Abstract
The characterisation of the single flower truss (sft) mutant phenotype of tomato (Lycopersicon esculentum Mill.), as well as its genetic interactions with other mutations affecting FALSIFLORA (FA) and SELF PRUNING (SP) genes, has revealed that SFT is a key gene in the control of floral transition and floral meristem identity. The single sft mutation produces a late-flowering phenotype in both long-day and short-day conditions. In combination with fa, a mutation affecting the tomato gene orthologous to LFY, sft completely blocks the transition to flowering in this species. Thus, the phenotype of the sft fa double mutants indicates that SFT and FA participate in two parallel pathways that regulate the switch from vegetative to reproductive phase in tomato, and that both genes are indispensable for flowering. On the other hand, the replacement of flowers by vegetative shoots observed in the sft inflorescence suggests that SFT regulates flower meristem identity during inflorescence development of tomato. In addition to these two main functions, SFT is involved in the development of both flowers and sympodial shoots of tomato. First, the mutation produces a partial conversion of sepals into leaves in the first floral whorl, and a reduction in the number of floral organs, particularly carpels. Secondly, the sympodial development in the mutant plants is altered, which can be related to the interaction between SFT and SP, a gene controlling the number of nodes in sympodial shoots. In fact, we have found that the sft phenotype is epistatic to that of sp, and that the level of SP mRNA in the apical buds of sft around flowering is reduced. SFT can therefore co-ordinate the regulation of two simultaneous developmental processes in the tomato apical shoot, the promotion of flowering in one sympodial segment and the vegetative development of the next segment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The transition from vegetative to reproductive development constitutes an important process during the life cycle of higher plants. This transition involves a change in the developmental program of the shoot apical meristem, which acquires a reproductive competence and starts the production of flowers instead of leaves. The duration of the vegetative phase of development or flowering time is known to be controlled by both internal and environmental signals that ensure reproduction during the most favourable developmental and environmental conditions (reviewed in Koornneef et al. 1998; Levy and Dean 1998).
Our present knowledge of the genetic control of floral transition is mainly derived from studies in the facultative long-day plant Arabidopsis thaliana. The existence of late- and early-flowering mutants has allowed the identification of several genes that promote or repress flowering in this species (Koornneef et al. 1998). Moreover, on the basis of mutant response to photoperiods and vernalisation, as well as from genetic interaction analyses, the existence of four pathways controlling flowering time in Arabidopsis has been established (Levy and Dean 1998). Two of these, the photoperiod and vernalisation pathways, mediate signals from environmental conditions. The other two, autonomous and gibberellin-dependent pathways, are constitutive pathways that promote or repress flowering independently of daylength.
The integration of these floral inductive signals from multiple pathways is not completely understood at the moment. It has been proposed that multiple promotion pathways converge in a floral repressor encoded by EMBRYONIC FLOWER genes (Koornneef et al. 1998). More recently, considerable evidence has indicated that multiple floral induction pathways are integrated in the transcriptional regulation of certain floral inductive genes such as the flower identity gene LEAFY (LFY) (Blázquez and Weigel 2000), as well as in flowering-time genes such as the FLOWERING LOCUS T (FT) and SUPRESSOR OF OVEREXPRESSION OF CONSTANS (SOC1) (Araki 2001). The existence of all of these different promotion pathways as well as the cross-talk between them could explain why no single or double mutation in Arabidopsis has been found that completely prevents the transition to flowering. For instance, mutations in both LFY and FT, or LFY and FWA, produce a late-flowering phenotype and a very altered inflorescence with no floral structures, but do not inhibit the transition to the inflorescence phase (Ruiz-Garcia et al. 1997). Nevertheless, there is evidence to suggest that this genetic model of flowering in Arabidopsis might not fit other species. In Petunia, for example, co-suppression mutants for PFG, a MADS-box gene related in sequence to AP1 and FRUITFULL (FUL), are unable to undergo the floral transition and plants maintain their vegetative growth indefinitely (Immnink et al. 1999).
