTAC-3′, and reverse primer, 5′-GGTACGTGCTGATGATTACACAA-3′; ACT1 forward primer, 5′-CTTCTAATTCTTCGGACCCA-3′, and reverse primer, 5′-TTGAAAACTTTGTCCACGCTAATC-3′.
Results
Morphology of spikelets in nsr plants
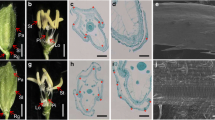
Wild-type rice spikelet comprised a pair of rudimentary outer glumes at its base and four whorls of floral organs, namely lemma/palea, a pair of lodicules, six stamens and a pistil from the periphery to the center, and lodicules were located inner to lemma (Fig. 1a). Wild-type spikelet was closed (Fig. 1b), and its palea/lemma was characterized by abundant and large epidermal cells and long trichomes (Fig. 1j). No obvious alteration was observed during vegetative stage, while abnormal spikelets were investigated after heading in nsr plants. Compared to wild-type plant, nsr mutant showed leafy and overdeveloped lemma and palea in spikelet, which was open (Fig. 1c), and the palea/lemma displayed fewer and smaller epidermal cells and shorter trichomes with higher density (Fig. 1k). nsr spikelet generated internal florets with palea-/lemma-like organ (Fig. 1d). Two pairs of lodicules became leafy and arranged inner to both lemma and palea, and three stamens and one pistil were formed in nsr spikelet (Fig. 1e), although eight stamens, three pistils and three pairs of lodicules were rarely found in nsr spikelet (Fig. 1f). Occasionally, an ovary tipped with five stigmas (Fig. 1g) or the conversion of anther into stigma (Fig. 1h) was observed in the nsr spikelet. Mature florets were formed on one rachilla axis of nsr mature spikelet (Fig. 1i), indicating that nsr spikelets had an incomplete determination of floral meristem.
Phenotypes of wild-type and naked seed rice (nsr) mutant rice. a–b the spikelet of wild-type rice. c–i phenotypic alteration of floral organs in nsr spikelets. Lemma and palea were removed from nsr spikelets (e–h), and the outer three whorls of floral organs were removed from the nsr spikelet (g). Arrowhead indicated the conversion of anther into stigma and internal floret in nsr spikelets (h) and (i), respectively. Arrow indicated trichome and arrowhead showed the epidermis of wild-type (j) and nsr (k) spikelet. g glume; l lemma; p palea; lo lodicule; plo palea-/lemma-like organ; s stamen; o ovary; pi pistil; st stigma. Bars from a–i=1 mm, Bars in j, k=100 μm
To study further on the floral development in the spikelets of nsr mutant, an investigation on 3,600 spikelets was conducted. 24.4% of nsr spikelets generated abnormal florets with palea-/lemma-like organs, whereas 91.3% of nsr spikelets contained two pairs of lodicules, which were transformed to palea-/lemma-like organs. 97.9% of spikelets contained less than six stamens and the number of stamens was reduced to three, on an average. One pistil was mostly observed in the nsr spikelets, of which 20.9% formed over one ovary. nsr spikelets were characterized by overdeveloped lemma and palea, an increased number of lodicules, a decreased number of stamens and extra pistils. nsr spikelets aslo formed internal florets, which contained floral organs of four whorls.
Microscopic investigation of nsr spikelets
Scanning electron microscopy was conducted to compare the morphogenesis of wild-type flowers with that of nsr at different differentiation stages (Fig. 2). In wild-type flower, floral primordium generated outer glumes, lemma and palea first (Fig. 2a). Then lodicule and six stamens primordia were generated and the former was located inner to the lemma (Fig. 2b). Floral primordium gave a rise to generate a gibbous carpel primordium, while other floral organs primordia were continuing to grow (Fig. 2c).
