Abstract
Endogenous Ca2+-activated Cl− channels (CaCC) demonstrate biophysical and pharmacological properties that are well represented in cells overexpressing anoctamin 1 (Ano 1, TMEM16A), a protein that has been identified recently as CaCC. Proteins of the anoctamin family (anoctamin 1–10, TMEM16A-K) are widely expressed. The number of reports demonstrating their physiological and clinical relevance is quickly rising. Anoctamins gain additional interest through their potential role in cell volume regulation and malignancy. Available data suggest that Ano 1 forms stable dimers and probably liaise with accessory proteins such as calmodulin or other anoctamins. In order to understand how anoctamins produce Ca2+-activated Cl− currents, it will be necessary to obtain better insight into their molecular structure, interactions with partner proteins, and mode of activation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ca2+-activated Cl− currents (CaCC) are abundant and are present in many different cell types, although with slight differences regarding their biophysical properties and pharmacology [30, 38]. CaCC mediates Ca2+-dependent Cl− secretion in glands and flat epithelia and modifies cellular responses to adequate stimuli in muscle, nerve and receptors (Table 1). This is therefore why the druggable channels may be ideal pharmacological targets to control physiological functions or to correct defects in diseases such as cystic fibrosis [51, 93].
There is hardly any tissue in which CaCC does not have a basic or at least a modulatory role (Table 1). Among these tissues are various sensory receptors, different types of smooth muscles, heart, endothelium, neuronal tissues, and epithelial organs (reviewed in [25, 34, 38, 48, 58, 68]). Anoctamins are expressed in all of these tissues (Table 1). As Ano 1 has been identified as a major component of the Ca2+-activated Cl− channel [12, 100, 121], one might speculate that all anoctamins participate in formation of Ca2+-activated Cl− currents in these organs. Cl− currents generated by expression of Ano 1 have characteristics that come very close to those described for endogenous channels. Remarkably, silencing of Ano 1 by siRNA in a number of different cell types abolished CaCC, and mice lacking Ano 1 have defects in Ca2+-dependent Cl− transport in a number of tissues, which supports the concept of Ano 1 forming an essential component of CaCC [82, 93, 113, 121]. As the basic molecular and physiological properties of anoctamins have been described already in previous reviews [30, 35, 51], we will focus in the present review on more recent findings and will speculate on additional functions of anoctamins, apart from their role as Ca2+-activated Cl− channels.
Molecular aspects of anoctamins
Molecular aspects of Ano 1 have been already discussed in earlier reviews [30, 51]. The family of anoctamins consists of ten different proteins but only Ano 1 has been examined in more detail. Anoctamins do not show any obvious homology to other ion channels. The 986 amino acids of Ano 1 (Homo sapiens) form a tertiary structure with eight predicted transmembrane helices, intracellular NH2− and COOH ends and a pore, formed by the 5th and 6th transmembrane helices together with a p-loop dipping back into the membrane (Fig. 1a). This still preliminary structural information is based on mutagenesis of amino acids critical to pore formation (R647, K671, K694 in the a, c, d splicing isoform) (Fig. 1a), which changed the anion selectivity [121]. Moreover, Ano 1 comes as different splice variants, which has an impact on ion channel properties [31]. Thus, skipping of exon 6b may increase Ca2+ sensitivity while skipping of exon 13 abolishes the characteristic time-dependent activation observed for Ca2+-activated Cl− channels. Interestingly, the pore loop contains three cysteines (at position 651, 656, 661 in the a, c, d isoform) and cysteine reagents were able to block Ca2+-activated Cl− currents generated by Ano 1 [1, 51, 121]. All anoctamins carry at least one consensus site for N-glycosylation in their fifth extracellular loop (Fig. 1a).