In contrast to Arabidopsis, flowering time of tomato is not influenced by photoperiod, and it is therefore considered a day-neutral plant (Atherton and Harris 1986). In addition, the main shoot of tomato has a sympodial growth habit and is formed by an initial segment bearing from 6 to 12 leaves (depending on cultivars), followed by a terminal inflorescence, as well as different sympodial segments each composed of 3 leaves and a terminal inflorescence. Although it would be very important to address whether the genetic model proposed for flower transition in Arabidopsis is also applicable to day-neutral plants such as tomato, little is known about the genetic control of flowering time in this species. To date, only two regulatory genes have been cloned that are involved in the control of floral transition in tomato: SELF PRUNING (SP) and FALSIFLORA (FA). SP, the TERMINAL FLOWER1 (TFL1) homologous gene of tomato, can be considered a floral repressor since mutations in this gene promote an early flowering phenotype of the sympodial shoots, reducing progressively the number of leaves in each sympodial segment and concluding its growth with the development of two consecutive inflorescences (Pnueli et al. 1998). Moreover, detailed studies have recently shown that interactions between SP and other regulatory proteins are required for the biological function of the SP gene (Pnueli et al. 2001). On the other hand, we have recently found that FA, the LFY orthologous gene of tomato, acts as a floral promoter, regulating both the identity of floral meristem and the floral transition of the initial and the successive sympodial segments of the tomato plant (Molinero-Rosales et al. 1999). In this paper we report the regulatory functions of the gene SINGLE FLOWER TRUSS (SFT), by analysing both the phenotypic effects of the sft mutation, and its genetic interactions with both FA and SP. The mutant has been provided by the Tomato Genetic Resource Centre (TGRC), and was first described by Kerr (1982) as a monogenic mutant with a reduced number of flowers per truss. The phenotypic characterisation of the sft mutant indicates that SFT promotes flowering in tomato, being also involved in the regulation of floral meristem identity, as well as in the number and identity of floral organs. Furthermore, based on the sft fa and sft sp double-mutant phenotypes, SFT acts in parallel with FA in the control of floral transition in tomato, but is epistatic to SP.
Materials and methods
Plant material and growth conditions
Tomato (Lycopersicon esculentum Mill.) seeds for the sft and sp mutants, and their background genotypes (cv. Platense and cv. Gardener, respectively) were provided by the Tomato Genetic Resource Center (Department of Vegetable Crops, University of California, Davis) under TGR accession numbers LA2460, LA3133, LA3243, and LA854, respectively. Plants were grown under standard greenhouse conditions. Phenotypic characterisation of sft and its background genotype cv. Platense was also carried out in plants growing in controlled chambers at 26 °C day/20 °C night, under either long-day (16 h light) or short-day (8 h light) conditions.
Generation and identification of sft fa and sft sp double mutants
Since fa mutants are completely sterile, sft fa double mutants were obtained by using the sft mutant flowers (sft/sft) as pollen donor to fertilise emasculated flowers of plants heterozygous for the fa mutation (+/fa). F1 progeny plants heterozygous for the fa mutation were selfed, and the resulting F2 populations used to identify double mutants. Since the fa allele has a 16-bp deletion in the coding region of the gene (Molinero-Rosales et al. 1999), the presence of the wild type (WT) and the mutated alleles in either parental, F1 or F2 plants were identified by PCR. Genomic DNA of each plant was used as template with primers lf-cDNA-for (5′-CGC AGA TAT TTC GGT GGG ACC-3′) and LFY-rev (5′-ATT CCT CCA CCT CCA CCT CCT TGG-3′), located in the FA coding region, and allowing the amplification of the gene region containing the deleted sequence.
The sft sp double mutant was identified from an F2 population generated by cross-pollinating plants homozygous for sft and sp mutations. Since none of the F2 plants analysed showed a novel phenotype, the double mutant was recognised from those plants that, as well as showing an sft phenotype, were homozygous for the sp allele. The lack of an MvaI restriction site in the mutated sp allele was used to genotype those F2 plants. Primers SP1F (5′-ATG GCT TCC AAA ATG TGT GAA CCC-3′) and SP4R (5′-AGA GCA ATC TGT AGT GCC TGG-3′) were designed flanking the MvaI site. The 1,027-bp PCR fragment derived from the SP WT allele was cleaved into two smaller fragments when digested with MvaI, but remained uncut when derived from the mutated sp allele, representing a cleavage amplified polymorphic (CAP) marker.
Phenotypic analyses
Flowering time was measured in at least 10 plants of each genotype, as either the number of days from sowing time until the first flower in each inflorescence opened, or the number of leaves below the first inflorescence and between inflorescences. For morphological characterisation, inflorescences were removed from plants of each genotype and examined with a Nikon stereomicroscope. Scanning electron microscopy of the inflorescence apex was performed as previously described by Huijser et al. (1992).
