Abstract
Arachidonic acid (AA) is a polyunsaturated fatty acid involved in a complex network of cell signaling. It is well known that this fatty acid can directly modulate several cellular target structures, among them, ion channels. We explored the effects of AA on high conductance Ca2+- and voltage-dependent K+ channel (BKCa) in vascular smooth muscle cells (VSMCs) where the presence of β1-subunit was functionally demonstrated by lithocholic acid activation. Using patch-clamp technique, we show at the single channel level that 10 μM AA increases the open probability (Po) of BKCa channels tenfold, mainly by a reduction of closed dwell times. AA also induces a left-shift in Po versus voltage curves without modifying their steepness. Furthermore, AA accelerates the kinetics of the voltage channel activation by a fourfold reduction in latencies to first channel opening. When AA was tested on BKCa channel expressed in HEK cells with or without the β1-subunit, activation only occurs in presence of the modulatory subunit. These results contribute to highlight the molecular mechanism of AA-dependent BKCa activation. We conclude that AA itself selectively activates the β1-associated BKCa channel, destabilizing its closed state probably by interacting with the β1-subunit, without modifying the channel voltage sensitivity. Since BKCa channels physiologically contribute to regulation of VSMCs contractility and blood pressure, we used the whole-cell configuration to show that AA is able to activate these channels, inducing significant cell hyperpolarization that can lead to VSMCs relaxation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arachidonic Acid (AA), a physiologically relevant 20-carbon omega-6 polyunsaturated fatty acid (PUFA), is an integral component of cell membrane phospholipids, which can be obtained from diet nutrients or synthesized in the liver. In humans, plasma concentration can normally be found in a range of 2.5–13.1 μM [8, 41] and could be significantly increased in pathological conditions, such as ischemia [1, 22] and preeclampsia [41]. AA can be released from phospholipids by activation of phospholipase A2, and it can then be transformed through different enzymatic pathways mediated by ciclooxigenase, lipooxigenase and cytochrome P450, into a great variety of metabolites (prostacyclins, leucotrienes and epoxyeicosatrienoic acids, respectively) involved in a complex network of cell signaling. These have been implicated in different physiological processes, such as endothelium-dependent blood vessel relaxation, platelet aggregation, stomach mucus and acid secretion, and smooth muscle tone regulation, among others. Moreover, the role of AA metabolites as pro-inflammatory factors has also been demonstrated in several pathological states [9, 13, 36, 38, 42].
Although AA was mainly thought only as a precursor of active metabolites, it is now well-known that AA and other fatty acids (FA) can directly modulate different particular protein structures. Among them, different ion channels (i.e., KV, Na+ V, Ca2+ V, K2P, and BKCa) can be inhibited or activated by AA [4, 12, 15, 29, 34] (reviewed by Meves (2008) [32]). In particular, the action of PUFAs involving the high conductance Ca2+- and voltage-dependent (BKCa) channel as the target is emerging as an important non-pharmacological mechanism able to lower blood pressure, since these channels are clearly involved in the physiological regulation of vascular smooth muscle contractility and blood pressure in animals and humans [37]. Furthermore, changes in BKCa expression or function are associated with human ageing, hypertension, and diabetes [17, 30, 39, 45]. BKCa channels are activated by membrane depolarization and intracellular Ca2+ concentration. They are tetramers of pore-forming α-subunits (encoded by KCNMA1 gene, also named Slo1) and can be associated to one of four auxiliary β-subunits codified by KCNMB1-4 genes. Each one of these confers distinctive functional and pharmacological properties to the resulting α–β channel complex [18, 26, 44]. The differential expression of β-subunits in different cell types could explain the diversity of functions and regulations on the BKCa channel among diverse tissues and could be used to obtain a tissue-selective BKCa channel activation, using the auxiliary subunit as a specific molecular target. An example of this is the activation of BKCa channel by lithocholate, which is only observed in the presence of β1-subunit [5, 7]. Particularly, in vascular smooth muscle cells (VSMCs), BKCa channels are highly expressed in association with the β1-subunit [24, 25]. Recently, Hoshi and colleagues demonstrated that BKCa channels associated with the auxiliary β1-subunit are directly activated by docosahexaenoic acid (DHA), a PUFA of omega-3 series and its administration on anesthetized wild-type mice lowers blood pressure. On the other hand, the same treatment has no effect on BKCa channel knockout mice [21].
In the case of AA, although it is known that it directly activates BKCa channels [1, 11, 43], the molecular mechanisms of channel activation are not completely understood. Sun et al. have shown in their work with BKCa expression in Xenopus laevis oocytes that AA activates the channel depending on the β-subunit that is present at the time [43]. These authors examined the action of AA on BKCa channels expressed with or without the β2-to β4-subunits, thus finding that it is possible to observe a significant activation of the channel only in the presence of the β2- or β3- subunits. Nevertheless, the effects of AA over this channel when found only with β1-subunit are still unknown.
In the present study, we evaluated the effect of AA on BKCa channel mediated currents in native human VSMCs where the presence of the β1-subunit was functionally tested. By using the patch-clamp technique at the single channel level, we observed that this FA induces a significant increase in the open probability of BKCa channels, mainly by reducing closed dwell time to a great extent. Moreover, we found that AA accelerates the kinetics of voltage channel activation. In whole cell configuration, we demonstrated that AA is able to activate the BKCa channel and induce significant cell hyperpolarization in a more physiological context. Finally, by testing the effect of AA on BKCa channel expressed in HEK293T cells with or without the β1-subunit, we demonstrated that AA needs the presence of this auxiliary subunit to effectively activate BKCa channel.
Methods
Smooth muscle cell isolation procedure for patch-clamp experiments
Umbilical cords were obtained from normal term pregnancies after vaginal and cesarean deliveries. They were placed in transport solution of the following composition (in millimolars)—130 NaCl, 4.7 KCl, 1.17 KH2PO4, 1.16 MgSO4, 24 NaCO3H, 2.5 CaCl2, pH 7.4 at 4 °C and immediately taken to our laboratory where they were stored at 4°C and used within the next 24 h. All procedures were in accordance with the declaration of Helsinki (1975).
The human umbilical arteries (HUA) were dissected from Wharton’s jelly just before the cell isolation procedure. HUA smooth muscle cells (HUASMCs) were obtained by a method based on Knocker’s et al. [23] description and later modified in our laboratory in order to diminish the enzyme content in the dissociation medium (DM) [40]. Briefly, a segment of HUA was cleansed of any residual connective tissue, cut in small strips, and placed for 15 min in a DM containing the following (in millimolars)—140 NaCl, 5 KH2PO4, 5 MgCl2, 6 glucose, 5 HEPES; pH was adjusted to 7.4 with NaOH. The strips were then placed in DM with 3 mg/ml collagenase type I during 15–30 min, with gentle agitation, at 35 °C. After the incubation period, the strips were washed with DM, and single HUASMCs were obtained by a gentle dispersion of the treated tissue using a Pasteur pipette. The supernatant, containing our isolated cells, was stored at room temperature (∼22 °C) until used. HUASMCs were allowed to settle onto the coverglass bottom of a 3 ml experimental chamber. Only well-relaxed, spindle-shaped smooth muscle cells were used for electrophysiological recordings. Data were collected within 4–6 h after cell isolation.