Molecular properties of Ano 1: a Model for Ano 1 indicating eight transmembrane helices and a pore loop containing several cysteine and charged amino acid residues. At least one glycosylation site is present at the last extracellular loop. Ano 1 is differentially spliced (blue ovals a, b, c, d) and may contain non-canonical CAM binding sites in the N terminus. The C terminus contains two putative Erk1,2 phosphorylation sites. b Ano 1 forms stable dimers, with either two separate pores or one common pore. Whether anoctamins form heterodimers is currently unknown
The sequence homology within the putative pore region of anoctamins is considerable, while the overall homology is only moderate [51, 72]. Ano 1 and Ano 2 (TMEM16B) are close relatives with about 60% amino acid identity. In contrast to Ano 1 and Ano 2, the other anoctamins demonstrate an identity generally of 30% or below that may indicate a possible functional divergence of these proteins. Obviously anoctamin paralogs evolved from subsequent gene duplication events which were followed by a functional divergence of vertebrate anoctamins [72]. Interaction with auxiliary proteins would be suggested by the presence of protein interaction domains, which however are only present in Ano 2, 5, 7, and 9 (PDZ and coiled-coil domain) [72]. So far, Cl− channel activity has been clearly identified for Ano 1 and Ano 2 [86, 96, 106, 108, 121]. We also found Ca2+-activated Cl− currents generated by Ano 6 and Ano 7, while Ano 5, 8, 9, and 10 did not produce any measureable currents [99]. Ano 1 is clearly different from the other anoctamins since it is able to produce Ca2+-dependent Cl− currents of much bigger amplitude when compared to Ano 2, 6, and 7 [99].
It is also entirely possible that anoctamins have diverse functions either as plasma membrane localized proteins or in intracellular compartments. In this respect, it is rather exiting that Ano 6 was found to induce scramblase activity in platelets [110]. Ano 6 induces Ca2+-dependent translocation of phosphatidylserine (PS) from the inner leaflet of the plasma membrane to the outer leaflet and is therefore essential for platelet aggregation. Patients suffering from the rare Scott syndrome, which is characterized by impaired blood coagulation, were shown to have a loss-of-function mutation in TMEM16F [15, 110]. Although Ano 1 does not have scramblase activity [110], it will be interesting to learn whether other anoctamins also facilitate PS scrambling.
Structural aspects of TMEM16 proteins were illuminated by two more recent publications, indicating that Ano 1 does not exist as a single protein [28, 102]. The data from both reports indicate that Ano 1 exists as obligate homodimer, similar to CLC-0 channels [62, 71]. As the quaternary structure of Ano 1 is not altered by changes in cytosolic Ca2+, dimerization appears to be permanent. Dimerization of Ano 1 seems to be stable, independent of Ca2+, and does not depend on the cytoskeleton [102]. Moreover, the present data do not indicate interactions with other proteins [28, 102]. It will now be interesting to study whether each Ano 1 subunit forms its own independent Cl− channel pore as described for ClC channels [24], or whether the homodimer forms only one pore (Fig. 1b). Also, future work will show if heterodimeric channels are formed, similar to the ClC channels [27]. A heterodimeric architecture could help to explain why some anoctamins influence each other. We found earlier that coexpression of Ano 9 reduces the activity of Ano 1 [99].
Typically several anoctamins are expressed in parallel in a given cell. A detailed analysis of expression of all ten anoctamins indicated that every cell type in mouse and human tissues expresses at least two, but often several different anoctamins, with Ano 6 being the most abundant paralog [51, 99]. Ano 6 and 10 are broadly expressed in mouse and human tissues. Ano 8 is also broadly expressed although at lower levels. Ano 2, 3, and 4 are preferentially expressed in sensory receptor cells and neuronal tissues, while Ano 5 was found in skeletal muscle and thyroid gland [99]. In addition, splice variants of one given anoctamin are coexpressed in cells. Moreover, we found that expression of anoctamins is not stable, i.e., it largely varies with age, cell density, and polarization (unpublished data from the author's laboratory), which may explain inconsistent findings regarding expression levels of Ano 1 [1, 102].
