Abstract
Aging-induced progressive decline of molecular and metabolic factors in the myocardium is suggested to be related with heart dysfunction and cardiovascular disease. Therefore, we evaluated the effects of exercise training and l-arginine supplementation on oxidative stress, inflammation, and apoptosis in ventricle of the aging rat heart. Twenty-four 24-month-aged Wistar rats were randomly divided into four groups: the aged control, aged exercise, aged l-arginine (orally administered with 150 mg/kg for 12 weeks), and aged exercise + l-arginine groups. Six 4-month-old rats were also considered the young control. Animals with training program performed exercise on a treadmill 5 days/week for 12 weeks. After 12 weeks, protein levels of Bax, Bcl-2, pro-caspase-3/cleaved caspase-3, cytochrome C, and heat shock protein (HSP)-70 were assessed. Tissue contents of total anti-oxidant capacity, superoxide dismutase, catalase, and levels of tumor necrosis factor alpha (TNF-α), interleukin (IL)-1β, and IL-6 were analyzed. Histological and fibrotic changes were also evaluated. Treadmill exercise and l-arginine supplementation significantly alleviated aging-induced apoptosis with enhancing HSP-70 expression, increasing anti-oxidant enzyme activity, and suppressing inflammatory markers in the cardiac myocytes. Potent attenuation in apoptosis, inflammation, and oxidative stress was indicated in the rats with the combination of l-arginine supplementation and exercise program in comparison with each group (p < 0.05). In addition, fibrosis percentage and collagen accumulation were significantly lower in the rats with the combination treatment of l-arginine and exercise (p < 0.05). Treadmill exercise and l-arginine supplementation provided protection against age-induced increase in the myocyte loss and formation of fibrosis in the ventricle through potent suppression of oxidative stress, inflammations, and apoptosis pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aging-induced progressive decline of molecular and metabolic factors in the myocardium is suggested to be related with heart dysfunction and cardiovascular disease. Biological aging is a regulated physiological process comprised of progressive alterations in the function of living cells, time-dependent loss of physiological integrity, senescence, and finally death. From molecular point of view, aging is characterized by several major features such as decrease in genome stability, which is caused by increased DNA damage and reduced DNA repair; significant alterations in the regulation of gene expression, alternative splicing, protein folding, and trafficking; telomere shortening; mitochondrial dysfunction and dysregulation in intercellular signaling pathways such as inflammation and nutrient sensors; and increased apoptosis rate [20]. With respect to cardiac aging, the number of heart tissue cells decreases dramatically, which may lead to a decrease in cardiac pacing [9]. Furthermore, elevated inflammatory response, increased oxidative stress, and high levels of apoptosis are suggested to be the most common features in cardiac aging.
Apoptosis is a well-regulated type of cell death with critical functions in the maintenance of cellular homeostasis and biological conditions, as well as some pathological events such as aging [38]. Chromatin condensation, cell shrinkage, and DNA fragmentation are the most characteristic features of cells undergoing apoptosis [38]. Accumulating studies have been reported a significant increase in the apoptotic rate of cardiomyocytes with aging because of the lack of myocyte substitutability [15, 25, 36, 37].
Vascular disorders in heart failure can result in disruption of nitric oxide (NO) production. Disturbance in NO production may be due to low levels of its precursor amino acid (l-arginine) or a decrease in the expression of the endothelial nitric oxide synthase (eNOS) enzyme [6]. Considering the important role of NO in endothelial vasodilation, regulation of vascular tone, and suppression of platelet aggregation and leukocyte adhesion, studies have been demonstrated the reduction of l-arginine levels in patients with cardiovascular diseases [35]. Therefore, several studies have examined the effect of l-arginine supplementation. An increase in blood circulation was observed in response to l-arginine supplementation, resulted in increased exercise tolerance and skeletal muscle mass [1]. In addition, l-arginine supplementation has been reported to improve anti-oxidant enzymes and inflammatory responses in the rat aorta [10]. l-Arginine alone or combined with exercise training was found to reduce tumor necrosis factor alpha (TNF-α), interleukin (IL)-1α, and IL-1β [10], which could potentially lower apoptosis and cellular aging.
Regular exercise plays a key role in preventing cardiovascular disease and mortality in adults and the elderly. Regular exercise improves aging-induced diminished cardiac capillary density, which it is thought to optimize energy cost of carrying oxygen in the heart [13]. Over the past decade, studies have shown that regular exercise reduces cell apoptosis. Kwak et al. showed a protective effect of 12 weeks of moderate-intensity aerobic training against apoptosis by decreasing levels of caspase-9, caspase-3, and Bax/Bcl-2 ratios in heart tissue of rats [16]. Peterson et al. found that 9 weeks of moderate-intensity aerobic activity can reduce Bax levels and decrease the activity of caspases and fragmentation of DNA in the heart tissue of obese rats [23]. Therefore, regarding the beneficial anti-oxidant and anti-inflammatory impacts of l-arginine and regular exercise training, we aimed to evaluate the effects of 12 weeks of treadmill exercise training and l-arginine supplementation on apoptosis, inflammation, and oxidative stress in the heart of aged rats.