Reverse transcription (RT)–PCR reactions
Total RNA was isolated from apices of the tomato once floral buds were visible, by using the Rneasy Plant Mini Kit (Qiagen). Reverse transcription was performed with 1 µg of total RNA using the First-Strand cDNA synthesis kit (Amersham Pharmacia Biotech) and following the manufacturer's instructions. The cDNA of each genotype was then used in PCR reactions to amplify simultaneously an FA or SP-specific fragment, and an UBIQUITIN 3 (UBI3)-specific fragment used as a control (Zegzouti et al. 1999). The number of cycles for the amplification of FA, SP or UBI3 was 30, 25 and 20, respectively, since these cycles of PCR reactions had previously been proven to maintain PCR products within the exponential range of amplification. Primers lf-cDNA-for (5′-CGC AGA TAT TTC GGT GGG ACC-3′) and lf-cDNA-rev (5′-GGC AGT GAA GTC GCG ATA GCA ATG C-3′) were used for the amplification of FA; SP-2F (5′-CGA CAA ATT AAA AGC ATC TAC-3′) and SP-3R (5′-GAT GAT ATT ACA TTA CAT TGT GC-3′) for SP; and Le-ubi5′ (5′-CTA ACG GGG AAG ATC ACC C-3′) and Le-ubi3′ (5′-TCC CAA GGG TTG TCA CAT ACA TC-3′) for UBI3. PCR products were resolved on agarose gels, blotted onto a nylon membrane and hybridised with a radiolabelled probe for FA, SP or UBI3 genes of tomato.
Results
single flower truss (sft ) is a late-flowering mutant
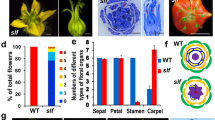
The primary shoot of tomato plants used in this work (cv. Platense) develops an initial vegetative segment composed of about ten leaves and terminated by an inflorescence. Then, an indeterminate number of sympodial segments, each composed of three leaves and a terminal inflorescence, are formed. To assess the time to flowering of the sft mutant, homozygous plants for the mutated allele were grown together with plants of its background genotype in controlled chambers under both short and long days. Compared to the WT genotype, the sft mutant flowered between 10 and 20 days later, and produced significantly more leaves in both long- and short-day conditions (Fig. 1). As observed in the fa mutant, the number of leaves below the first inflorescence in sft, although slightly lower in short days than in long days, did not differ significantly (Fig. 1). On the basis of these observations, sft can be classified as a constitutive late-flowering mutant of tomato.
sft mutation alters floral meristem identity and flower development
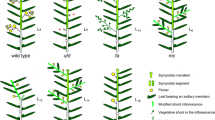
The development of both inflorescence and sympodial meristem was affected by the sft mutation. In WT tomato, flowering results in a determinate inflorescence that is displaced to a lateral position by the growth of the sympodial meristem, a vegetative apex appearing in the axil of the last-formed leaf. This sympodial shoot grows vigorously, producing a vegetative sympodial segment of about three leaves and a new terminal inflorescence (Sawhney and Greyson 1972; Gómez et al. 1999). The reiteration of this developmental pattern results in a main axis where one inflorescence is separated from another by about three leaves (Fig. 2a, b). After flowering, mutant sft plants produced, however, a terminal segment characterised by a reiteration of one or two individual flowers and two to three leaves (Fig. 2c, d). This altered development could be interpreted as being caused by the conversion of each inflorescence into an individual flower, with a normal sympodial development, giving rise to the vegetative segments among flowers. Nevertheless, a detailed characterisation of WT and mutant plants during early stages of development demonstrated that the inflorescence of sft corresponds to the entire terminal segment of the plant rather than to each single flower. Compared to WT inflorescences, where each floral meristem emerges from the base of the preceding flower (Fig. 2e, and particularly Fig. 3), the initiation of the sft inflorescence is normal, but after producing one or two flowers, the subsequent floral meristem is completely replaced by a vegetative meristem (Fig. 2f, and particularly Fig. 3). Therefore, the sft inflorescence loses floral meristem identity after producing one or two flowers and reverts to a vegetative developmental program being the position of the next flower occupied by a vegetative shoot (Fig. 2f). This ectopic shoot within the inflorescence grows vigorously as a sympodial meristem, acquiring apical dominance and displacing flowers to a lateral position (Fig. 2d). On the other hand, the sympodial bud, which normally develops in the leaf just below the inflorescence and allows the plant to grow indeterminately, is arrested in its growth (Fig. 2d). The resultant phenotype is a terminal vigorous inflorescence shoot in which one or two individually attached flowers are followed by two to three leaves. (Fig. 2c, d).