Regulation of Ano 1
Although Ano 1 is activated by a rise in intracellular [Ca2+], a canonical Ca2+ binding domain has not yet been identified. The team led by Galietta proposed a cluster of four joined glutamic acid residues in the first intracellular loop, which may serve as Ca2+ binding site [30, 31] (Fig. 1a). We found recently that calmodulin (CAM) is required for activation of Ano 1 [113]. CAM was shown to bind to Ano 1, possibly within the N terminus. Moreover, we found a requirement for cytosolic ATP to fully activate Ano 1 [113]. When membrane patches were excised after stimulation of Ano 1 expressing HEK293 cells, the activated Cl− currents rapidly inactivated [17, 113]. Similar results were found for HeLa cells overexpressing Ano 1. Purinergic stimulation (100 μM ATP) activated a whole cell current in Ano 1 expressing HeLa cells but not in mock transfected cells (Fig. 2a). Cl− currents could also be activated in cell attached patches from Ano 1-transfected HeLa cells, but currents inactivated immediately after excision of the cell membrane (Fig. 2b). Thus, cellular components are required to keep the channel active.
Ano 1 channels require cytosolic components to maintain activity: a Whole cell patch clamp experiments in mock-transfected and Ano 1-expressing HeLa cells. Stimulation of purinergic receptors by ATP (10 μM) activates a substantial whole cell current only in Ano 1-expressing cells as indicated by the current/voltage relationships. b Patch clamp experiments with Ano 1-expressing and mock-transfected HeLa cells. Activation of Ano 1 currents by ATP in cell-attached patches of Ano 1-expressing cells. After excision of the membrane patch, the current inactivates immediately. Patch clamp conditions were as described in [113]
Although Ano 1 carries multiple putative phosphorylation sites for protein kinase (PK) A and C, CAMK and CK2, none of these kinases seem to be relevant for gating of Ano 1 [51]. Thus, although the presence of calmodulin and ATP appears important for activation of Ano 1, calmodulin-dependent kinase (CAMKII) is not required [32, 113]. However, we identified two extracellular regulated kinase (Erk1,2) sites at the C terminus which are relevant for receptor-mediated activation of Ano 1 (Fig. 1a), while activation through direct increase in intracellular Ca2+ does not seem to require phosphorylation by Erk1,2 [113]. This finding could have important implications for gating of Ano 1 and suggests that Ano 1 can be activated through other Ca2+-independent pathways.
When expressed in Fisher rat thyroid cell (FRT), we found Ano 1, 2, and 6 well expressed in the plasma membrane, while other Ano 5, 7, 8, 9, and 10 were much less membrane localized, which probably has a substantial impact on the current size [99, 113]. We further noticed that overexpression of Ano 1 in FRT cells [99], HeLa cells (Fig. 3a), HEK293 cells [52], and oocytes from Xenopus laevis (Fig. 3b–f) induced large baseline Cl− currents, which were due to spontaneously active Ano 1 channels. Enhanced baseline conductance that is found upon expression of Ano 1 is not due to enhanced intracellular Ca2+ concentrations; since they are at similar levels or even lower in cells expressing anoctamins (data not shown). Large baseline Cl− conductance is not due to possible patch clamp artifacts since it was also found in fluorescence measurements with HeLa cells. In HeLa cells overexpressing Ano 1, I− influx induced quenching of YFP-I152L, while additional stimulation of the cells with 100 μM ATP did not further increase influx of iodide and YFP-quenching. This suggests that no additional Cl− conductance is activated by an increase in intracellular Ca2+ by ATP-stimulation (Fig. 3a). This could indicate a higher Ca2+ sensitivity of the overexpressed channel, which therefore may be already active at baseline Ca2+ levels. We may also suggest that additional regulatory subunits are missing, which keep the channel shut under control conditions.