Material methods
Animals
Twenty-four-month-old (n = 24) and 4-month-old (n = 6) male Wistar rats were provided from the Laboratory Animal House, Urmia University of Medical Sciences. The protocol was performed according to the guidelines for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication), and approved by the Animal Care and Use Committee at the Urmia University of Medical Sciences, Urmia, Iran (ethical code: IR.UMSU.REC.1396.434). All rats were housed under standard conditions (22 °C ± 2 °C, 50% humidity, and low noise) with 12/12-h light/dark cycles and had access to commercial chow and water ad libitum.
Experimental protocols
The aged rats were randomly divided into four groups (n = 6 in each group): the old-aged control, the old-aged exercise, the old-aged l-arginine, and the old-aged exercise + l-arginine groups. A young-aged control group was also considered. The exercise training program was performed on a treadmill with 0% grade for 12 weeks and 5 days in every week. For familiarization with exercise program, all rats ran on a rodent treadmill (Maze Router, Tabriz, Iran) for 1 week, 10 min/day, on a 0% grade in a room with a controlled temperature (23 ± 1 °C). The main protocol began 1 week after familiarization. The duration and intensity of the exercise were gradually increased, in which the duration and intensity of each session from 10 min and 17 m/min in the first week was increased to 60 min and 27 m/min in the sixth week and then remained steady until the end of the intervention [11]. All of the experiments took place at the same time in the morning. The remaining rats without exercise program were put on the treadmill similar with the exercise group but performed no training. They were exposed to similar handling and noise in an attempt to control for extraneous stresses. l-Arginine (Merck, Darmstadt, Germany) was mixed with saline (0.9% w/v NaCl) and orally administered with 150 mg/kg once a day for 5 days in a week during 12 weeks [11]. For the animals of the other groups, it was performed placebo gavage with the same volume of solution, however, containing only saline.
Tissue preparation
At the end of 27th month, the experimental animals were sacrificed 24 h after the last exercise program and treatment. They were anesthetized using 5% ketamine (100 mg/kg; Alfasan, Woerden, Netherlands) and 2% xylazine (40 mg/kg; Trittau, Germany) intraperitoneally, and the ventricle area in the cardiac muscle was removed. Tissues were fixed in 10% neutral-buffered formalin for 24 h and embedded in paraffin, then dehydrated in ascending concentrations of ethanol for 1 h, cleared in xylene for 1 h, and impregnated with paraffin wax for 4 h using a wax-embedding machine (Shandon Histocentre 2, UK). Transverse sections of 5-μm thickness were made by a paraffin microtome, mounted on coated slides, and then dried at 37 °C on a slide dryer. All stages of tissue processing and staining were performed by a technician blinded to the treatments.
Immunohistochemistry for cytochrome C
Following cutting and drying, the ventricle cross sections were deparaffinized in xylene and rehydrated using descending concentrations of ethanol; then, the antigen retrieval process was performed in 10 mM sodium citrate buffer (pH 7.2). IHC staining was conducted according to the manufacturer’s protocol detection IHC kit rabbit-specific HRP/DAB (Abcam, UK). Briefly, endogenous peroxidase was blocked in a peroxidase blocking solution (0.03% hydrogen peroxide) for 10 min. Tissue sections were then washed gently with phosphate-buffered saline (PBS, pH 7.4). Following this step, we applied the cytochrome C–biotinylated primary antibody (Santa Cruz, UK, 1:300) overnight at 4 °C. Subsequently, after a washing step with PBS, the biotinylated goat anti-rabbit secondary antibody was applied on the slides for 10 min. After that, we added a DAB chromogen to the tissue sections and incubated them for 10 min. The slides were counter-stained with hematoxylin for 15 s and dipping in ammonia (0.037 ml) 10 times and rinsing with distilled water. In continue, the sections were cover-slipped. Then, tissue sections were examined using a light microscope and photomicrographs. They were captured and analyzed using the ImageJ software. In brief, the positive DAB-stained area as the positive area was automatically separated from hematoxylin which represents the total area, in each digital photomicrograph. By using color deconvolution plug-in, images were processed into a binary color image (black and white). The percentage of positively stained area (represented by the black color) was determined. Immunoreactivity for cytochrome C was evaluated in ten consecutive sections representative to the whole tissue section in each.
Masson’s trichrome stain for collagen and myocytes
Masson’s trichrome technique (Pajohesh Asia, Iran) was used for evaluation of collagen fibers in heart muscle. The staining was conducted based on the manufacturer’s protocol. This technique was carried out from cardiomyocytes stain a bright red, collagen fibers blue, and nuclei black. Briefly, ventricle cross sections were deparaffinized and rehydrated through decreasing concentrations of ethanol and then washed in distilled water. In continuation, we re-fixed in Bouin’s solution for 1 h at 56 °C and rinsed in running tap water for 5–10 min to remove the yellow color. Following this step, tissues were stained with Weigert’s iron hematoxylin working solution for 10 min and washed again. Afterward, Biebrich scarlet–acid fuchsin solution was used for 15 min. The sections were differentiated in phosphomolybdic-phosphotungstic acid solution for 15 min after another washing step. For staining the sections, they were incubated in aniline blue solution for 10 min, then rinsed in distilled water and differentiated in 1% acetic acid solution for 3 min. Ethanol 95% and xylene were used for quick dehydrating and clearing, respectively. Images were captured on a microscope (Olympus, Germany, CH-02) and quantified using the ImageJ analysis program. Serial sections in 6 rats/group were analyzed for percentage connective tissue area.