Clones and transient transfection
HEK293T cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) and 10 % fetal bovine serum (FBS) and split when 70–80 % confluent. Human Slo1 α-subunit (KCNMA1, U11058), the auxiliary subunit β1-(KCNMB1, AF035046), and enhanced green fluorescent protein cDNAs (eGFP) were transiently transfected into HEK cells using FuGene 6. Transfections were done with cDNA encoding for α-subunits alone or α + β1-subunit both cloned in pcDNA3 plasmid. The α + β1-subunit were mixed at 1:3 molar ratios to ensure an excess of β1-subunit over α-subunit. When α-subunits were transfected alone, β1-cDNA was replaced by empty plasmid. After transfection, cells were kept in culture for 60 h. The electrophysiological measurements were performed 60–70 h after transfection. eGFP clone and vector were kindly provided by Dr. J. Raingo (IMBICE, Argentina).
Electrophysiology
The isolated cells were observed with a mechanically stabilized inverted microscope (Telaval 3, Carl Zeiss, Jena, Germany) equipped with a ×40 objective lens. The test solutions were applied through a multibarreled pipette positioned close to the target cell. After each experiment on a single cell, the experimental chamber was replaced by another one containing a new sample of cells. All experiments were performed at room temperature (∼22 °C).
The standard tight-seal inside-out and whole-cell configurations of the patch-clamp technique [19] were used to record single-channel and macroscopic currents. Glass pipettes were drawn from WPI PG52165-4 glass on a two-stage vertical micropipette puller (PP-83, Narishige Scientific Instrument Laboratories, Tokyo, Japan) and pipette resistance ranged from 2 to 4 MΩ.
Single-channel recordings in HUASMCs
Single-channel currents were recorded in the inside-out configuration (IO), filtered with a four-pole lowpass Bessel filter at 2 kHz (Axopatch 200A amplifier, Axon Instruments, Foster City, CA) and digitized (Digidata 1200 Axon Instruments, Foster City, CA) at 16 kHz. Voltage-clamp recordings were taken up to 30–60 s at different membrane potentials to obtain the single channel current amplitude, the open probability (NPo), and the mean open and closed dwell times of the channels. The identification of BKCa channel presence in the patch was based on its unitary conductance value, voltage-dependent activity, and activation by an increase in intracellular Ca2+ concentration, as we described before [31]. The control pipette solution used for single-channel recordings contained the following (in millimolars)—140 KCl, 0.5 MgCl2, 10 HEPES, 6 glucose, 1 CaCl2, 5 4-aminopirydine (4-AP), pH adjusted to 7.4 with HCl. 4-AP was added to block Kv channels present is HUASMCs [33]. The bath solution (BS) in contact with the cytosolic face of the membrane contained the following (in millimolars)—140 KCl, 0.5 MgCl2, 10 HEPES, 6 glucose, 1 EGTA, pH adjusted to 7.4 with KOH. Different amounts of CaCl2 were added to the BS so as to establish the desired free Ca2+ concentrations, according to the BKCa channel activity needed for each protocol (100, 50, or 3 nM; calculated using Maxchelator software from Stanford University: http://maxchelator.stanford.edu).
The effects of lithocholic acid (LT) and AA on BKCa channels were tested by adding the adequate quantity to the BS so as to reach a final concentration of 150 and 10 μM, respectively.
Single-channel currents were analyzed with Clampfit software (Molecular Devices, version 10.3). Open probability, as well as closed and open dwell times were studied in steady state conditions. Open probability is expressed as NPo below, where N is the number of single channels present in each patch, and its values were calculated using the following expression:
where T is the recording duration and t j is the time spent with j = 1,2,3,…n channels open. Stationary conditions of single channel recordings were controlled by plotting the NPo values calculated for intervals of 5 s of recording as time function (stability plot). The effect of AA on BKCa channel open and closed dwell times was tested only in patches containing a single BKCa channel. Stability plots were also obtained in order to confirm stationary values in both conditions. They were constructed to represent the time spent in the closed (or open) state for every channel closure (or aperture) plotted as a function of recording time. NPo and dwell time values in the presence and absence of AA were obtained from single channel event detection by the half-amplitude threshold criterion. The mean values of these parameters, in control conditions and after AA perfusion, were calculated when these reached stable values.
A voltage ramp protocol was used to measure the effect of AA on BKCa single channel open probability at different voltages. This method was based on that described by Carl and Sanders [10], in which multiple voltage ramps are applied to an IO patch in order to obtain a single channel average current \( \left(\overline{\iota}\right) \) versus voltage (volts) relationship (\( \overline{\iota} \)-V curve). Considering that, at a given potential, \( \overline{\iota} \) is equal to NPo × i (where i is the channel current amplitude at this potential), Po vs voltage relationship (Po-V curve) can be obtained by dividing the \( \overline{\iota} \)-V curve by the N × i values at each potential. Before testing AA effects, we adapted this protocol to HUASMC BKCa channels. First, we tested different intracellular Ca2+ concentrations (100, 50, and 3 nM) in order to establish adequate channel basal activity (data not shown). Then, we tested both depolarizing and hyperpolarizing voltage ramp protocols at different rates of applied potential change (0.02, 0.04, 0.08, or 0.16 mV/ms) so as to select a protocol that would be slow enough to avoid Po-V distortion by the time constants of voltage-dependent channel activation or deactivation phenomena (data not shown). With the information provided by these experiments, we constructed a voltage protocol consisting of a 500 ms voltage step from a holding potential of −80 mV to +80 mV (to obtain steady-state activation) followed by a 0.08 mV/ms hyperpolarizing voltage ramp to −80 mV. This protocol was applied between 15 and 75 times in a BS with 50 nM free Ca2+ concentration, both before and after AA perfusion. After that, \( \overline{\iota} \)-V and Po-V curves were constructed. Finally, the Po-Vs were fitted using the GraphPad Prism (version 5.03) with a Boltzmann relationship assuming a Pomax of 1, since the membrane patches was disrupted in extremely depolarized steps, where maximal Po could be observed. The equation used to fit these curves was:
where \( {V}_{\raisebox{1ex}{$1$}\!\left/ \!\raisebox{-1ex}{$2$}\right.} \) is the potential at which Po is 0.5, z is a constant value that describes voltage sensibility (steepness of the curve), F is the Faraday constant (96,500 C/mol), R is the gas constant (8.314 J/(mol × K)), and T is room temperature (approximately 298 K).