Receptor activation of Ano 1: a Summary curves of fluorescence quenching experiments with HeLa cells stably expressing I− sensitive YFP in the absence or presence of Ano 1. Application of extracellular I− to Ano 1-expressing cells (right panel) leads to quenching of YFP-fluorescence without Ca2+-dependent stimulation. Additional increase of intracellular Ca2+ by extracellular ATP (10 μM) does not further increase quenching (not shown), indicating that overexpression of Ano 1 leads to a spontaneously active anion conductance in these cells. b Double electrode voltage clamp experiments with Xenopus laevis oocytes expressing P2Y2 receptors (left panel) or coexpressing P2Y2 receptors and mAno 1 (right panel). Oocytes expressing P2Y2-R only, activate endogenous xANO 1 upon stimulation with 100 μM ATP. Much larger currents are activated in mANO 1 expressing oocytes, which are slightly inwardly rectifying (insert). These oocytes also demonstrate a large baseline Cl− conductance even in the absence of ATP. c Summary of the ATP-activated and ionomycin (1 μM)-activated conductances measured in P2Y2 (−Ano 1) and P2Y2 + Ano 1 (+Ano 1) expressing cells. d Summary of ATP-activated conductances in and P2Y2 + Ano 1 expressing cells and inhibition by DIDS (100 μM) and NFA (10 μM). e, f Effect of removal of extracellular Cl−, Na+, and Ca2+ on ANO 1 currents (e) and conductance (f). Experimental conditions as described in [29, 99]
Receptor activation of Ano 1
Xenopus laevis oocytes are known for their endogenous Ca2+ sensitive Cl− currents (Fig. 3b). It is this endogenous Xenopus Ano 1 that made expression cloning of Ano 1 in oocytes difficult and that prompted Schroeder and colleagues to move to oocytes from Axolotl, which are free of CaCC [100]. Ca2+-activated Cl− currents typically show more or less outward rectification, depending on the increase in intracellular Ca2+ [38]. An example for receptor (P2Y2) mediated activation of endogenous CaCC is shown in the left panel in Fig. 3b. Extracellular ATP (100 μM) activates a transient outwardly rectifying Cl− current. Expression of mouse Ano 1 in oocytes largely augmented Cl− currents activated by ATP (or ionomycin; Fig. 3b, c). Interestingly, these Cl− currents induced by overexpression of Ano 1 were linear or even slightly inwardly rectifying (Fig. 3b, right panel), when compared to endogenous currents, which show slight outward rectification (Fig. 3b, left panel). A similar current behavior was noticeable in the cloning papers by Schroeder and Yang [100, 121]. Thus, there seem clear differences in current properties between endogenous CaCC and overexpressed anoctamins. Notably, a differential time and voltage dependence was reported recently, depending on whether anoctamin was examined as whole cell currents or in cell excised inside/out membrane patches [17]. When we determined the ion selectivity of overexpressed mAno 1 under bi-ionic conditions, i.e., after ion replacements in the bath, we found a substantial ATP (Ca2+)-activated Na+ conductance in addition to the Cl− conductance (Fig. 3e, f). This pronounced Na+ permeability is not explained by the only moderate selectivity of Ca2+-activated Cl− channels for Cl− over Na+ by only 0.1 [88]. It is possible that expression of Ano 1 translocates additional Ca2+/Na+ influx pathways to the cell membrane that may shape the current voltage relationship of whole cell currents. Also, depending on the number of Cl− channels activated by increase in intracellular Ca2+, the cell membrane potential will be more or less depolarized which will activate voltage gated Ca2+ influx pathways.
Ano 1 and more
Not much is known currently about the interaction of TMEM16A with other proteins. Current data indicate dimerization of Ano 1 and interaction with components of the intracellular signaling pathways such as CAM [28, 52, 102, 113]. An interesting finding concerns the interference of Ca2+-activated Cl− channels and cystic fibrosis transmembrane conductance regulator (CFTR). CFTR is a cAMP/PKA/ATP-regulated Cl− channel that also controls the function of other membrane proteins [56]. It has been reported that CFTR inhibits endogenous Ca2+-activated Cl− currents (CaCC) in Xenopus oocytes, bovine pulmonary artery endothelium, and isolated parotid acinar cells, by an unknown mechanism [54, 85, 118, 119]. Moreover, also volume-regulated anion channels were inhibited by expression of CFTR, which shares some properties with Ca2+-activated Cl− currents [1, 116]. Additional studies demonstrated that upregulation or downregulation of CFTR resulted in a parallel up- and downregulation of cAMP-, Ca2+-, and volume-regulated Cl− conductance [50]. Although these data clearly suggested a relationship of these different anion conductances, they have been regarded as separate molecular entities [2]. Recently, we could recapitulate the CFTR-dependent inhibition of CaCC by demonstrating inhibition of Ano 1 by coexpressed CFTR after cAMP-dependent activation. However, we found no evidence for a direct molecular interaction, as both proteins did not coimmunoprecipitate (data not shown). A recent paper examined a possible molecular interaction of both channels by Förster resonance energy transfer (FRET), but detected only minimal FRET between CFTR and Ano 1 [102]. Thus, the authors suggested that regulation of Ano 1 by CFTR is not due to a direct interaction.