Western blotting
One hundred milligrams of tissues was harvested from the ventricle and washed with PBS. The samples were homogenized in RIPA cold lysis buffer, containing protease and phosphatase inhibitors. Protein concentration was assessed by the Bradford method with bovine serum albumin (BSA) as standard (Bio-Rad, USA). Fifty micrograms of protein was separated on 10% SDS-PAGE gel and transferred onto polyvinylidene difluoride (PVDF) membrane [41]. After blocking with 5% skim milk in TBST (0.1% Tris-buffered saline and Tween 20) at room temperature for 1.5 h, the membrane was washed with TBST and incubated with primary antibody followed by secondary antibody. Protein bands were detected using the enhanced chemiluminescence system (ECL) Western Blotting Kit (Amersham Pharmacia Biotech, USA). Antibodies for Bax, Bcl2, pro-caspase-3 (Cas-3), cleaved Cas-3, and β-actin were obtained from Santa Cruz (UK).
Measurements of TNF-α, IL-1β, and IL-6
Rat-specific commercially available enzyme-linked immunosorbent assay (ELISA) kits were used to quantify the serum levels of TNF-α (Elabscience, Cat: E-EL-R0019), IL-1β (Elabscience, Cat: E-EL-R0012), and IL-6 (Elabscience, Cat: E-EL-R0015) proteins. All measurements were performed according to the manufacturer’s instructions.
Measurements of total anti-oxidant capacity, superoxide dismutase, and catalase
Total anti-oxidant capacity (TAC) and enzyme activities were assessed over the supernatant part of 0.5% tissue homogenates. TAC levels were measured by the FRAP method using the TAC assay kit (Elabscience, CAT: E-BC-K225) according to the manufacturer’s instruction. Fe3+-TPTZ was reduced by anti-oxidants and produced blue Fe2+-TPTZ under acid condition. The anti-oxidant capacity of samples was calculated by detecting the absorbance value at 593 nm and stated as millimoles per liter. Total SOD activities were also measured by the hydroxylamine method using the SOD assay kit (Elabscience, CAT: E-BC-K019). The superoxide anion was produced by the xanthine and xanthine oxidase reaction systems. The oxidized hydroxylamine formed nitrite, which turned to purple under the reaction developer. When samples contain SOD, the SOD specifically inhibited free radical. The inhibitory effect of SOD reduced the formation of nitrite, so the absorbance values of samples were lower. The absorbance values of samples were measured at 550 nm and stated as units per milligram protein. The catalase assay kit (Elabscience, CAT: E-BC-K031) was used to evaluate the catalase activities in tissue homogenate of all groups. The reaction that catalase decomposes H2O2 can be quickly stopped by ammonium molybdate. The residual H2O2 reacts with ammonium molybdate to generate a yellowish complex. Catalase activities were calculated by production of the yellowish complex at 405 nm and stated as units per milligram protein.
Statistical analysis
SPSS for windows (version 20, SPSS Inc., Chicago, IL, USA) was used to analyze data. The values were reported as mean ± SD. The Kolmogorov-Smirnov and Levene tests were used to investigate the normality of the data and homogeneity of variances in the groups. Given the existence of parametric conditions, one-way ANOVA post hoc (Tukey and Dunnett) tests were used to compare the mean between the study groups. The statistical significance level was considered less than 0.05.