Comparison of WT and sft mutant phenotypes of tomato. a Diagram showing a WT tomato plant (yellow closed circles flowers, central column main shoot composed of different sympodial segments, small arrows vegetative axillary meristems, le leaf, i inflorescence). After the first inflorescence (i 1 ), the sympodial segment (white) is composed of three leaves and a terminal inflorescence (i 2 ). b Apical shoot of a WT tomato plant with two consecutive inflorescences (i 1 and i 2 ). The first inflorescence developed after the seventh leaf (le 7 ) was formed (removed). After flowering, the plant continued its growth through the sympodial shoot; a vegetative shoot developed in the axil of the last-formed leaf. c Diagram of an sft mutant plant of tomato. The apical shoot (light green) represents the only terminal inflorescence of sft plants (symbols are the same as indicated in a). Note that sft plants flower later (le18) than WT plants (le7). d Apical shoot of an sft plant showing a single terminal inflorescence and the arrested development of the sympodial shoot (SS). After the first flower (lower arrow), the inflorescence continued its growth by developing two leaves, followed by the production of a new secondary inflorescence (upper arrow). This secondary inflorescence has reiterated the same developmental pattern as the primary one. e Architecture of a WT inflorescence with six flowers (f1 to f6). f Architecture of an sft inflorescence. After the first two flowers (f1 and f2) have been initiated, the position of the next flower is occupied by a vegetative shoot (vs), where two leaves are formed before producing a new secondary inflorescence (arrow). g Comparison of WT and sft flowers. Note that sepals of mutant flowers are larger, and one of them has particularly leafy features. h The ovary of WT flowers is formed by six to seven carpels while that of sft flowers only develops two carpels. Comparison of the multilocular fruit observed in WT tomato plants (cv. Platense) with the bilocular fruit produced by sft mutant plants
Early development and morphology of inflorescences from WT and sft tomato plants. a Scanning electron microscopy (SEM) image of a WT inflorescence showing the sympodial meristem (sm) and three flower buds (fm1 to fm3). Each flower in the inflorescence is developed from a floral meristem in the base of the preceding one. b SEM image of an sft inflorescence. Note that after the production of the first two flowers (fm1 and fm2), the position of the next flower is replaced by a vegetative meristem (vm), in which two leaf primordia are visible. Bars = 100 μm
During flower development, the sft mutation also alters the identity of first floral whorl organs and the morphology of the gynoecium. Many of the flowers analysed displayed a transformation of sepals into leaf-like structures, with one of the leafy-sepals much larger than the others (Fig. 2g). In comparison with WT flowers, the number of floral organs in sft flowers was also reduced in all the whorls, although the most dramatic change affected the number of carpels. Most of the sft flowers analysed developed only two carpels compared to the seven or eight carpels produced by WT flowers of its background genotype (cv. Platense). Therefore, tomato fruits of cv. Platense were all multilocular while those of sft were bilocular (Fig. 2h).
Flowering is abolished in sft fa double-mutant plants
Loss of function of FA, the tomato LFY ortholog, produces a late-flowering phenotype with an indeterminate inflorescence in which flowers are replaced by vegetative shoots (Molinero-Rosales et al. 1999). To analyse the genetic interaction between SFT and the floral meristem identity gene FA, we generated and characterised the phenotypic effects of sft fa double mutants. Since the fa mutant is completely sterile, double mutants were obtained by crossing sft with heterozygous plants for the fa allele. From a phenotypic analysis performed on 90 F2 plants, 52 had a WT phenotype, 18 showed an fa phenotype and 17 an sft phenotype. Three of the 90 F2 plants were identified as double mutants since they showed a completely new phenotype and were genotyped as homozygous for the fa allele by PCR. After almost 1 year of growth these three plants were not able to undergo the floral transition, with no inflorescence development observed after the production of more than 100 leaves (Table 1). The phenotype of these plants was also confirmed in F3 populations obtained by selfing sft plants of the F2. As expected, of 30 F3 plants analysed, 24 showed an sft phenotype, and 6 the non-flowering phenotype of the double mutant. The phenotype of the sft fa double mutant was found, therefore, to be additive for flowering time, indicating that SFT and FA participate in two parallel pathways that are necessary to promote flowering in tomato.