To study the effect of AA on BKCa channel voltage activation kinetics, we carried out a protocol to examine the latency to the first channel opening after a voltage depolarizing step. Since most patches recorded in HUASMCs contained more than a single BKCa channel, we proceeded as described by Aldrich et al. [2]. A 500 ms voltage step from a holding potential of −80 to +80 mV was applied on IO patches with less than four copies of BKCa channels, and this was repeated at least 50 times for each patch with and without 10 μM AA. Latencies were then measured to represent the delay of the first channel openings after the voltage step. A cumulative histogram was then constructed with the latencies of such first openings measured in both conditions, thus resulting in the cumulative density function (cdf). The count was expressed in probabilities, and for patches containing more than a single channel, the true first latency distribution was obtained by taking the N th root of the apparent distribution function, where N is the number of channels present in the patch [2]. Further on, the distributions were fitted with Clampfit software with the following first-order exponential decay function:
where τ act is the time constant of voltage activation.
Whole-cell recordings in HUASMCs
Whole-cell currents were filtered with a four-pole lowpass Bessel filter (Axopatch 200A amplifier) at 2 kHz and digitized (Digidata 1200 Axon Instruments) at a sample frequency of 20 kHz. The experimental recordings were stored on a computer hard disk for later analysis. The pipette solution contained the following (in millimolars)—130 KCl, 10 HEPES, 0.1 EGTA, 1 MgCl2, 0.1 CaCl2, 5 ATP-Na2; pH was adjusted to 7.4 with KOH yielding a calculated free Ca2+ concentration of 750 nM. The BS contained the following (in millimolars)—130 NaCl, 5.4 KCl, 1.2 MgCl2, 2.5 CaCl2, 5 HEPES, 6 glucose, pH was adjusted to 7.4 with NaOH. In some experiments, 5 mM 4-AP or 500 nM paxilline were added to the BS in order to inhibit the KV or BKCa channel contribution to the macroscopic current, respectively [31, 33]. Whole-cell current stability was monitored by applying successive 500 ms voltage steps (from a holding potential of −50 mV to a test potential of +40 mV), discarding cells in which the current amplitude did not remain constant in time. After the current was stable, the same voltage-clamp step protocol was applied in the control or in the presence of AA. After current stabilization, a voltage-clamp step protocol, which consisted in a family of 500 ms voltage steps between −70 and +60 mV from a holding potential of −50 mV, was applied both in the control and in the presence of AA, for further current–voltage relationship (I-V) analysis. Cell membrane capacitance was calculated from the capacity current obtained from the recording of a single 10 mV hyperpolarizing step. With the aim to quantify the macroscopic kinetics of activation, a first-order exponential function was used to fit these recordings. The equation that was used to fit with the Clampfit software was:
where τ x is the time constant of activation from the current evocated (I x ) by a voltage pulse to x mV.
Inside-out macroscopic current recordings in heterologously expressed BKCa channels
Macroscopic currents of BKCa channel expressed in HEK293T cells were recorded using the cell-free inside-out configuration. Currents were filtered with a four-pole lowpass Bessel filter (Axopatch 200A amplifier) at 2 kHz and digitized (Digidata 1200 Axon Instruments) at a sample frequency of 20 kHz. The experimental recordings were stored on a computer hard disk for later analysis. The pipette solution contained the following (in millimolars)—140 KCl, 0.5 MgCl2, 10 HEPES, 6 glucose, 1 CaCl2; pH was adjusted to 7.4 with KOH. The BS contained the following (in millimolars)—140 KCl, 0.5 MgCl2, 10 HEPES, 6 Glucose, 1 EGTA, pH adjusted to 7.4 with KOH yielding a calculated free Ca2+ concentration of 3 nM (calculated using Maxchelator software from Stanford University: http://maxchelator.stanford.edu). The effect of AA on BKCa channels was tested by adding the adequate quantity to the BS so as to reach a final concentration of 10 μM. Current stability was monitored by applying successive 150 ms voltage steps (from a holding potential of 0 mV to a test potential of +120 mV), discarding cells in which the current amplitude did not remain constant in time. The same voltage-clamp step protocol was applied in control conditions as in presence of AA. After current stabilization, a voltage-clamp step protocol, which consisted in a family of 180 ms voltage steps between −50 and 190 mV from a holding potential of 0 mV followed by a 50 ms voltage step to −50 mV, was applied both in the control and in the presence of AA, for further conductance–voltage relationship (G-V) analysis. The G-V curves were fitted using the GraphPad Prism (version 5.03) with a Boltzmann relationship. The equation used to fit these curves was:
where \( {V}_{\raisebox{1ex}{$1$}\!\left/ \!\raisebox{-1ex}{$2$}\right.} \) is the potential at which \( \frac{G}{G_{\max }} \) is 0.5, z is a constant value that describes voltage sensibility (steepness of the curve), F is the Faraday constant (96,500 C/mol), R is the gas constant (8.314 J/(mol × K)), and T is room temperature (approximately 298 K).
Drugs and reagents used
AA, LT, paxilline, 4-AP, EGTA, Na2ATP, and collagenase used for cell isolation were purchased from Sigma Chemical Co. All other reagents were of analytical grade and purchased from local suppliers as well as DMEM medium and FBS. AA, LT, and paxilline were dissolved in dimethyl sulfoxide (DMSO). Fresh aliquots of stock solutions of AA, LT, and paxilline were added to the bath solution on the day of the experiment. An appropriate amount of DMSO was added to all control solutions without AA, LT, and paxilline. AA stock solution was stored at −20 ºC in an N2-atmosphere to prevent drug oxidation.
Statistics
The results are expressed as mean ± standard error of the mean. Paired or unpaired Student t tests were used to compare two groups. ANOVA test was used to compare three groups. For the comparison of frequency of instantaneous kinetics observed in whole cell currents before and after AA perfusion, the Chi-square test was used. In all cases, a P value lower than 0.05 was considered for establishing statistically significant differences.
Results
Lithocholic acid (LT) effect on BKCa channels in human umbilical artery (HUA) smooth muscle cells (SMCs): a functional evidence for the presence of the β1-accessory subunit
Bukiya et al. (2007–2011) have clearly demonstrated that LT activates BKCa channels only in the presence of accessory β1-subunits [5, 7]. This effect is mediated by a direct interaction between the lithocholate and the steroid interaction site in transmembrane domain 2 of the β1-subunit [6]. Considering that the BKCa channel is commonly associated with β1-subunits in native VSMCs [24, 25], we tested the effect of 150 μM LT on BKCa channel activity in freshly isolated HUASMCs, so as to confirm the presence of this accessory subunit in these cells. The effects of LT on BKCa channels were studied at the single channel level in the IO configuration under symmetrical K+ concentration, which is an experimental condition where we have largely characterized BKCa channels in these cells [31]. Figure 1 presents a typical recording and mean NPo values showing a significant BKCa channel activation induced by LT, indicating that BKCa channel α subunits are likely to be associated with accessory β1-subunits in this cell type. Since LT activation occurs in all copies (n = 1–3) of BKCa channels present in all patches (n = 7) tested, the probability of finding a channel formed by α-alone, α-β2, α-β3, or α-β4 subunits was negligible. Therefore, the AA effects described below on BKCa channel activity and kinetics corresponds to this α–β1 association.