Results
Effect of l-arginine supplementation and exercise on the expression levels of apoptotic genes in the aging cardiac muscle
The expression levels of Bcl-2, Bax, and pro-Cas-3/cleaved Cas-3 were determined by western blotting (Fig. 1). The levels of Bcl-2, Bax, and Cas-3 were set as 1.00 in the young control. The expression levels of Bax were 1.74 ± 0.04, 1.12 ± 0.02, 1.20 ± 0.04, and 0.88 ± 0.03 in the old-aged control, old-aged l-arginine, old-aged exercise, and old-aged l-arginine + exercise groups, respectively (Fig. 1b). Aging resulted in the significant increase in the expression levels of Bax as compared with that in the young-aged group (p < 0.001). Accordingly, the expression levels of Bax were significantly suppressed in rats with l-arginine supplementation and exercise program (p < 0.001). Not surprisingly, co-administration of l-arginine and exercise training program resulted in more potent effects on the expression levels of Bax. In this case, the Bcl-2 levels were 0.87 ± 0.02 in the old-aged group, 0.99 ± 0.03 in the old-aged l-arginine group, 0.95 ± 0.02 in the old-aged exercise group, and 1.25 ± 0.05 in the old-aged l-arginine + exercise group (Fig. 1c). The expression levels of Bcl-2 were significantly lower than those of the old-aged rats in comparison with the young-aged group (p < 0.001). Therefore, l-arginine and exercise administration alone resulted in the increased expression of Bcl-2 in the old-aged rats (p < 0.001). This effect was more potent when the old-aged rats were treated with combination of l-arginine and exercise program (p < 0.001). In addition, the evaluation of pro-Cas-3 and its cleaved form as an important trigger of apoptosis showed that the expression levels of cleaved Cas-3 were significantly higher in the old-aged rats in comparison with those in the young-aged group (p < 0.001). The mean levels of cleaved Cas-3 expression were 1.76 ± 0.04, 1.9 ± 0.05, and 0.99 ± 0.02 in the l-arginine, exercise, and l-arginine+ exercise groups, respectively. Also, the cleaved Cas-3 expression was remarkably lower than pro-Cas-3 in the l-arginine and exercise groups, as compared with that in the old-aged control (p < 0.001; Fig. 1d). Most importantly, the simultaneous administration of l-arginine and exercise program resulted in the potent suppression in activation of pro-Cas-3 to cleaved Cas-3 in comparison with the l-arginine or exercise alone groups (p < 0.001). Furthermore, in the calculation of Bax/Bcl-2 ratio, we found that the results of l-arginine and exercise showed the significant decrease for this ratio in the old-aged rats (p < 0.001; Fig. 1e).
l-Arginine supplementation and exercise training change in Bax, Bcl-2, pro-Cas-3, and cleaved Cas-3 levels after 12 weeks of intervention. a Western blotting of Bax, Bcl-2, pro-Cas-3, and cleaved Cas-3 protein levels. β-Actin was used as the loading control. b–d Quantitative analysis of Bax, Bcl-2, and cleaved Cas-3 levels. e Quantitative analysis of Bax/Bcl-2 ratio. n = 6 in each group. *p < 0.05 compared with the young control; **p < 0.05 compared with the aged control; ***p < 0.05 compared with the l-arginine and exercise group. Cas-3, caspase-3
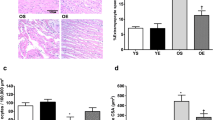
The expression levels of cytochrome C was also evaluated by IHC (Fig. 2). In this study, paraffin-embedded cardiac tissue sections were used to detect IHC staining of cytochrome C. We found a significant difference in immunostaining for cytochrome C between the groups. The old-aged group showed greater immunoreactivity for cytochrome C compared with the young-aged control group (p < 0.05). Additionally, combination of l-arginine and exercise program resulted in a significant decrease in immunoreactivity for cytochrome C in comparison with each group alone (p < 0.05). Cytochrome C releasing from the mitochondria results in the activation of Cas-3 and hence induction of apoptosis. Therefore, cytochrome C is a marker of apoptosis and cardiac damage. In other words, we showed that cytochrome C levels and hence cardiac damage were significantly decreased in the l-arginine + exercise program group.
l-Arginine supplementation and exercise training change in cytochrome C–positive staining after 12 weeks of intervention. a Immunohistochemical staining of cardiac tissue with cytochrome C (screen magnification = × 400). b Quantitative analysis of cytochrome C. n = 6 in each group. *p < 0.05 compared with the young control; **p < 0.05 compared with the aged control; ***p < 0.05 compared with the l-arginine group
Effect of l-arginine supplementation and exercise on the expression levels of HSP-70 in the aging cardiac muscle
In the next part of study, we measured the expression levels of HSP-70, as a stress-induced protein (Fig. 3). When the level of HSP-70 in the young-aged group was set at 1.00, the level of HSP-70 was 0.67 ± 0.02 in the old-aged group, 1.64 ± 0.02 in the old-aged l-arginine group, 1.74 ± 0.05 in the old-aged exercise group, and 1.87 ± 0.06 in the old-aged l-arginine + exercise group. Hence, the expression levels of HSP-70 were significantly lower in the old-aged rats compared with those in the young-aged groups (p < 0.001). Treadmill exercise and l-arginine enhanced the expression of HSP-70 in the old-aged rats (p < 0.001). l-Arginine and exercise program, in combination, exerted more potent increase in the expression levels of HSP-70 (p < 0.001).
l-Arginine supplementation and exercise training change in HSP-70 levels after 12 weeks of intervention. a Western blotting of HSP-70 protein levels. β-Actin was used as the loading control. b Quantitative analysis of HSP-70 levels. n = 6 in each group. *p < 0.05 compared with the young control; **p < 0.05 compared with the aged control; ***p < 0.05 compared with the l-arginine and exercise group. HSP-70, heat shock protein-70
Effect of l-arginine supplementation and exercise on oxidant status in the aging cardiac muscle
Figure 4 showed the effects of l-arginine, exercise training program, and their combination of the TAC content and SOD and catalase activities. Our results showed a decrease in the levels of TAC and enzymatic activities of SOD and catalase in the old-aged groups in comparison with those in the young-aged groups (p < 0.05). Moreover, we found an increase in the TAC content in the l-arginine and exercise groups compare with that in the old-aged rats. However, the differences were not statistically significant (1.72 ± 0.72 vs. 1.59 ± 0.12 and 1.87 ± 0.25 vs. 1.59 ± 0.12 mmol/L). The mean activity of SOD in the l-arginine, exercise, and l-arginine + exercise groups was 108.77 ± 9.54, 112.31 ± 12.74, and 162.15 ± 14.01 U/mg protein, respectively. In addition, the mean activity of catalase was 5.49 ± 0.83, 5.20 ± 0.61, and 6.86 ± 0.75 U/mg protein in the l-arginine, exercise, and l-arginine + exercise groups, respectively. Statistical analysis revealed more significant effect for the combination of l-arginine and exercise program in comparison with l-arginine and exercise groups alone (p < 0.05) for SOD and catalase activities.