To investigate whether the floral meristem identity defects observed in sft mutant were caused by down-regulation of FA, the expression of FA in single fa and sft mutants was analysed by RT–PCR. The levels of FA transcripts in the apices of the different mutants were similar to those in their WT background genotypes (Fig. 4). These results indicate that SFT does not control the accumulation of FA mRNA in the analysed tissues. Furthermore, the similar level of FA expression in WT and fa plants suggests that this gene does not seem to regulate its own expression, as has been reported for its orthologous gene FLORICAULA (FLO) in Antirrhinum (Carpenter et al. 1995). Similarly, no change in the expression of FA was detectable in the sp mutant (Fig. 4).
RT–PCR analysis of FA and SP expression in tomato plants bearing fa, sft or sp mutations. RNA was isolated from apices of each mutant (on the right) and its WT genotype (on the left; Pl cv. Platense, Gar cv. Gardener), once flowering had occurred and inflorescences were visible. Reverse transcription was performed from 1 μg of total RNA. A fragment of the constitutively expressed UBIQUITINE-3 (UBI-3) gene was amplified in the same PCR reaction and used as an internal control. From each sample, the amplified fragments of FA and UBI-3, or SP and UBI-3 were transferred to a nylon membrane and hybridised with FA, SP or UBI-3 probes. The detected level of FA transcripts in the three mutant genotypes remains unchanged with respect to the WT genotypes. The levels of the SP transcripts do not change in either fa or sp mutants, but are lower in sft
Genetic interaction between sft and sp
Given that the sft mutation disturbs the developmental program of the sympodial shoot, a program known to be controlled by SP (Pnueli et al. 1998), we also studied the genetic interactions of these two genes by producing and characterising plants with both mutations. The double mutants sft sp (same phenotype as the sft single mutant, cf. Fig. 2d) were produced by pollinating sft plants with pollen obtained from sp flowers. The resulting F1 generation showed a unique WT phenotype, and in an F2 population composed of 107 plants, the segregation was 61:17:29 for the observed phenotypes WT, sft and sp, respectively. No additional phenotype was detected for the double mutant. To confirm that a proportion of sft plants also contained the sp mutation, these plants were genotyped using a CAP marker designed to distinguish between SP and sp alleles (see Materials and methods). The analysis demonstrated that 5 of the 17 F2 plants with an sft phenotype were double mutants since they were homozygous for the sp mutated allele. Flowering time as well as inflorescence development in these double-mutant plants did not differ from plants with the single sft mutation (Table 1), indicating that sft is epistatic to sp. Therefore, SFT may act upstream of SP in the same transductional pathway, or alternatively, SFT is required earlier than SP during flower transition.
To clarify the relation between SFT and SP genes, we tested whether SFT controls the transcription of SP. The level of SP mRNA in the sft mutant and in its background genotype (cv. Platense) was analysed by RT–PCR in apical buds once floral meristems were visible. As shown in Fig. 4, the level of SP mRNA in the apical meristems of the sft mutant is lower than that found in the WT cv. Platense, indicating that SFT could control the expression of SP. The levels of SP mRNA remained unchanged either in fa or sp mutants with respect to their WT genotypes (Fig. 4).
Discussion
SFT promotes flower transition independently of FA
Similar to the regulatory function of FA as flowering promoter gene (Molinero-Rosales et al. 1999), the late-flowering phenotype displayed by the sft mutant clearly indicates a key role for the SFT gene in the control of floral transition in tomato. Both sft and fa mutants develop almost twice as many leaves before flower initiation as their corresponding WT genotypes, in a photoperiod-independent manner. The latter observation suggests that FA and SFT could act as regulatory components of an autonomous pathway of flowering in tomato, although it may also be a consequence of the day-neutral behaviour characteristic of tomato. More interestingly, fa sft double-mutant plants showed a complete abolishment of flowering and absence of any reproductive trait, indicating that, in the absence of FA, SFT is required for floral transition to take place. The double-mutant phenotype also demonstrates an additive effect of both mutations, and therefore that FA and SFT participate in two parallel pathways that promote flowering in tomato. It is possible that the two autonomous pathways represented by these genes are the only ones that promote flowering in a day-neutral species such as tomato. Nevertheless, given that both FA and SFT regulate the identity of the floral meristem, it is likely that these genes are targets that integrate signals from different promoting pathways. In accordance with this last hypothesis, it is known that, as in Arabidopsis, a gibberellin (GA)-dependent flowering pathway also exists in tomato, as proved by the non-flowering phenotype showed by GA-deficient mutants (Koornneef et al. 1990). Thus, the absence of flowering in the fa sft double mutants might indicate a function of FA and/or SFT in the integration of flowering signals, including those promoted by gibberellins, in a similar way to LFY in Arabidopsis (Blázquez et al. 1998).