Lithocholic acid activates the high-conductance voltage- and Ca2+-activated K+ channels (BKCa) in human umbilical artery (HUA) smooth muscle cells (SMC) in the inside-out (IO) single-channel configuration a Typical recording of lithocholic acid (150 μM) effect in an IO patch containing 2 BKCa channel copies clamped at +40 mV. The closed level is indicated as C. b Mean activity values (NPo) of BKCa channels recorded as shown in a in control conditions and with 150 μM lithocholic acid (n = 7 patches from six umbilical cord donors). The asterisk indicates statistically significant difference from controls (Student’s t test, P < 0.05)
Arachidonic acid (AA) increases single channel BKCa activity in SMCs freshly isolated from HUA
The effects of AA on BKCa channels were studied at the single channel level in the IO configuration in the same conditions described before [31]. First, the effect of 10 μM AA was tested on steady-state channel activity at +40 mV membrane potential. AA produces a reversible increase in BKCa channel open probability without modifying its conductance value. Figure 2 shows a typical recording of channel activation and after 4 min washout (Fig. 2a), followed by its stability plot (Fig. 2b), where the open probability (expressed as NPo) was calculated every 5 s and plotted as a function of the recording time. It is possible to observe that AA slowly increases the NPo of the BKCa channel, reaching maximal steady-state activation after about 4–5 min of AA perfusion. AA induced a significant rise in NPo values from 0.01113 ± 0.00741 under control conditions to 0.10992 ± 0.06129 after stable AA effect; this activation was reversible after 4 min of AA washout where the NPo fell to 0.00875 ± 0.00417, returning to control values (P < 0.05, n = 6 patches from three umbilical cord donors).
Time course of BKCa channel activation produced by arachidonic acid (AA) in the IO configuration. a Representative traces of a single BKCa channel in the HUASMC in an IO patch clamped at +40 mV in control condition, during 10 μM AA perfusion and after 4 min of AA washout. The closed level is indicated as C. b Temporal course of the AA effect on BKCa channel activity (NPo) obtained from channel recording shown in a. NPo values were calculated every 5 s and plotted as function of recoding time. c, d Dwell time stability plots of the BKCa channel activation by AA obtained from channel recording shown in a. Each graph represents the time spent in closed (c) and open (d) state in every channel closure and aperture, respectively, plotted as function of recording time. The inset in c shows with high resolution the final portion of closed dwell time stability plot
Kinetic analysis was performed by detecting single channel events based on the half-amplitude threshold criterion in all patches containing a single BKCa channel (n = 4). Stability plots of closed and open dwell times, both in control conditions and during AA perfusion, were constructed by plotting the time that the channel remained in every closed (or open) state event as a function of time recording (Fig. 2c and d, respectively). In accordance to the NPo analysis, these graphs show that open and closed dwell times of AA-induced BKCa channel activation reach a stable value after about 4 min of perfusion. The analysis of mean closed and open times before and after AA indicates that both significantly changed with AA. Specifically, there was a reduction of mean closed time from 2,090.9 ± 604.2 ms to 208.3 ± 53.7 ms and an increase in mean open time from 7.8 ± 0.3 ms to 13.2 ± 1.3 ms in tested cells (P < 0.05, n = 4 patches from three umbilical cord donors). These results indicate that the increase of NPo induced by AA is primarily caused by a tenfold decrease in the mean duration of closed dwell time.
Next, in order to study the voltage dependence of AA-induced BKCa channel activation, we performed a series of experiments using a voltage ramp protocol similar to that described by Carl and Sanders [10]. Working with 50 nM of Ca2+ in the solution in contact with the channel intracellular side, the patches were slowly hyperpolarized from +80 to −80 mV using a 2 s voltage ramp protocol. The rate of hyperpolarization (0.08 mV/ms) was slow enough to avoid data distortion by the deactivation time constant (see “Methods”, for more detail). This procedure was repeated between 15 and 75 times in each tested condition to obtain the mean current response. Typical superimposed recordings in the control condition and in the presence of 10 μM AA are shown in Fig. 3a, and the corresponding mean current–voltage relationships (\( \overline{\iota} \)-V) drawn from the average currents are presented in Fig. 3b. The voltage-dependent activation of the BKCa channel can be best characterized by the relationship between Po and V (Po-V curves). Accordingly, the mean \( \overline{\iota} \)-V curves were transformed into a Po-V curve by dividing each data point of the averaged current by the number of copies of the channel in the patch and the single channel current amplitude at each potential. Figure 3c shows the mean Po-V curves of the BKCa channel in the absence and presence of AA obtained from five patches. AA induced a shift in Po-V curves to more hyperpolarized membrane potentials, achieving statistically significant difference in all voltages higher than +23 mV (P < 0.05). It was not possible to observe maximal activation in Po-V curves because more depolarized potentials, in general, disrupted the membrane patch. However, in order to quantify the shift effect of AA in Po-V curves, we assumed Pomax as 1 and fitted these relationships by a Boltzmann function, thus observing that AA induces a significant 20.13 mV change in V1/2 (from 124.7 ± 1.5 mV in control conditions to 83.9 ± 0.5 mV in AA, P < 0.05) without significantly modifying the z value (1.407 ± 0.060 and 1.286 ± 0.034 in control conditions and in AA respectively; NS, P = 0.1178).
AA induces a shift to more hyperpolarized values in the voltage activation curve of BKCa channel in HUA SMC in the IO configuration. a 30 superimposed representative recordings of BKCa channels IO currents obtained by applying 80 mV/s hyperpolarizating voltage-ramp protocol from +80 to −80 mV (see “Methods”) in control conditions (upper panel) and after 10 μM AA perfusion (lower panel). The patch contains four channel copies. b Averaged current versus voltage curve obtained from recording shown in a in control conditions and with 10 μM AA. c Mean voltage activation curves (Po vs. voltage) of BKCa channels in control conditions and with 10 μM AA (n = 5 patches from two umbilical cord donors). Po was calculated from the averaged current, after correcting for the number of channels in the patch, the unitary conductance and the driving force (see “Methods”). Data points were fitted by a Bolzmann function (solid lines) taking Po = 1 as maximal activation. Mean Po values of BKCa channel in AA showed statistically significant difference from controls in all voltages greater than +23 mV (Student’s t test, P < 0.05)
Finally, we studied the effect of AA on BKCa channel voltage activation kinetics by analyzing the latency to the first channel opening. A 500 ms voltage step from a holding potential of −80 to +80 mV was applied in IO patches and repeated at least 50 times for each patch with and without 10 μM AA. Representative single channel traces in both conditions are shown in Fig. 4a. It was observed that AA produces an increase of about a 20 % in the number of pulses with at least one single channel opening during voltage step (data not shown) and a reduction in the time delay to first channel opening (first latency).