l-Arginine supplementation and exercise training affect tissue levels of oxidative markers after 12 weeks of intervention. a Total anti-oxidant capacity (TAC), b superoxide dismutase (SOD), and c catalase. n = 6 in each group. *p < 0.05 compared with the young control; **p < 0.05 compared with the aged control; ***p < 0.05 compared with the l-arginine and exercise group
Effect of l-arginine supplementation and exercise on the pro-inflammatory cytokines in the aging cardiac muscle
To gain insight into the inflammatory effects of our interventions, we performed ELISAs to measure the levels of inflammatory mediators TNF-α, IL-1β, and IL-6 (Fig. 5). l-Arginine supplementation and exercise program resulted in the suppression of inflammatory responses, which were higher in the old-aged rats in comparison with those in the young-aged group and indicated the marked reduction in the levels of TNF-α, IL-1β, and IL-6 in the heart muscle of rats rather than in the old-aged rats. Moreover, the expression levels of all three inflammatory markers were more potently reduced when rats were treated with the combination of l-arginine and exercise program in comparison with each intervention alone (p < 0.05).
l-Arginine supplementation and exercise training affect tissue levels of inflammatory markers after 12 weeks of intervention. a Interleukin-6 (IL-6), b interleukin-1β (IL-1β), and c tumor necrosis factor alpha (TNF-α). n = 6 in each group. *p < 0.05 compared with the young control; **p < 0.05 compared with the aged control; l-arginine supplementation and exercise training; ***p < 0.05 compared with the l-arginine and exercise group
Effect of l-arginine supplementation and exercise on connective tissue and collagen in the aging cardiac muscle
Initially Masson’s trichrome staining was used to visualize fibrillar collagen that might be located in the extramyocyte space. Our analysis showed an enhanced fibrosis in the heart of aged rats in comparison with that in the heart of young group (Fig. 6a). The positive staining for total collagen (Blue) was greater in ventricle samples from the aged rats compared with young rats. Both exercise training and treatment with l-arginine reduced myocardial fibrosis and age-related elevation in collagen-positive staining (Fig. 6). Clear protection against age-related alterations in collagen fiber network was observed, and a more potent effect was determined in group treated with the combination of exercise program and l-arginine.
l-Arginine supplementation and exercise training affect myocardial fibrosis after 12 weeks of intervention. a Paraffin-embedded heart sections were Masson-trichrome-stained for evaluation of fibrosis. b Graphs showed the fibrosis area. n = 6 in each group. *p < 0.05 compared with the young control; **p < 0.05 compared with the aged control; ***p < 0.05 compared with the l-arginine and exercise group
Discussion
Progressive loss of cardiac myocytes associated with aging is due to programmed cell death or apoptosis, enhanced inflammatory cytokines, and increased oxidative stress, hence impaired cardiac function [34]. In the present study, we evaluated the effects of l-arginine supplementation and exercise program, alone or in combination, on apoptosis, inflammation, and oxidative stress of cardiac muscle of the old-aged rats. We found that administration of l-arginine combined with exercise exerted potent impacts on the reduction of apoptosis, significant induction in the expression of HSP-70, reduction in inflammatory response, enhancement of myocardial anti-oxidant defense system, and reversing of cardiac damage. Increased level of apoptosis is demonstrated to be a common event in aging process [15, 22]. Subsequently, aging also results in the dysregulation of key component of apoptosis including Bcl-2 family and caspase signaling [24]. In spite of numerous efforts to reveal the underlying mechanisms of apoptotic signaling in aging, the exact signaling is still unclear. Since the heart is a post-mitotic tissue and myocytes lost cannot be compensated, aging-induced progressive apoptosis is a deleterious condition in the heart [19]. An accumulating body of recent studies has evaluated the effects of exercise training on the various aspects of aging such as apoptosis rate, oxidative stress, inflammatory response, and potent signaling pathways and reported promising findings [3, 7, 29]. Kwak et al. [16] showed that exercise training significantly protected against the loss of cardiac myocytes and enhanced connective tissue in ventricle of the aging rat heart. Exercise training also significantly reduced apoptosis rate in the ventricle, through reducing caspase-9 levels and Bax/Bcl-2 ratio by lowering Bax protein expression and increasing Bcl-2 levels. Ko et al. [12] showed that treadmill exercise alleviated aging-induced apoptosis by lowering Bax/Bcl-2 ratio. In a study by Zhao et al. [42], a short-duration swimming exercise significantly augmented ventricular function, increased survival rate, and suppressed myocardial apoptosis via regulating the decreasing activity of caspase-3. In accordance with aforementioned studies, we showed that exercise training program resulted in the significant attenuation of apoptosis in aged rats, which was evident from reducing the expression levels of Bax and enhancing Bcl-2 levels, hence lowering Bax/Bcl-2 ratio, as well as suppressing the expression levels of cleaved Cas-3.