The observation that FA expression is not affected by the sft mutation supports the conclusion that SFT and FA control the floral transition independently. In Arabidopsis, it has also been observed that the expression of LFY is not altered in the single late-flowering mutants ft or fwa, or in the double mutants lfy ft or lfy fwa (Ruiz-Garcia et al. 1997). Nevertheless, although these double mutants show alterations in flowering time and a lack of flower-like structures in the inflorescence, flower transition is never completely abolished. More recently, Reeves and Coupland (2001) have demonstrated that flower transition was completely prevented only in the co-2 fca-1 ga1-3 triple mutant, which indicates that the three pathways altered by these mutations, i.e. autonomous (represented by FCA), long-day (CO) and gibberellin-dependent (GA1), are required for flowering under long days. In contrast, inhibition of expression of a single gene in Petunia, PFG, also promoted the same non-flowering phenotype of either the tomato sft fa double mutant or Arabidopsis triple mutants (Immink et al. 1999). Taking all these results into account, the models proposed to explain gene interactions controlling flower transition in Arabidopsis would appear to be different in other species, despite the fact that the functional roles of individual genes may be similar.
SFT controls floral meristem identity and floral development of tomato
The phenotype of the sft inflorescence is characterised by the conversion of the floral meristem into a leaf-producing vegetative meristem in later stages of inflorescence development. Since in the tomato the inflorescence each new flower emerges in the base of the preceding flower, this conversion blocks the production of new flowers, reverting the inflorescence to a leaf-producing vegetative shoot. Inflorescence reversions have also been found in the jointless tomato mutant, besides its major effect on the abscission zone of the flower pedicel (Szymkowiak and Irish 1999). The JOINTLESS gene encodes a MADS-box transcription factor and maps on chromosome 11 (Mao et al. 2000), while the SFT locus is located on chromosome 3 (Kerr 1982), indicating that sft and jointless are not allelic. A leafy indeterminate inflorescence is also produced by mutations in the tomato AP1-like gene LeMADS-MC, but this also maps in a different chromosome with respect to SFT (Vrebalov et al. 2002). The production of flowers during early sft inflorescence development indicates that SFT is not absolutely necessary during this initial developmental program of the inflorescence, but its activity is required later to confer floral meristem identity, maintaining flowering and repressing vegetative development within the inflorescence of tomato. A similar function has been attributed to the MADS-box gene PFG of petunia (Immink et al. 1999), a species close related to tomato, since they both belong to the family Solanaceae. In fact, in strong homozygous pfg co-suppression mutants and the sft fa double mutant the floral transition is completely blocked, and the less-severe phenotype of the hemizygous pfg co-suppression plants resembles that of sft (Immink et al. 1999). The cloning of SFT will establish whether it is homologous to PFG and other MADS-box-related genes such as AP1 and FUL of Arabidopsis or SQUAMOSA of Antirrhinum.
The conversion of flowers into vegetative shoots during sft inflorescence development is not due to a down-regulation of FA, a key tomato gene for floral meristem identity (Molinero-Rosales et al. 1999), since we have detected that the sft mutation does not alter the level of FA mRNA. The initial flowers formed in sft inflorescences could therefore be attributed to the activity of FA, although in later inflorescence development this activity does not seem to be enough to maintain the identity of floral meristems. Although our data indicate that SFT does not regulate the transcription of FA, this does not exclude a positive regulation of SFT by FA in the establishment of floral meristem identity. In Arabidopsis, it is known that LFY is one of the first floral identity genes activated in the floral meristem, and that this activates the transcription of other genes involved in this same function, such as AP1 and CAL (Pidkowich et al. 1999). Similarly, FA could promote the floral initiation program by itself, or by the activation of other genes, which, like SFT, participate in the control of floral meristem identity during later stages of tomato inflorescence development. As fa sft double mutants do not initiate flowering, it is therefore impossible to prove the genetic interaction between the loci affected by these mutations. The activation of SFT by FA, however, may explain the complete absence of flowers in the fa inflorescence and the fact that a loss of FA function cannot be recovered by other identity genes such as SFT.