AA reduces the latency to the first channel open in depolarizating voltage step activation of BKCa channel in HUA SMC in the IO configuration. a Ten representative recordings of BKCa channels IO currents obtained by applying a potential step from −80 to +80 mV in control conditions and after 5 min of 10 μM AA perfusion. The patch contains a single BKCa channel. b Cumulative distributions of first latencies corresponding to the same patch show in a, obtained after correcting for the number of channels in the patch (see “Methods”). These distributions show the probabilities that the channel remained closed by the times indicated. Data points were fitted by a first-order exponential function (solid lines) in order to obtain the time constants of voltage activation (τ act) in both conditions. c Mean time constants of voltage activation (τ act) obtained as shown in b under control conditions and after 5 min of 10 μM AA perfusion (n = 3–5 patches from five umbilical cord donors). The asterisk indicates statistically significant difference from control (Student’s t test, P < 0.05)
A cumulative histogram was then constructed with latencies to first openings measured in both conditions, so as to obtain the cumulative density function. For patches containing more than a single channel, the true first latency distribution was established by taking the N th root of the apparent distribution function, where N is the number of channels present in the patch [2] (see “Methods”, for more details). These distributions show the probability that the channel still remains closed at the times indicated. As is shown in Fig. 4b, AA induced a shift to shorter first latencies in the cumulative distributions obtained from the recordings presented in Fig. 4a. The distributions from three to five different patches were fitted by a first-order exponential decay function, and the mean time constants of voltage activation τ act values were obtained for both conditions. The data presented in Fig. 4c shows that AA significantly decreases the τ act consistent with the acceleration of BKCa channel voltage activation kinetics (P < 0.05). These results are consistent with the ones obtained in the analysis of steady-state dwell times, since AA reduced the time that the channel remained closed in both cases, thus increasing the opening rate.
AA effects in whole-cell K+ currents in HUASMCs
To analyze the effect of this FA on K+ currents under a more physiological context, we tested the AA effect on macroscopic currents from HUASMCs. In a previous report, we demonstrated that the macroscopic voltage-dependent K+ current in HUASMCs is mostly carried through two different types of ion channels: BKCa channels and the voltage-dependent K+ channels (KV) [33]. We also observed that this macroscopic current is completely inhibited when using 500 nM paxilline (a BKCa channel blocker) and 5 mM 4-AP (a classical KV channels blocker) [31]. Consequently, to investigate AA effects on both components of macroscopic currents, we tested the effect of 10 μM AA on a family of currents evoked by 500 ms voltage steps between −70 and +60 mV from a holding potential of −50 mV in the presence of 5 mM 4-AP (Fig. 5) or 500 nM paxilline (Fig. 6). In agreement with single channel results, extracellular perfusion of 10 μM AA (in the presence of 5 mM 4-AP) significantly increased the amplitude of the outward whole-cell current. Mean current–voltage relationships (I-V curves, Fig. 5b) showed that current increments are statistically significant at membrane potential values between −30 and +50 mV. These results suggest that the AA-activated macroscopic current was mediated by BKCa channels, so we confirmed them by testing the AA effect in the presence of 500 nM paxilline (without 4-AP). In such conditions AA had no effect, hence indicating that this FA is able to activate BKCa channels as we observed at the single channel level, and it did not affect KV channel activity (Fig. 6).
AA increases the BKCa channel component of whole-cell (WC) K+ currents in HUA SMC (recorded in 5 mM 4-aminopyridine) and accelerates its voltage activation kinetic. a Superimposed representative recordings of WC currents obtained by applying 10 mV voltage steps from −70 to +60 mV from a holding potential of −50 mV in control conditions (first panel), after 3 and 5 min of 10 μM AA perfusion (second and third panel, respectively) and after AA washout (fourth panel). b Mean current versus voltage (I-V) curves corresponding to the control conditions and 5 min of 10 μM AA perfusion obtained in the same conditions as in a (n = 11 cells from two umbilical cord donors). c Mean E reversal values corresponding to the control conditions and 5 min of 10 μM AA perfusion obtained from the I-V curves recorded in the same conditions as in a (n = 11 cells from two umbilical cord donors). d Mean time constants of activation (τ act) obtained from a first-order exponential function fit of the recording traces evoked by a potential step from a holding of −50 to +10–60 mV, corresponding to control conditions and after 3 min of 10 μM AA perfusion (n = 7 cells from two umbilical cord donors). The inset in d shows with high resolution the typical recording traces evoked by a potential step from −50 to +60 mV in control conditions, after 3 and 5 min of 10 μM AA perfusion and after AA washout. All experiments were made in presence of 5 mM 4-aminopyridine in order to inhibit KV channels. The asterisk indicates statistically significant difference from control (Student’s t test, P < 0.05)
AA failed to activate the non-BKCa channel component of WC K+ currents in HUA SMC (recorded in 500 nM paxiline). a Superimposed representative recordings of WC currents obtained by applying 10 mV voltage steps from −70 to +60 mV from a holding potential of −50 mV in control conditions (upper panel) and after 5 min of 10 μM AA perfusion (bottom panel) b Mean I-V curves corresponding to the control conditions and 5 min of 10 μM AA perfusion obtained in the same conditions as in a (n = 10 cells from six umbilical cord donors). All experiments were made in presence of 500 nM paxilline in order to inhibit BKCa channels. No statistically significant difference were observed (Student’s t test, P < 0.05)
As it is possible to see in Fig. 5b, the AA effect produces a left shift in the I-V curve, inducing significant cell membrane hyperpolarization. The effect of AA on resting cell membrane potentials was evaluated by measuring the reversal potential (E rev) of the I-V relationships, where 10 μM AA changed this value by about 25 mV with respect to the control condition (Fig. 5c, P < 0.05).
Consistent with the results obtained at the single channel level, AA induces a significant increase in activation kinetics of the voltage-induced whole-cell current. Time constants of activation (τ act) were obtained by fitting the beginning of the whole-cell current at all membrane potentials, where possible, with a single exponential function. In Fig. 5d, the mean τ act values before and after 3 min of AA perfusion were plotted versus membrane potential. AA accelerated activation kinetics at all membrane potentials analyzed. Moreover, after 5 min of AA perfusion, the current kinetics changed from a time-dependent activation without inactivation to an instantaneous activation with a tight inactivation at most depolarized steps in 9 out of 11 tested cells (Fig. 5a) (chi square test, P < 0.05).
Effect of the modulatory β1-subunit on AA activation of BKCa channels expressed in HEK cells
In order to study the relevance of β1-subunit presence on AA BKCa channel activation, we expressed the channel α-subunit in HEK293T cells with or without the β1-subunit and tested the AA effect on them. Figure 7 shows typical traces of macroscopic BKCa channels currents recorded in cell-free inside-out configuration before and after AA perfusion. The AA had no effect on BKCa expressed without the β1-subunit in all tested patches (n = 3) but clearly activated the channel in presence of the modulatory β1-subunit (Fig. 7a and b, respectively). When G-V curves were constructed and fitted with a Boltzmann equation, we observed that the activation of α–β1 complex is characterized by a significant left shift on G-V curves without modification of apparent voltage sensitivity in accordance with results observed in native HUASMCs (Fig. 7c). The \( {V}_{\raisebox{1ex}{$1$}\!\left/ \!\raisebox{-1ex}{$2$}\right.} \) was changed from 141.3 ± 2.0 mV under control conditions to 86.1 ± 3.9 mV in presence of AA (P < 0.0001), while the z value was unaffected by AA (1.047 ± 0.085 and 1.098 ± 0.156 in control conditions and in AA, respectively; NS, P = 0.7755). These results suggest that presence of the auxiliary β1-subunit is necessary to mediate the AA effect on BKCa channel described above.