Scanning electron microscopy of wild-type and nsr spikelets. a–c and d–l, the differentiation of floral primordium in wild-type spikelets and nsr spikelets, respectively. Lemma was ripped off the nsr spikelet (h), and lemma and palea were removed from nsr spikelet (i). g glume; l lemma; p palea; lo lodicule; plo palea-/lemma-like organ; s stamen; c carpel; fm floral primordium. Bars=100 μm
The morphogenesis of nsr spikelet was different from that of wild-type spikelet. In nsr spikelet, outer glumes, palea and lemma were formed (Fig. 2d). Then lodicule primordium was initiated inner to the lemma (Fig. 2e). Alternatively, two lodicule primordia were formed and located inner to both the lemma and palea, respectively (Fig. 2f). Lodicule began to overdevelop at the early stage of flower development (Fig. 2h). Stamens and carpel primordia were generated irregularly, with the number of stamens varying from two to four (Fig. 2g, h). Interestingly, floral primordium was elongated in the direction of lemma and palea and divided (Fig. 2i). Two separate floral primordia were formed in nsr spikelet (Fig. 2j), and two newly formed floral primordia generated palea-/lemma-like organs, respectively (Fig. 2k). Three floral primordia developed in one nsr spikelet (Fig. 2l). The floral primordium of nsr spikelet differentiated under the irregular pattern and incomplete determination.
Genetic analysis and gene mapping
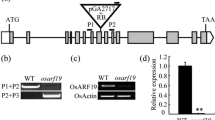
The F1 cross was made between nsr and japonica rice Zhenongda 104, which showed normal spikelet phenotype. The F2 population of 460 plants segregated wild-type and mutant plants in a ratio of 3:1 (χ2=0.23, P>0.95), indicating nsr was a monogenic recessive trait. nsr locus was primarily mapped to chromosome 3 between microsatellite markers, RM3548 (2.26 cM) and RM2326 (1.7 cM). Subsequent fine mapping showed that nsr locus was located between RM3417 (0.26 cM) and RM7576 (0.21 cM) (Fig. 3a). With the sequence of RM3417 and RM7576, a blast homology search was carried out and identified two BAC clones, AC105928 and AC135138, between which there were three overlapping BAC clones (AC104179, AC146619 and AC134241). Alignment of sequence of the five overlapping BAC clones showed that the physical distance between RM3417 and RM7576 was about 400 kb. Annotation of this region identified an open reading frame (ORF) encoding a MADS-box protein, OsMADS1 (http://www.gramene.org).
Jeon et al. (2000) reported that lhs1 was a homeotic mutation in OsMADS1, which was mapped between RG100 and RZ313. The region included nsr locus. Thereby, the coding regions of the OsMADS1 gene were amplified from nsr and the wild-type rice with primers located at OsMADS1 cDNA sequence. Sequence analysis of the amplified fragments showed that 5 nucleotides of A, G, A, A and T at positions 58, 80, 287, 527 and 666 in coding region were changed to G, A, G, G and C in the nsr mutant, respectively. Consequently, the deduced amino acids of Thr20, Gly27, Lys96 and Asn176 were replaced with Ala, Asp, Arg and Ser, respectively, and His222 was not changed in OsMADS1 protein of nsr (Fig. 3b). nsr contained both missense and nonsense mutations in the coding region of OsMADS1. In lhs1 mutant, the nucleotides C and G at position 70 and 80 in the coding region of OsMADS1 were changed to T and A, respectively (Jeon et al. 2000). Thereby, nsr was a novel mutation of homeotic gene OsMADS1.
Floral organs alteration in transgenic rice plants overexpressing OsMADS1
To address the role of OsMADS1 in floral organ development, the cDNA clone containing the full-length OsMADS1 ORF was placed under the cauliflower mosaic virus 35S promoter. The construct was introduced into rice cells by Agrobacterium-mediated transformation. Two independent transgenic lines were generated and each included a number of transgenic plants.
The wild-type rice spikelet had a pair of rudimentary outer glumes at its base and comprised lemma, palea, two lodicules, six stamens and one pistil with two stigmas (Fig. 4a, b). In 35S-OsMADS1 transgenic lines, no obvious abnormality was observed during vegetative stage, but the alternations of flower phenotypes were investigated after heading. A significant proportion (17.9%) of flowers in 35S-OsMADS1 transgenic lines generated the enlarged outer glumes, which approached the size of lemma or palea (Fig. 4c). The stamen was characterized by the decreased number, which was mostly five (Fig. 4e). Occasionally, the flowers did not bear any internal floral organs (Fig. 4d). RT-PCR analysis revealed that the transgenic lines expressed the OsMADS1 transcript in their leaves, while no transcript was detected in the wild-type leaves (Fig. 4j), suggesting that ectopic expression of OsMADS1 was responsible for the alteration of floral organs in transgenic rice plants.