Another major consequence of aging is increased oxidative stress and chronic inflammation, which are major causes of tissue damages and hence induction of apoptosis, particularly in heart tissues, which works continuously and is more susceptible to oxidative stress and inflammation-induced damages [32]. Increased oxidative stress is demonstrated to induce the expression of stress sensors such as HSPs particularly HSP-70, an activating factor of cell protection signaling pathways [26]. HSP-70 is also involved in the suppression of apoptosis, hence promotion of heart survival [26, 43].
Various studies reported the beneficial effects of exercise training on the suppression of the inflammatory response, boosting anti-oxidant defense system and decreasing the production of ROS [2, 4, 27, 30, 40]. Lisa et al. reported that a 14-week aerobic exercise program resulted in the significant suppression of arterial inflammation in aged mice, as evident from the significant decrease in the expression levels of IL-1 and IL-6, IFN-γ, and TNF-α [17]. Siu et al. [31] showed that after 8 weeks of training resulted in the increased expression levels of SOD and suppression of apoptosis in the ventricle of aged rats. Soufi et al. [33] found that regular exercise modulated age-induced changes in SOD and HSP-70. Old-aged rats performing exercise program showed increased expression levels of HSP-70 and SOD activities. Through these changes, exercise effectively suppressed apoptosis in the rat myocardium. In a study by Rinaldi et al. [28], it was reported exercise-mediated protection against heart failure linked to aging is related to higher expression levels of the HSP-70, and HSP-70-induced suppression of apoptosis. Another study showed that HSP-70 is involved in the Fas-mediated apoptosis to protect the cardiomyocyte from stress-induced injury [21]. The results of this study were in agreement with the abovementioned studies. We found that exercise attenuated aging-induced decreases in the TAC levels and SOD and catalase activities as major enzymatic anti-oxidants, and decreases in the expression levels of IL-1β, IL-6, and TNF-α, as well as reduced expression levels of HSP-70. Therefore, increasing oxidative stress and inflammatory response and decreasing levels of HSP-70 during aging may be important factors in increasing apoptosis and tissue damage of the heart. Exercise could induce increase in anti-oxidant capacity of cardiomyocytes through upregulation of major anti-oxidant enzymes such as SOD and catalase, as well as overexpression of HSP-70. Additionally, exercise could attenuate aging-induced chronic inflammation in cardiomyocytes, hence may effectively suppress apoptosis and protect cardiomyocytes against aging-induced tissue damages, as evident from the large reduction in the collagen accumulation in ventricle and fibrosis.
l-Arginine is the biological precursor of nitric oxide (NO), which plays a key role in the cardiovascular system [35]. A grown body of studies has focused on the anti-oxidative, anti-inflammatory, and anti-apoptotic functions associated with aging. For example, in rats with chronic renal failure, l-arginine supplementation improved kidney functions, decreased systolic blood pressure, and decreased inflammatory cytokine levels including IL-1α, IL1-β, IL-6, and TNF-α [10, 14]. The enzymatic and non-enzymatic anti-oxidant parameters like SOD, catalase, and ascorbic acid along with pro-oxidant parameters, such as xanthine oxidase, as well as index of oxidative stress as protein carbonyl content and malondialdehyde were modulated by l-arginine [18, 39]. Anti-apoptotic function of l-arginine is reported to be related to inhibitory effects on the expression of the major apoptotic genes including Bcl-2 family, caspase family, and cytochrome C [5, 8]. To the best of our knowledge, the beneficial effects of l-arginine in aging through inhibition of the apoptosis, inflammations, and oxidative stress have not been studied. We showed that l-arginine supplementation resulted in the significant increase in the TAC levels, SOD and catalase activities, and HSP-70 expression, hence boosting anti-oxidative status of the myocardium. In line with this, the expression levels of inflammatory markers including IL-1β, IL-6, and TNF-α were also suppressed. In response to l-arginine supplementation, we observed that apoptosis was inhibited by decreasing the ratio of Bax to Bcl-2, and the decreased Bax/Bcl-2 ratio inhibited cytochrome C release and caspase-3 activation and consequently decreased apoptosis. Therefore, l-arginine and exercise program modulated the enzymatic activities of anti-oxidants, the expression levels of inflammatory markers, and apoptosis key proteins in the aged-heart tissue. It was also found that exercise training combined with l-arginine supplementation induced more pronounced modulatory effects in aged rats (p < 0.05).