The presence of accessory β1-subunits is necessary for AA activation in BKCa expressed in HEK cells. Superimposed representative IO currents of BKCa channels, expressed in HEK cells with (b) or without the β1-subunit (a) before (left) and after 5 min of 10 μM AA perfusion (right). The currents were elicited in response to 20 mV voltage steps from −70 to +190 mV from a holding potential of 0 mV. c Mean normalized conductance versus voltage (G-V) curves corresponding to the control conditions and 5 min of 10 μM AA perfusion obtained in BKCa channel associated to β1-subunit (n = 4 patches). Data points were fitted by a Bolzmann function (solid lines)
Discussion
The present study demonstrates that AA, an endogenous PUFA, directly activates BKCa channels present in HUASMCs. Moreover, taking into account that LT is able to activate BKCa channels only if they are associated to β1-subunits [5, 7], BKCa channel activation by LT (showed in Fig. 1) denotes functional evidence of such association. Together, our results provide the first evidence that AA is able to activate BKCa channels modulated by the β1-subunit in human vascular smooth muscle cells. Additionally, the effect of AA on the BKCa channel expressed with or without the β1-subunit demonstrates the requirement of β1-subunit modulation to obtain the channel activation by this fatty acid.
The AA-induced BKCa channel activation observed in the excised patch IO configuration (in HUASMCs and in HEK cells) indicates that this PUFA has a direct effect on such channel activity, without metabolic modification by the different pathways that could produce AA metabolites [36, 38, 42]. Direct activation of the native BKCa in IO patches was already reported so as to discard the effect of AA by its metabolites in rabbit coronary [1] and pulmonary [11] SMCs, guinea pig gastric myocytes [46], and GH3 cells [12]. In the present study, our experiments performed at the single channel level allowed us to analyze in depth the mechanism of the stimulatory effect of AA on BKCa channels present in HUASMCs.
When the voltage ramp protocol was applied on the IO configuration in HUASMCs, to study the effect of AA on BKCa channel open probability at different membrane potentials, we observed that the fatty acid induces a left-shift in Po-V curves. This indicates that AA can activate the channel at more negative membrane potential values, where the channel is usually closed, without modifying the steepness of the curve. This result was also observed in I-V curves from HUASMCs macroscopic WC currents and in G-V curves from BKCa channels co-expressed with β1-subunit in HEK cells. Previously, Ahn et al. as well as Denson et al. observed a similar effect of AA on BKCa channel Po-V curves from rabbit coronary SMCs and GH3 cells, respectively [1, 12]. The fact that AA induces this left shift on Po-V and G-V curves without modifying the slope in all cases suggests that the AA effect is not mediated by a modification of channel voltage sensitivity. However, up to now, the mechanism of AA-induced BKCa channel activation can be said to be poorly understood.
Our single channel analysis showed that the increase in BKCa channel activity is mainly due to a decrease in closed state dwell times and, in a minor way, to an increase in open state dwell times. Since there are no reports in the literature about AA effects on BKCa single channel kinetics, these results are the first to indicate that AA can activate the BKCa channels through a destabilization of its closed state to greatly facilitate channel opening and also by a stabilization of its open state to prolong their open time duration (Fig. 8b). A similar effect on closed state dwell times has been previously reported by Morin et al. for the AA-metabolite 20-HETE on BKCa channels from human airway SMC [35].
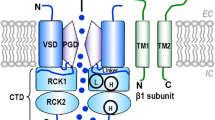
Summary of the action mechanism proposed to explain the AA-dependent activation of the BKCa channel associated with the β1-subunit in human VSMCs. a Arachidonic acid (AA) interacts with the β1-subunit of BKCa channel and modifies the operation of the gating ring. Moreover, the channel voltage sensitivity was unaffected by AA. Activator effect of AA induces a membrane hyperpolarization that can close VOCC (dotted black arrow) and lead to VSMC relaxation. PD pore domain, GR gating ring, VSD voltage sensor domain, Ca 2+ SD calcium sensor domain, VOCC voltage-operated calcium channel, VSMC vascular smooth muscle cell, EC extracellular side, IC intracellular side, TM1-2 transmembrane domain 1-2, S0-6 transmembrane segment 0-6. b Two states model can explain AA activator effect where AA binding to BKCa channel destabilizes its close state and stabilizes its open state. C: close state, O: open state, C AA : AA-bonded close state, O AA : AA-bonded open state
The decrease in BKCa channel closed state stability induced by AA also generates acceleration in the activation kinetics of voltage-induced currents in HUASMCs, where AA clearly reduces latency to first BKCa channel opening (in the IO configuration) and diminishes the activation time constants (in both IO and WC configurations). This acceleration in activation rates induced by AA has also been previously observed in different types of K+ channels: by Gavrilova-Ruch et al. in KV1.5, hEAG1 and hEAG2 [16] and by Ferroni et al. in TREK-2 channels [14].
Since the kinetic analysis performed in HUASMCs reflects that AA modifies the gating ring behavior by destabilization of closed channel conformation, it is possible to postulate that this effect could be independent of β-subunit presence. However, it is known that auxiliary β1-subunit can interact with the channel α-subunit affecting the gating ring as well as the voltage sensor domain [3, 27, 28]. Thus, another possible mechanism to explain the AA effect on BKCa channel is the interaction of the fatty acid with the ion channel by means of the auxiliary β1-subunit. In order to confirm the relevance of β1-subunit in AA BKCa channel activation, we expressed the channel α-subunit with or without the β1-subunit in HEK cells, and we tested the effect of AA on them. Similarly to Sun et al. experiments [43], AA did not affect the BKCa channel without any β-subunit expression, but accordingly with our HUASMCs results, in the presence of the β1-subunit, the AA induces a significant channel activation (Fig. 7). Therefore, AA activation of BKCa channels requires the presence of β1-, β2-, or β3-subunits. However, our experimental results also suggest that the mechanism of AA activation of the α–β1 complex differs from the demonstrated by Sun et al. for the α–β2 complex where AA prevents the β2-dependent channel inactivation.
Considering the experimental evidence presented in HUASMCs at single channel level and in BKCa channels expressed in HEK, we are able to propose a more detailed action mechanism for AA activation of the BKCa channel summarized in Fig. 8a. First, the AA acts by interacting with the β1-subunit of BKCa channel. Second, based on AA modification of BKCa channel kinetics, this interaction is able to modify the operation of the gating ring (GR). Third, AA-BKCa channel activation is independent of modification of the channel voltage sensitivity. Thus, the AA mainly produces, at the level of the BKCa channel gating ring, a reduction on closed channel state stability, which increases the channel opening rate and the open probability (Fig. 8b).