Developmental aspects of anoctamins
Members of the anoctamin family are widely expressed during vertebrate embryogenesis [37, 91]. This is perhaps not surprising given that functions typically associated with CaCC, including secretion and smooth muscle contraction, are active during development [79, 97]. Despite their abundant expression, little is known about the functions of anoctamin family members during embryogenesis. This is, in part, because null mice have been reported only for Ano 1 [90]. Further, only Ano 5 has been associated with a congenital defect in humans so far [9, 41].
Because of its widespread expression and the fundamental importance of CaCC, Ano 1 has been identified by a number of investigators examining diverse questions in a number of tissues. For example, Ano 1 was identified in a screen for genes expressed in the zone of polarizing activity (ZPA) of the embryonic mouse limb bud [92]. Subsequent studies have found that this gene is expressed in a number of tissues of all three germ layers in the developing embryo [37, 91]. Mice homozygous for a null mutation of Ano 1 die within the first month of life and exhibit decreased calcium-activated chloride currents in a number of tissues [44, 82, 93, 94]. These mice demonstrated a severe failure to thrive and therefore it has been complicated to identify the precise cause of death. As early as embryonic day (E)14.5, all homozygous mutant mice exhibit abnormal tracheal morphology and develop gaps in the cartilage rings that normally surround the ventrolateral trachea [90, 93]. Intriguingly, expression of Ano 1 was never detected in the embryonic chondrogenic mesenchyme and so it was hypothesized that the cartilage defects are secondary to defects of the respiratory epithelium or smooth muscle where Ano 1 is abundantly expressed. A similar tracheal patterning defect has been observed in CFTR knockout mice, CFTR deficient pigs, and perhaps in CF patients [70, 93]. It remains to be seen if this defect is attributable to decreased chloride secretion in epithelial or mesenchymal cells. The generation of a conditional null allele of Ano 1 will facilitate the characterization of this and other developmental defects observed in Ano 1 null mice. Preliminary data suggest that deletion of Ano 1 specifically from the embryonic airway epithelium does not lead to tracheal malformation (JRR unpublished data).
Ano 5 is one of the members of the family that has been associated with a disease other than cancer in humans. During embryogenesis, Ano 5 is expressed in developing mesenchymal cells including the somites, myotome-derived skeletal muscle precursors, cardiac myofibers, and chondrocytes [73]. Mutations in Ano 5 have been linked to gnathodiaphyseal dysplasia, a disease of the bones, and two forms of muscular dystrophy [9, 115]. The function of Ano 5 in musculoskeletal cells and the mechanisms by which these mutations contribute to disease remain to be identified. As described, only Ano 1 and Ano2 have been reported to encode channels with CaCC activity and endogenous and overexpressed Ano 5 have been localized primarily to intracellular vesicles [73, 115]. The development of animal models and the identification of other human mutations in anoctamin family proteins will facilitate the identification of functions for these proteins during normal development.
Also postnatal expression of anoctamins may vary considerably with age. This is of particular interest for Cl− secretion in the intestinal epithelium. A Ca2+-dependent Cl− secretion has been detected in the colon and small intestine of neonatal mice, which disappeared in older mice [74]. In both mouse proximal and distal colon, Ca2+-dependent Cl− secretion through muscarinic stimulation fades with increase in animal age (Fig. 4a). This Ca2+-dependent Cl− secretion was shown to be activated during infection with rotavirus, which is probably the reason for infantile gastroenteritis in more than 600,000 patients every year worldwide [5, 61]. We found recently activation of Ca2+-dependent Cl− secretion in the distal colon of young mouse pups, which disappeared completely in older animals [83]. We further demonstrated activation of Ano 1 by an active peptide of the rotavirus toxin NSP4. Interestingly, expression of Ano 1 is found in the neonatal colon throughout epithelial cells, but appears to be expressed only in basolateral membranes in epithelial cells of adult mouse colon (Fig. 4b, c). Similarly expression of Ano 1 was found in the basolateral membrane of colonic epithelial cells of adult guinea pigs where it may contribute to production of electrogenic K+ secretion [39]. In contrast, clear luminal staining is seen in the distal colon of younger animals (Fig. 4c). Taken together, Ano 1 appears as a major player in rotavirus-induced diarrhea in the infant/juvenile intestine and may become a major pharmacological target for the treatment of the worldwide common rotavirus diarrhea.