Conclusion
Twelve weeks of exercise training combined with l-arginine supplementation provided protection against age-induced increase in the myocyte loss and formation of fibrosis in the ventricle through potent suppression of oxidative stress, inflammations, and apoptosis pathways. We concluded that exercise and l-arginine supplementation improved specific cellular signaling mechanisms involved in cardiac aging.
References
Barcelos GT, Rossato DD, Perini JL, Pinheiro LP, Carvalho C, Jaenisch RB, Rhoden CR, Lago PD, Nunes RB (2017) Effects of L-arginine supplementation associated with continuous or interval aerobic training on chronic heart failure rats. Metabolism 76:1–10
Belaya I, Suwa M, Chen T, Giniatullin R, Kanninen KM, Atalay M, Kumagai S (2018) Long-term exercise protects against cellular stresses in aged mice. Oxidative Med Cell Longev 2018(2894247):1–10
Bergland A, Fougner M, Lund A, Debesay J (2018) Ageing and exercise: building body capital in old age. Eur Rev Ageing Phys Act 15:7
Bouzid MA, Filaire E, Matran R, Robin S, Fabre C (2018) Lifelong voluntary exercise modulates age-related changes in oxidative stress. Int J Sports Med 39:21–28
Chattopadhyay P, Shukla G, Wahi AK (2009) Protective effect of L-arginine against necrosis and apoptosis induced by experimental ischemic and reperfusion in rat liver. Saudi J Gastroenterol 15:156–162
Feletou M, Köhler R, Vanhoutte PM (2012) Nitric oxide: orchestrator of endothelium-dependent responses. Ann Med 44:694–716
Finkel T, Holbrook NJ (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408:239–247
Greene JM, Feugang JM, Pfeiffer KE, Stokes JV, Bowers SD, Ryan PL (2013) L-arginine enhances cell proliferation and reduces apoptosis in human endometrial RL95-2 cells. Reprod Biol Endocrinol 11:15
Gude NA, Broughton KM, Firouzi F, Sussman MA (2018) Cardiac ageing: extrinsic and intrinsic factors in cellular renewal and senescence. Nat Rev Cardiol 15:523–542
H-j K, Son J, Jin E, Lee J, Park S (2016) Effects of exercise and L-arginine intake on inflammation in aorta of high-fat diet induced obese rats. J Exerc Nutr Biochem 20:36
Kim H-J, Son J, Jin E, Lee J, Park S, Joen J, biochemistry (2016) Effects of exercise and L-arginine intake on inflammation in aorta of high-fat diet induced obese rats. J Exerc Nutr Biochem 20:36
Ko I-G, Kim S-E, Kim C-J, Jee Y-S (2013) Treadmill exercise alleviates ageing-induced apoptosis in rat cardiac myocytes. Int J Gerontol 7:152–157
Ko I-G, Kim S-E, Kim C-J, Jee Y-SJIJG (2013) Treadmill exercise alleviates ageing-induced apoptosis in rat cardiac myocytes. Int J Gerontol 7:152–157
Korish AA (2010) Multiple antioxidants and L-arginine modulate inflammation and dyslipidemia in chronic renal failure rats. Ren Fail 32:203–213
Kwak H-B (2013) Effects of ageing and exercise training on apoptosis in the heart. J Exerc Rehabil 9:212–219
Kwak H-B, Song W, Lawler JM (2006) Exercise training attenuates age-induced elevation in Bax/Bcl-2 ratio, apoptosis, and remodeling in the rat heart. FASEB J 20:791–793
Lesniewski LA, Durrant JR, Connell ML, Henson GD, Black AD, Donato AJ, Seals DR (2011) Aerobic exercise reverses arterial inflammation with ageing in mice. Am J Phys Heart Circ Phys 301:H1025–H1H32
Liang M, Wang Z, Li H, Cai L, Pan J, He H, Wu Q, Tang Y, Ma J, Yang L (2018) l-Arginine induces antioxidant response to prevent oxidative stress via stimulation of glutathione synthesis and activation of Nrf2 pathway. Food Chem Toxicol 115:315–328
Liao P-H, Hsieh DJ-Y, Kuo C-H, Day C-H, Shen C-Y, Lai C-H et al (2015) Moderate exercise training attenuates ageing-induced cardiac inflammation, hypertrophy and fibrosis injuries of rat hearts. Oncotarget 6:35383
Majidinia M, Reiter RJ, Shakouri SK, Yousefi B (2018) The role of melatonin, a multitasking molecule, in retarding the processes of ageing. Ageing Res Rev 47:198–213
Moran M, Delgado J, Gonzalez B, Manso R, Megias A (2004) Responses of rat myocardial antioxidant defences and heat shock protein HSP72 induced by 12 and 24-week treadmill training. Acta Physiol Scand 180:157–166
Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD (2005) Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309:481–484
Peterson JM, Bryner RW, Sindler A, Frisbee JC, SEJJoap A (2008) Mitochondrial apoptotic signaling is elevated in cardiac but not skeletal muscle in the obese Zucker rat and is reduced with aerobic exercise. J Appl Physiol 105:1934–1943
Phaneuf S, Leeuwenburgh C (2002) Cytochrome c release from mitochondria in the ageing heart: a possible mechanism for apoptosis with age. Am J Phys Regul Integr Comp Phys 282:R423–RR30
Pollack M, Phaneuf S, Dirks A, Leeuwenburgh C (2002) The role of apoptosis in the normal ageing brain, skeletal muscle, and heart. Ann N Y Acad Sci 959:93–107
Powers SK, Quindry JC, Kavazis AN (2008) Exercise-induced cardioprotection against myocardial ischemia–reperfusion injury. Free Radic Biol Med 44:193–201
Bloomer RJ. (2008) Effect of exercise on oxidative stress biomarkers. Advances in clinical chemistry. 1;46:1-50.