The flower developmental abnormalities observed in the sft mutant indicate that SFT is not only required for floral meristem identity but also for the identity and number of floral organs. In fact, we have observed that many of the sepals in sft flowers have leaf identity, and that the number of floral organs is reduced in comparison with WT flowers. Genes that control floral meristem identity and floral development, such as SFT, are also known in Arabidopsis. Thus, the development of leaves in the first whorl of the sft flowers resembles the phenotype of mutants in the floral identity gene AP1 of Arabidopsis, a MADS-box gene that controls not only the identity of the floral meristem but also the identity of the two outer whorls of the flower (Bowman et al. 1993). In the same way, the Arabidopsis FUL gene is required for carpel and fruit development but also functions as a flowering promoter in a pathway independent of LFY, and acts redundantly with AP1 and CAULIFLOWER (CAL) in the specification of floral meristem identity (Ferrándiz et al. 2000). The same dual function in the control of floral meristem identity and fruit development has also been recently attributed to the DEFH28 MADS-box gene of Antirrhinum (Müller et al. 2001).
SFT regulates the sympodial development of tomato
The sft mutation arrests the growth of the sympodial buds and allows the plant to grow from the ectopic vegetative meristems appearing in the inflorescence. These alterations to normal growth of the sympodial shoots may indicate that SFT is required for the normal development of the sympodial meristem. The growth pattern of the sympodial meristem in tomato is regulated by SP, the TFL1 homologous gene of tomato (Pnueli et al. 1998). In fact, the sp mutant has a determinate growth habit, progressively reducing the number of leaves in each sympodial segment of the plant until two consecutive terminal inflorescences are produced. Given that SP expression is reduced in the apical buds of the sft mutant during flowering, it is possible that the defect observed in the sympodial shoots is caused by a down-regulation of SP in the sympodial meristem. The observation that the flowering phenotype of sft is epistatic to that of sp can also support this conclusion, indicating that SFT could act upstream of SP in the same pathway that leads to the vegetative development of the sympodial shoots. Recently, it has been suggested that the SP protein functions by interacting with a variety of signalling proteins, including 14-3-3 proteins (Pnueli et al. 2001). Thus, it is also possible that the function of SFT controlling sympodial development depends on its interaction with SP. In this case, SFT could act as an upstream regulator of SP in the sympodial meristem.
The fact that SFT can act as both a flowering promoter and an activator of genes involved in the maintenance of the vegetative program, such as SP, may seem contradictory. In tomato, however, this is not so, as flowering in a given segment and vegetative development of the next sympodial segment occur simultaneously. In this sense, SFT may co-ordinate different signals in the apical buds of the plant, promoting flowering of the apical meristem as well as activating genes that maintain the vegetative program in the new emerging sympodial meristem.
Abbreviations
- CAP:
-
cleavage amplified polymorphic (marker)
- fa:
-
falsiflora
- RT:
-
reverse transcription
- sft:
-
single flower truss
- sp:
-
self pruning
- WT:
-
wild type
References
Araki T (2001) Transition from vegetative to reproductive phase. Curr Opin Plant Biol 4:63–68
Atherton JG, Harris GP (1986) Flowering. In: Atherton JG, Rudich J (eds) The tomato crop. Chapman and Hall, London, pp 167–200
Blázquez MA, Weigel D (2000) Integration of floral inductive signals in Arabidopsis. Nature 404:889–892
Blázquez MA, Green R, Nilsson O, Sussman MR, Weigel D (1998) Gibberellins promote flowering of Arabidopsis by activating the LEAFY promoter. Plant Cell 10:791–800
Bowman HL, Alvarez J, Weigel D, Meyerowitz EM, Smith DR (1993) Control of flower development in Arabidopsis thaliana by APETALA1 and interacting genes. Development 119:721–743
Carpenter R, Copsey L, Vicent C, Doyle S, Magrath R, Coen E (1995) Control of flower development and phyllotaxy by meristem identity genes in Antirrhinum. Plant Cell 7:2001–2011