Floral alterations of transgenic rice carrying 35S-OsMADS1 and 35S-dsRNAiOsMADS1 construct. a–b wild-type flower; c–e the flowers of 35S-OsMADS1 transgenic plants; f–i the flowers of 35S-dsRNAiOsMADS1 transgenic plants. Lemma and palea were removed from spikelet (b), (e), (h) and (i). j–k RT-PCR analysis of OsMADS1 transcripts in the 35S-OsMADS1 and 35S-dsRNAiOsMADS1 transgenic plants; Lane 1, RT-PCR product from wild-type rice; the other lanes, RT-PCR products of transgenic lines. g glume; l lemma; p palea; lo lodicule; plo palea-/lemma-like organ; s stamen; pi pistil. Bars=1 mm
The real-time quantitative reverse transcriptase technique offers both high sensitivity and specificity (Bustin 2000), and it was applied in an analysis of the expression level of genes belonging to a very conserved gene family (Yokoyama and Nishitani 2001). Therefore, real-time PCR was applied to investigate the transcript levels of several MADS-box genes, which presumably specify floral organ identity in rice. Figure 5 illustrated the relative transcript levels of several MADS-box genes in the young panicles (5 cm) of 35S-OsMADS1 transgenic rice and the control plants. The transcript level of OsMADS1 was about two times higher than that of wild-type rice. The expression of RAP1A, OsMADS4, OsMADS16, OsMADS13 and OsMADS15 were increased significantly, but OsMADS3 transcript was affected less.
Floral organs alteration in 35S-dsRNAiOsMADS1 transgenic rice plants
In plants, the transgenic plants expressing dsRNAs (RNA hairpins) can significantly knock down the transcription level of their targeted endogenous genes in Arabidopsis, and mimic their loss-of-function mutants (Chuang and Meyerowitz 2000). In rice, RNAi was applied to address the function of AP3 homologue OsMADS16 (Xiao et al. 2003). In this study, the OsMADS1 cDNA fragment was introduced into an RNAi construct under 35S promoter. Three transgenic rice lines (independent transformants) were successfully recovered.
All 35S-dsRNAiOsMADS1 transgenic lines grew normally as wild-type rice during vegetative stage. Obvious changes in spikelets were observed after heading. Compared with that in wild-type rice (Fig. 4a, b), the overdevelopment of lemma and palea was observed in 35S-dsRNAiOsMADS1 spikelet, which was open (Fig. 4f). 35S-dsRNAiOsMADS1 transgenic spikelet generated one extra palea-/lemma-like organ inside (Fig. 4g) or the transformation of two lodicules into four palea-/lemma-like organs (Fig. 4h). The stamens were characterized by the diverse number, which varied from two (Fig. 4i) to six (data not shown). Occasionally, two separate pistils appeared in one spikelet (Fig. 4h).
An investigation on floral organs was made to study further on the flowers development of the 35S-dsRNAiOsMADS1 transgenic plants. The averaged length of lemmas and paleas amounted to 0.90 and 0.73 cm, respectively, and were significantly longer than those of wild-type spikelets. 36.7% of the investigated flowers had five stamens, 11.4% of flowers contained two, three or four stamens. Whereas 5.1% of lodicules in investigated flowers was transformed into palea-/lemma-like organs. The different efficiencies of the floral organ variation indicated that lodicules and stamens might have different responses to the introduced dsRNA of OsMADS1 cDNA fragment.