Rinaldi B, Corbi G, Boccuti S, Filippelli W, Rengo G, Leosco D, Rossi F, Filippelli A, Ferrara N (2006) Exercise training affects age-induced changes in SOD and heat shock protein expression in rat heart. Exp Gerontol 41:764–770
Ross MD, Malone E, Florida-James G (2016) Vascular ageing and exercise: focus on cellular reparative processes. Oxidative Med Cell Longev 2016:1–15
Simioni C, Zauli G, Martelli AM, Vitale M, Sacchetti G, Gonelli A et al (2018) Oxidative stress: role of physical exercise and antioxidant nutraceuticals in adulthood and ageing. Oncotarget 9:17181
Siu PM, Bryner RW, Martyn JK, Alway SE (2004) Apoptotic adaptations from exercise training in skeletal and cardiac muscles. FASEB J 18:1150–1152
Snoeckx LH, Cornelussen RN, Van Nieuwenhoven FA, Reneman RS, Van der Vusse GJ (2001) Heat shock proteins and cardiovascular pathophysiology. Physiol Rev 81:1461–1497
Sonfi FG, Farajnia S, Aslanabadi N, Ahmadiasl N, Alipour M, Alipour M et al (2008) Long-term exercise training affects age-induced changes in HSP70 and apoptosis in rat heart. Gen Physiol Biophys 27:263
Steenman M, Lande G (2017) Cardiac ageing and heart disease in humans. Biophys Rev 9:131–137
Sudar-Milovanovic E, Obradovic M, Jovanovic A, Zaric B, Zafirovic S, Panic A, Radak D, Isenovic ER (2016) Benefits of L-arginine on cardiovascular system. Mini-Rev Med Chem 16:94–103
Tarhriz V, Eyvazi S, Musavi M, Abasi M, Sharifi K, Ghanbarian H et al Transient induction of Cdk9 in the early stage of differentiation is critical for myogenesis. J Cell Biochem 2019 120(11):18854–18861
Tarhriz V, Wagner KD, Masoumi Z, Molavi O, Hejazi MS, Ghanbarian H (2018) CDK9 regulates apoptosis of myoblast cells by modulation of microRNA-1 expression. J Cell Biochem 119:547–554
Thukral H, Soin S, Rani R, Sarkar A (2016) Molecular and cellular parameters of ageing: an overview. Al Ameen J Med Sci 9(1):4–14
Tripathi P, Pandey S (2013) L-arginine attenuates oxidative stress condition during cardiomyopathy. Indian J Biochem Biophys 50(2):99–104
Woods JA, Wilund KR, Martin SA, Kistler BM (2012) Exercise, inflammation and ageing. Ageing Dis 3:130
Yazdani P, Mansouri E, Eyvazi S, Yousefi V, Kahroba H, Hejazi MS, Mesbahi A, Tarhriz V, Abolghasemi MM (2019) Layered double hydroxide nanoparticles as an appealing nanoparticle in gene/plasmid and drug delivery system in C2C12 myoblast cells. Artif Cells Nanomed Biotechnol 47:436–442
Zhao D, Sun Y, Tan Y, Zhang Z, Hou Z, Gao C, Feng P, Zhang X, Yi W, Gao F (2018) Short-duration swimming exercise after myocardial infarction attenuates cardiac dysfunction and regulates mitochondrial quality control in aged mice. Oxidative Med Cell Longev 2018(4079041):1–16
Zhao Y, Wang W, Qian L (2007) Hsp70 may protect cardiomyocytes from stress-induced injury by inhibiting Fas-mediated apoptosis. Cell Stress Chaperones 12:83–95
Funding
This study was supported by the Research Council of Urmia University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to this work.
Corresponding author
Ethics declarations
The protocol was performed according to the guidelines for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication), and approved by the Animal Care and Use Committee at the Urmia University of Medical Sciences, Urmia, Iran (ethical code: IR.UMSU.REC.1396.434).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the special issue on Exercise Physiology: future opportunities and challenges in Pflügers Archiv—European Journal of Physiology
Rights and permissions
About this article
Cite this article
Darband, S.G., Sadighparvar, S., Yousefi, B. et al. Combination of exercise training and l-arginine reverses aging process through suppression of oxidative stress, inflammation, and apoptosis in the rat heart. Pflugers Arch - Eur J Physiol 472, 169–178 (2020). https://doi.org/10.1007/s00424-019-02311-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-019-02311-1