Ferrándiz C, Gu Q, Martienssen R, Yanofsky MF (2000) Redundant regulation of meristem identity and plant architecture by FRUITFULL, APETALA1 and CAULIFLOWER. Development 127:725–734
Goméz P, Jamilena M, Capel J, Zurita S, Angosto T, Lozano R (1999) Stamenless, a tomato mutant with homeotic conversions in petal and stamens. Planta 209:172–179
Huijser P, Klein J, Lönning W-E, Meijer H, Saedler H, Sommer H (1992) Bracteomania, an inflorescence anomaly, is caused by the loss of function of the MADS-box gene squamosa in Antirrhinum majus. EMBO J 11:1239–1249
Immink RGH, Hannapel DJ, Ferrario S, Busscher M, Franken J, Lookeren Campagne MM, Angenent GC (1999) A Petunia MADS box gene involved in the transition from vegetative to reproductive development. Development 126:5117–5126
Kerr EA (1982) Single flower truss "sft" appears to be on chromosome 3. Rep Tomato Genet Coop 32:31
Koornneef M, Besma TDG, Hanhart CJ, van der Veen JH, Zeevaart JAD (1990) The isolation and characterization of gibberellin-deficient mutants in tomato. Theor Appl Genet 80:852–857
Koornneef MK, Alonso-Blanco C, Peeters AJM, Soppe W (1998) Genetic control of flowering time in Arabidopsis. Annu Rev Plant Physiol Plant Mol Biol 49:345–370
Levy YY, Dean C (1998) The transition to flowering. Plant Cell 10:1973–1989
Mao L, Begum D, Chuang H, Budiman MA, Szymkowiak EJ, Irish EE, Wing RA (2000) JOINTLESS is a MADS-box gene controlling tomato flower abscission zone development. Nature 406:910–913
Molinero-Rosales N, Jamilena M, Zurita S, Gómez P, Capel J, Lozano R (1999) FALSIFLORA, the tomato orthologue of FLORICAULA and LEAFY, controls flowering time and floral meristem identity. Plant J 20:685–693
Müller BM, Saedler H, Zachgo S (2001) The MADS-box gene DEF28 from Antirrhinum is involved in the regulation of floral meristem identity and fruit development. Plant J 28: 169–179
Pidkowich MS, Klenz JE, Haughn GW (1999) The making of a flower: control of floral meristem identity in Arabidopsis. Trends Plant Sci 4: 64–70
Pnueli L, Carmel-Goren L, Hareven D, Gutfinger T, Alvarez J, Ganal M, Zamir D, Lifschitz E (1998) The SELF-PRUNING gene of tomato regulates vegetative to reproductive switching of sympodial meristems and is the ortholog of CEN and TFL1. Development 125:1979–1989
Pnueli L, Gutfinger T, Hareven D, Ben-Naim O, Ron N, Adir N, Lifschitz E (2001) Tomato SP-interacting proteins define a conserved signaling system that regulates shoot architecture and flowering. Plant Cell 13:2678–2702
Reeves PH, Coupland G (2001) Analysis of flowering time control in Arabidopsis by comparison of double and triple mutants. Plant Physiol 126:1085–1091
Ruiz-García L, Madueño F, Wilkinson M, Haughn G, Salinas J, Martínez-Zapater J (1997) Different roles of flowering-time genes in the activation of floral initiation genes in Arabidopsis. Plant Cell 9:1921–1934
Sawhney VK, Greyson RI (1972) On the initiation of the inflorescence and floral organs in tomato (Lycopersicon esculentum). Can J Bot 50:1493–1495
Szymkowiak EJ, Irish EE (1999) Interactions between jointless and wild-type tomato tissues during development of the pedicel abscission zone and the inflorescence meristem. Plant Cell 11:159–175
Vrebalov J, Ruezinsky D, Padmanabhan V, White R, Medrano D, Drake R, Schuch W, Giovannoni J (2002) A MADS-box gene necessary for fruit ripening at the tomato Ripening-Inhibitor (Rin) locus. Science 296:343–346
Zegzouti H, Jones B, Frasse P, Marty C, Maitre B, Latché A, Pech JC, Bouzayen M (1999) Ethylene-regulated gene expression in tomato fruit: characterization of novel ethylene-responsive and ripening-related genes isolated by differential display. Plant J 18:589–600
Acknowledgements
We thank J.A. Jarillo and M.A. Blázquez for critical reading of the manuscript; Lola Martín, Francisco Lupiañez and Rafael Bueno for their technical assistance. We are grateful to the Tomato Genetic Resource Center (TGRC, California, USA) for providing the seed stocks used in this research, and Rijk Zwaan Ibérica for helpful growing of the F2 progenies. This work has been supported by grants from the Comisión Interministerial de Ciencia y Tecnología (AGF98-0206-C02-02 and BIO2001-2787).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Molinero-Rosales, N., Latorre, A., Jamilena, M. et al. SINGLE FLOWER TRUSS regulates the transition and maintenance of flowering in tomato. Planta 218, 427–434 (2004). https://doi.org/10.1007/s00425-003-1109-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-003-1109-1