RT-PCR analysis was conducted to compare the transcripts of OsMADS1 in the 35S-dsRNAiOsMADS1 transgenic plants with the control plants. As shown in Fig. 4k, a weaker band OsMADS1 transcripts were detected in flowers of transgenic plants after 35 cycles of amplification, compared to that in wild-type panicles. When 28 cycles was applied, the OsMADS1 transcript was not detected in the flowers of transgenic plants (data not shown). This result showed that the reduced level of OsMADS1 transcription led to the changes in floral organs of transgenic plants.
The real-time PCR was applied to investigate the transcription levels of several MADS-box genes in young panicles (5 cm) of 35S-dsRNAiOsMADS1 transgenic plants and the control plants (Fig. 6). In 35S-dsRNAiOsMADS1 lines, OsMADS1 transcript was reduced to approximately 20% of that in wild-type rice. The remaining OsMADS1 transcript indicated that the introduced dsRNA did not completely suppress the endogenous transcription in this transgenic plant. The transcript levels of OsMADS16, OsMADS3 and OsMADS13 were significantly reduced when compared with those of wild-type rice. In contrast, RAP1B expression was increased greatly. OsMADS4 and OsMADS15 transcript levels were affected slightly.
Discussion
Naked seed rice was characterized by the overdeveloped palea and lemma, leafy lodicules, decreased stamens, occasionally extra pistils and internal florets. The observation on the morphogenesis of floral organs indicated that floral primordium of nsr spikelets differentiated into floral organs under the irregular pattern, compared with that of wild-type rice. First, floral primordium initiated one lodicule inner to lemma, or one more lodicule inner to palea alternatively after the proper formation of palea and lemma primordia. As a result, two pairs of lodicules were observed in most nsr spikelets. Second, floral primordium in nsr spikelets formed stamens and carple primordia with an irregular shape and a decreased number of stamens. Third, the division floral primordium was also observed in the nsr spikelets. Consequently, the syncarpy or abnormal florets might be formed, which resulted in an increased number of floral organs subsequently. The results suggested that the mutant spikelets of nsr plants resulted from the irregular differentiation pattern of floral primordium and incomplete determination of floral meristem.
In rice, several floral mutants have been studied on a molecular basis, such as fon1, spw1, dl (Nagasawa et al. 2002; Suzaki et al. 2004). The lhs1 mutant is derived from japanica rice and caused by missense mutations of code Arg24 and Gly27 in MADS domain of OsMADS1 (Kinoshita et al. 1977; Jeon et al. 2000). nsr has been reported to be allelic to lhs1 (Khush and Librojo 1985). This result was supported by the findings in this study, but missense mutations of Thr20, Gly27, Lys96 and Asn176 and nonsense mutation of His222 of OsMADS1 in nsr were different from the missense mutations in lhs1. Therefore, nsr was a novel mutant of homeotic gene OsMADS1. Pellegrini et al. (1995) reported that the Gly27 is located at the DNA binding position. Similarly, the missense mutation of code Gly27 to Asp results in ap1–2 and cal-3 mutants (Mandel et al. 1992; Kempin et al. 1995). Transgenic lines from the introduced gene expressing double-stranded RNA with the OsMADS1 cDNA fragment generated mutant spikelets, which comprised overdeveloped palea and lemma, increased lodicules, decreased stamens and occasionally extra pistil. The spikelets of 35S-dsRNAiOsMADS1 lines were similar to those of nsr plants, providing further evidence that nsr was a homeotic mutant in OsMADS1.
The overdeveloped lemma and palea were present in the 35S-dsRNAiOsMADS1 transgenic plants, in which the transcript level of endogenous OsMADS1 was reduced significantly. No significant changes were observed in the length of lemma and palea, and the transcripts of OsMADS1 were expressed abundantly in 35S-OsMADS1 transgenic plants. In consideration of a later expression of OsMADS1, which is confined to lemma and palea (Chung et al. 1994; Prasad et al. 2001), it was suggested that wild-type OsMADS1 might function as a repressor of the overdevelopment of lemma and palea. Although the transcript level of mutant OsMADS1 in nsr spikelets appeared higher than wild-type OsMADS1 in the control plants (data not shown), the missense mutations contributed to the loss of proper function of OsMADS1 in nsr plants, as a result, the overdeveloped lemma and palea were observed.