Similar BKCa channel activation in rat vascular SMCs by alteration of closed state stability, independent from voltage sensor activation and Ca2+ binding, was recently reported by Hoshi et al. for DHA, a long-chain 22-carbon polyunsaturated omega-3 fatty acid [21]. Furthermore, they showed that the effect was enhanced by co-expression of α-subunit with β1- and β4-subunits, compared with the α-subunit alone or co-expressed with β2- and γ-subunits [20]. By using mutagenesis, the authors proved that the TM1 N terminus domain from the β1-subunit is essential for the DHA effect, thus suggesting that a direct interaction between the S6 domain (α-subunit) and the TM1 N terminus (β1-subunit) may be able to destabilize the closed state and increase open probability. This resemblance in both AA and DHA effects on BKCa channel activation by destabilization of the closed state could involve a common binding site. However, the differential roles of β-auxiliary subunits on PUFA channel activation reported by Sun et al. and Hoshi et al. suggest that a more complex interaction is involved [20, 43].
Finally, on WC configuration, a more physiological recording condition where the cell remains with its membrane resting potential, it was possible to observe that the activator effect of AA generates a hyperpolarization that can lead to smooth muscle relaxation (Fig. 8a). Since in this configuration the three metabolic pathways remain intact, the effect of AA could be mediated by its metabolites. However, the effect of this fatty acid on BKCa channel in the IO configuration (in HUASMCs and expressed in HEK cells) suggest that AA itself can contribute, in part, to smooth muscle hyperpolarization. These results highlights the role of AA itself as a possible second messenger molecule that can regulate channel activity under physiological and pathological conditions, leading to vasodilation and lower blood pressure values. Moreover, this last effect could explain the benefits of dietary consumption of the omega-6 fatty acid to obtain a cardiovascular protective effect.
References
Ahn DS, Kim YB, Lee YH, Kang BS, Kang DH (1994) Fatty acids directly increase the activity of Ca(2+)-activated K+ channels in rabbit coronary smooth muscle cells. Yonsei Med J 35(1):10–24
Aldrich RW, Corey DP, Stevens CF (1983) A reinterpretation of mammalian sodium channel gating based on single channel recording. Nature 306(5942):436–441
Bao L, Cox DH (2005) Gating and ionic currents reveal how the BKCa channel’s Ca2+ sensitivity is enhanced by its beta1 subunit. J Gen Physiol 126(4):393–412. doi:10.1085/jgp.200509346
Bendahhou S, Cummins TR, Agnew WS (1997) Mechanism of modulation of the voltage-gated skeletal and cardiac muscle sodium channels by fatty acids. Am J Physiol 272(2 Pt 1):C592–C600
Bukiya AN, Liu J, Toro L, Dopico AM (2007) Beta1 (KCNMB1) subunits mediate lithocholate activation of large-conductance Ca2+-activated K+ channels and dilation in small, resistance-size arteries. Mol Pharmacol 72(2):359–369. doi:10.1124/mol.107.034330
Bukiya AN, Singh AK, Parrill AL, Dopico AM (2011) The steroid interaction site in transmembrane domain 2 of the large conductance, voltage-and calcium-gated potassium (BK) channel accessory beta1 subunit. Proc Natl Acad Sci U S A 108(50):20207–20212. doi:10.1073/pnas.1112901108
Bukiya AN, Vaithianathan T, Toro L, Dopico AM (2009) Channel beta2-4 subunits fail to substitute for beta1 in sensitizing BK channels to lithocholate. Biochem Biophys Res Commun 390(3):995–1000. doi:10.1016/j.bbrc.2009.10.091
Burtis CA, Ashwood ER, Bruns DE (2006) Tietz textbook of clinical chemistry and molecular diagnostics, 4th edn. Elsevier Saunders, St.Louis
Campbell WB, Falck JR (2007) Arachidonic acid metabolites as endothelium-derived hyperpolarizing factors. Hypertension 49(3):590–596. doi:10.1161/01.HYP.0000255173.50317.fc
Carl A, Sanders KM (1990) Measurement of single channel open probability with voltage ramps. J Neurosci Methods 33(2–3):157–163
Clarke AL, Petrou S, Walsh JV Jr, Singer JJ (2002) Modulation of BK(Ca) channel activity by fatty acids: structural requirements and mechanism of action. Am J Physiol Cell Physiol 283(5):C1441–C1453. doi:10.1152/ajpcell.00035.2002
Denson DD, Wang X, Worrell RT, Eaton DC (2000) Effects of fatty acids on BK channels in GH(3) cells. Am J Physiol Cell Physiol 279(4):C1211–C1219
Feletou M, Vanhoutte PM (2009) EDHF: an update. Clin Sci (Lond) 117(4):139–155. doi:10.1042/CS20090096
Ferroni S, Valente P, Caprini M, Nobile M, Schubert P, Rapisarda C (2003) Arachidonic acid activates an open rectifier potassium channel in cultured rat cortical astrocytes. J Neurosci Res 72(3):363–372. doi:10.1002/jnr.10580
Fink M, Lesage F, Duprat F, Heurteaux C, Reyes R, Fosset M, Lazdunski M (1998) A neuronal two P domain K+ channel stimulated by arachidonic acid and polyunsaturated fatty acids. EMBO J 17(12):3297–3308. doi:10.1093/emboj/17.12.3297
Gavrilova-Ruch O, Schonherr R, Heinemann SH (2007) Activation of hEAG1 potassium channels by arachidonic acid. Pflugers Arch 453(6):891–903. doi:10.1007/s00424-006-0173-3
Gonzalez-Corrochano R, La Fuente J, Cuevas P, Fernandez A, Chen M, Saenz de Tejada I, Angulo J (2013) Ca2+ -activated K+ channel (KCa) stimulation improves relaxant capacity of PDE5 inhibitors in human penile arteries and recovers the reduced efficacy of PDE5 inhibition in diabetic erectile dysfunction. Br J Pharmacol 169(2):449–461. doi:10.1111/bph.12143
Gonzalez C, Baez-Nieto D, Valencia I, Oyarzun I, Rojas P, Naranjo D, Latorre R (2012) K(+) channels: function-structural overview. Compr Physiol 2(3):2087–2149. doi:10.1002/cphy.c110047
Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ (1981) Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch 391(2):85–100
Hoshi T, Tian Y, Xu R, Heinemann SH, Hou S (2013) Mechanism of the modulation of BK potassium channel complexes with different auxiliary subunit compositions by the omega-3 fatty acid DHA. Proc Natl Acad Sci U S A 110(12):4822–4827. doi:10.1073/pnas.1222003110
Hoshi T, Wissuwa B, Tian Y, Tajima N, Xu R, Bauer M, Heinemann SH, Hou S (2013) Omega-3 fatty acids lower blood pressure by directly activating large-conductance Ca(2)(+)-dependent K(+) channels. Proc Natl Acad Sci U S A 110(12):4816–4821. doi:10.1073/pnas.1221997110