Genetic studies on antisense suppression mutants of OsMADS4 and the maize silky1 mutant suggest that lodicules are equivalent to petals, although lodicules are morphologically different from petals (Schmidt and Ambrose 1998). Transgenic plants expressing double-stranded RNA with OsMADS16 cDNA fragment displays the conversion of two lodicules into palea-/lemma-like organs (Xiao et al. 2003). In this study, most of the nsr spikelets contained two pairs of lodicules, which were converted to palea-/lemma-like organs. The similar phenotypic conversion was observed in the 35S-dsRNAiOsMADS1 transgenic plants, in which the transcript level of OsMADS16 was reduced greatly. However, the conversion and increased number of lodicules were not observed in 35S-OsMADS1 transgenic plants, which had a higher transcript level of OsMADS16 than the wild-type rice. These results indicated that OsMADS1 played its role in upregulation of transcript activity of OsMADS16, an AP3 homologous gene. The transcripts of OsMADS16 are present in the lodicules and stamens (Moon et al. 1999). OsMADS1 is expressed uniformly in young flower primordia and its expression is confined to the lemma and palea later, with weak expression in the carpel (Chung et al. 1994; Prasad et al. 2001). It was speculated that the role of OsMADS1 in affecting the OsMADS16 transcripts might require a co-factor, which was expressed in both floral meristem and primordia of lodicule and stamens.
Abbreviations
- AG :
-
AGAMOUS
- AP :
-
APETALA
- DEF :
-
DEFICIENS
- GLO :
-
GLOBOSA
- nsr :
-
Naked seed rice
- PI :
-
PISTILLATA
- SEM:
-
Scanning electron microscopy
- SEP :
-
SEPALLATA
- SQUA :
-
SQUAMOSA
References
Angenent GC, Colombo L (1996) Molecular control of ovule development. Trends Plant Sci 1:228–232
Bustin SA (2000) Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol 25:169–193
Chuang CF, Meyerowitz EM (2000) Specific and heritable genetic interference by double-stranded RNA in Arabidopsis thaliana. Proc Natl Acad Sci USA 97:4985–4990
Chung YY, Kim SR, Finkel D, Yanofsky MF, An G (1994) Early flowering and reduced apical dominance result from ectopic expression of a rice MADS box gene. Plant Mol Biol 26:657–665
Clark SE, Running MP, Meyerowitz EM (1993) CLAVATA1, a regulator of meristem and flower development in Arabidopsis. Development 119:397–418
Coen ES, Meyerowitz EM (1991) War of the whorls: genetic interactions controlling flower development. Nature 353:31–37
Hiei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6:271–282
Honma T, Goto K (2001) Complexes of MADS-box proteins are sufficient to convert leaves into floral organs. Nature 409: 525–529
Jeon JS, Jang S, Lee S, Nam J, Kim C, Lee SH (2000) Leafy hull sterile 1 is a homeotic mutation in a rice MADS Box gene affecting rice flower development. Plant Cell 128:871–884
Kang HG, Jeon JS, Lee S, An G (1998) Identification of class B and class C floral organ identity genes from rice. Plant Mol Biol 38:1021–1029
Kempin SA, Savidge B, Yanofsky MF (1995) Molecular basis of the cauliflower phenotype in Arabidopsis. Science 267:522–525
Khush GS, Librojo AJ (1985) Naked seed rice (NSR) is allelic to op and lhs. Rice Genet News 2:71
Kinoshita T, Hidano Y, Takahashi M (1977) A mutant “long hull sterile” found in the rice variety, Sorachi. Memoirs of Faculty and Agriculture, Hokkaido University 10: 247–268
Kyozuka J, Kobayashi T, Morita M, Shimamoto K (2000) Spatially and temporally regulated expression of rice MADS box genes with similarity to Arabidopsis class A, B and C genes. Plant Cell Physiol 41:710–718
Laux T, Meyer KFX, Berger K, Jürgens G (1996) The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development 122:87–96