Katz AM, Messineo FC (1981) Lipid-membrane interactions and the pathogenesis of ischemic damage in the myocardium. Circ Res 48(1):1–16
Klockner U (1993) Intracellular calcium ions activate a low-conductance chloride channel in smooth-muscle cells isolated from human mesenteric artery. Pflugers Arch 424(3–4):231–237
Knaus HG, Folander K, Garcia-Calvo M, Garcia ML, Kaczorowski GJ, Smith M, Swanson R (1994) Primary sequence and immunological characterization of beta-subunit of high conductance Ca(2+)-activated K+ channel from smooth muscle. J Biol Chem 269(25):17274–17278
Knaus HG, Garcia-Calvo M, Kaczorowski GJ, Garcia ML (1994) Subunit composition of the high conductance calcium-activated potassium channel from smooth muscle, a representative of the mSlo and slowpoke family of potassium channels. J Biol Chem 269(6):3921–3924
Latorre R, Brauchi S (2006) Large conductance Ca2+-activated K+ (BK) channel: activation by Ca2+ and voltage. Biol Res 39(3):385–401
Liu G, Niu X, Wu RS, Chudasama N, Yao Y, Jin X, Weinberg R, Zakharov SI, Motoike H, Marx SO, Karlin A (2010) Location of modulatory beta subunits in BK potassium channels. J Gen Physiol 135(5):449–459. doi:10.1085/jgp.201010417
Liu G, Zakharov SI, Yang L, Wu RS, Deng SX, Landry DW, Karlin A, Marx SO (2008) Locations of the beta1 transmembrane helices in the BK potassium channel. Proc Natl Acad Sci U S A 105(31):10727–10732. doi:10.1073/pnas.0805212105
Liu L, Rittenhouse AR (2003) Arachidonic acid mediates muscarinic inhibition and enhancement of N-type Ca2+ current in sympathetic neurons. Proc Natl Acad Sci U S A 100(1):295–300. doi:10.1073/pnas.0136826100
Marijic J, Li Q, Song M, Nishimaru K, Stefani E, Toro L (2001) Decreased expression of voltage-and Ca(2+)-activated K(+) channels in coronary smooth muscle during aging. Circ Res 88(2):210–216
Martin P, Enrique N, Palomo AR, Rebolledo A, Milesi V (2012) Bupivacaine inhibits large conductance, voltage-and Ca2+-activated K+ channels in human umbilical artery smooth muscle cells. Channels (Austin) 6(3):174–180. doi:10.4161/chan.20362
Meves H (2008) Arachidonic acid and ion channels: an update. Br J Pharmacol 155(1):4–16. doi:10.1038/bjp.2008.216
Milesi V, Raingo J, Rebolledo A, Grassi de Gende AO (2003) Potassium channels in human umbilical artery cells. J Soc Gynecol Investig 10(6):339–346
Moreno C, Macias A, Prieto A, De La Cruz A, Valenzuela C (2012) Polyunsaturated fatty acids modify the gating of kv channels. Front Pharmacol 3:163. doi:10.3389/fphar.2012.00163
Morin C, Sirois M, Echave V, Gomes MM, Rousseau E (2007) Functional effects of 20-HETE on human bronchi: hyperpolarization and relaxation due to BKCa channel activation. Am J Physiol Lung Cell Mol Physiol 293(4):L1037–L1044. doi:10.1152/ajplung.00145.2007
Munaron L (2011) Shuffling the cards in signal transduction: calcium, arachidonic acid and mechanosensitivity. World J Biol Chem 2(4):59–66. doi:10.4331/wjbc.v2.i4.59
Nelson MT, Quayle JM (1995) Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol 268(4 Pt 1):C799–C822
Qiu Y, Quilley J (1999) Vascular effects of arachidonic acid in the rat perfused heart. Role of the endothelium, cyclooxygenase, cytochrome P450, and K(+) channels. J Lipid Res 40(12):2177–2184
Radenkovic M, Grbovic L, Radunovic N, Momcilov P (2007) Pharmacological evaluation of bradykinin effect on human umbilical artery in normal, hypertensive and diabetic pregnancy. Pharmacol Rep PR 59(1):64–73
Raingo J, Rebolledo A, Iveli F, Grassi de Gende AO, Milesi V (2004) Non-selective cationic channels (NSCC) in smooth muscle cells from human umbilical arteries. Placenta 25(8–9):723–729. doi:10.1016/j.placenta.2004.01.028
Shouk TA, Omar MN, Fayed ST (1999) Essential fatty acids profile and lipid peroxides in severe pre-eclampsia. Ann Clin Biochem 36(Pt 1):62–65
Smyth EM, Burke A, FitzGerald GA (2006) Lipid-derived autacoids: eicosanoids and platelet-activating factor. In: Brunton LL (ed) Goodman and Gilman’s the pharmacological basis of therapeutics, 11th edn. Mc Graw-Hill, New York, pp 653–670
Sun X, Zhou D, Zhang P, Moczydlowski EG, Haddad GG (2007) Beta-subunit-dependent modulation of hSlo BK current by arachidonic acid. J Neurophysiol 97(1):62–69. doi:10.1152/jn.00700.2006
Sweet TB, Cox DH (2009) Measuring the influence of the BKCa {beta}1 subunit on Ca2+ binding to the BKCa channel. J Gen Physiol 133(2):139–150. doi:10.1085/jgp.200810129
Yang Y, Li PY, Cheng J, Mao L, Wen J, Tan XQ, Liu ZF, Zeng XR (2013) Function of BKCa channels is reduced in human vascular smooth muscle cells from Han Chinese patients with hypertension. Hypertension 61(2):519–525. doi:10.1161/HYPERTENSIONAHA.111.00211
Zheng HF, Li XL, Jin ZY, Sun JB, Li ZL, Xu WX (2005) Effects of unsaturated fatty acids on calcium-activated potassium current in gastric myocytes of guinea pigs. World J Gastroenterol 11(5):672–675
Acknowledgments
The authors wish to thank Ms. Anabel Poch and the staff of the Instituto Central de Medicina for the collection of umbilical cords. This study was financially supported by grant PIP 0202 from the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina, and by FONDECYT Grants 1120802, Anillo ACT 1104 (to Carlos Gonzalez), and the Chile–Argentina exchange grant 2011-665 CH/11/12 (to Veronica Milesi and Carlos Gonzalez from the Centro Interdisciplinario de Neurociencia de Valparaíso (The Interdisciplinary Center for Neuroscience in Valparaiso), a Millennium Institute.
Dr. Jesica Raingo generously contributed to this work by providing HEK293T cells, eGFP, and vector. We are grateful to Dr. Alejandro Rebolledo for critical feedback on the manuscript and to Francina Agosti for her helpful assistance in cell culturing and transfection.
Author information
Authors and Affiliations
Corresponding author
Additional information
P. Martín and M. Moncada contributed equally to this work.
Rights and permissions
About this article
Cite this article
Martín, P., Moncada, M., Enrique, N. et al. Arachidonic acid activation of BKCa (Slo1) channels associated to the β1-subunit in human vascular smooth muscle cells. Pflugers Arch - Eur J Physiol 466, 1779–1792 (2014). https://doi.org/10.1007/s00424-013-1422-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-013-1422-x