Lee S, Jeon JS, An K, Moon YH, Lee S, Chung YY, An G (2003) Alteration of floral organ identity in rice through ectopic expression of OsMADS16. Planta 217:904–911
Lim J, Moon YH, An G, Jang SK (2000) Two rice MADS domain proteins interact with OsMADS1. Plant Mol Bio 44:513–527
Mandel MA, Gustafson BC, Savidge B, Yanofsky MF (1992) Molecular characterization of the Arabidopsis floral homeotic gene APETALA1. Nature 360:273–277
Moon YH, Jung JY, Kang HG, An G (1999) Identification of a rice APETALA3 homologue by yeast two-hybrid screening. Plant Mol Biol 40:167–177
Nagasawa N, Miyoshi M, Sano Y, Satoh H, Hirano H, Sakai H, Nagato Y (2002) SUPERWOMAN1 and DROOPING LEAF genes control floral organ identity in rice. Development 130:705–718
Pelaz S, Gary SD, Baumann E, Wisman E, Yanofsky MF (2000) B and C floral organ identity functions require SEPALLATA MADS-box genes. Nature 405:200–203
Pellegrini L, Tan S, Richmond TJ (1995) Structure of serum response factor core bound to DNA. Nature 376:490–498
Prasad K, Sriram P, Kumar CS, Kushalappa K, Vijayraghavan U (2001) Ectopic expression of rice OsMADS1 reveals a role in specifying the lemma and palea, grass floral organs analogous to sepals. Dev Genes Evol 211:281–290
Running MP, Meyerowitz EM (1996) Mutations in the PERIANTHIA gene of Arabidopsis specifically alter floral organ number and initiation pattern. Development 122:1261–1269
Running MP, Fletcher JC, Meyerowitz EM (1998) The WIGGUM gene is required for proper regulation of floral meristem size in Arabidopsis. Development 125:2545–2553
Schmidt R, Ambrose BA (1998) The blooming of grass flower development. Curr Opin Plant Biol 1:60–67
Suzaki T, Sato M, Ashikari M, Miyoshi M, Nagato Y, Hirano H (2004) The gene FLORAL ORGAN NUMBER1 regulates floral meristem size in rice and encodes a leucine-rich repeat receptor kinase orthologous to Arabidopsis CLAVATA1. Development 131:5649–5657
Tang XH, Zhu ZP, Huang QL (1981) Some aspects of morphological and physiological characters of ‘naked seed’ rice. Acta Genetica Sinica 8:350–355
Theissen G, Saedler H (2001) Floral quartets. Nature 409:469–471
Thomas J (2001) Relearning our ABCs: new twists on an old model. Trends Plant Sci 6:310–316
Weigel D, Meyerowitz EM (1994) The ABCs of floral homeotic genes. Cell 78: 203–209
Wu JG, Shi CH, Chen SY, Xiao JF (2004) The cytological mechanism of low fertility in the naked seed rice. Genetica 121:259–267
Xiao H, Wang Y, Liu D, Wang W, Li X, Zhao X, Xu J, Zhai W, Zhu L (2003) Functional analysis of the rice AP3 homologue OsMADS16 by RNA interference. Plant Mol Biol 52:957–966
Yamaguchi T, Nagasawa N, Kawasaki S, Matsuoka M, Nagato Y, Hirano HY (2004) The YABBY gene DROOPING LEAF regulates carpel specification and midrib development in Oryza sativa. Plant Cell 16:500–509
Yokoyama R, Nishitani K (2001) A comprehensive expression analysis of all the members of a gene family encoding cell-wall enzymes allowed us to predict cis-regulatory regions involved in cell-wall construction in specific organs of Arabidopsis. Plant Cell Physiol 42:1025–1033
Acknowledgements
This work was supported by National Natural Science Foundation of China (no. 30500319 and no. 30240030), the Science and Technology Office of Zhejiang Province (no. 011102471) and 151 Foundation for the Talents of Zhejiang Province.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, ZX., Wu, JG., Ding, WN. et al. Morphogenesis and molecular basis on naked seed rice, a novel homeotic mutation of OsMADS1 regulating transcript level of AP3 homologue in rice. Planta 223, 882–890 (2006). https://doi.org/10.1007/s00425-005-0141-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-005-0141-8