Role of Ano 1 for rotavirus toxin induced Cl - secretion: a Age dependence of Ca2+-activated Cl− secretion (muscarinic basolateral stimulation with 100 μM carbachol) in mouse proximal and distal colon. Cl− secretion was detected as CCH-activated short circuit currents (I sc). b, c Immunohistochemistry of Ano 1 in colon of neonatal, younger, and older mice. Ano 1 is found in the apical membrane only in colon mucosa of younger animals but is located basoalerally in adult animals. d Model for rotavirus toxin NSP4 induced Cl− secretion, which occurs through Ca2+-activated Ano 1 channels. The receptor for NSP4 and the ATP-release mechanism are unknown
An intracellular function of anoctamins?
Regulated secretion in exocrine cells occurs through exocytosis of secretory granules and the subsequent release of vesicular proteins. Secretory granules of pancreatic acinar cells express ClC Cl− channels which are believed to facilitate acidification and exocytosis of these granules [112]. A number of transport proteins have been identified in secretory granules of exocrine glands and it thus could be possible that other Cl− channels, apart from ClC channels, contribute to granular acidification and exocytosis. Ano 1 is clearly plasma membrane localized in overexpressing HEK293 and Cos-7 cells, according to fluorescence images and electron microscopy [113]. However, in some fluorescence images, a portion of Ano 1 appears to be localized also intracellularly, particularly those from mouse pancreatic and submandibular acinar cells [121] (Fig. 5a). In immunofluorescence, Ano 1 was detected in apical as well as basolateral membranes and also in the cytosol of exocrine cells from mouse pancreas and salivary glands. When analyzing proteins in whole cell lysates and the isolated granular fraction of pancreatic and submandibular glands, we detected Ano 1 in both fractions, supporting a possible role of Ano 1 in intracellular granules (Fig. 5b).
An intracellular function of Ano 1? a Immunohistochemistry of Ano 1 in mouse submandibular acinar cells indicates predominant basolateral, but also luminal and intracellular granular expression. b Western blot analysis of Ano 1 in total lysates or intracellular granules only of mouse pancreas and submandibular gland cells. In all samples, the expected 110 kDa band is detected. Only pancreatic acinar cells show a 150 kDa band which may reflect glycosylated Ano 1. c Cell swelling and light scattering by isolated granules in suspension. Because the granular membrane contains Cl− channels, incorporation of the K+ ionophore valinomycin leads to KCl-induced osmotic swelling of the granules and change in light scattering
The Cl− transport in isolated granules can be examined using light scattering (LS). LS is changed during application of the K+ ionophore valinomycin, when Cl− channels are activated and vesicles are swelling (Fig. 5c, upper panel). An example for a change in LS in isolated mouse pancreatic granules is shown in the lower panel of Fig. 5. Using this technique, it will be interesting to compare Cl− conductances and LS of granules isolated from pancreatic acini of Ano 1 null mice and from wild-type animals. A possible intracellular role of anoctamins has also been postulated for Ano 8, 9, and 10, as these anoctamins have been found exclusively in intracellular compartments when expressed in Fisher rat thyroid (FRT) and HEK293 cells [99]. Interestingly Ano 1, which is upregulated in human gastrointestinal stromal tumors (GIST, c.f. below), has been detected to a significant level in intracellular compartments [3, 26, 120].
Anoctamins in health and disease
Table 1 summarizes the role of Ca2+-activated Cl− channels in epithelial and non-epithelial tissues. It is evident that CaCC has a role in most tissues, and thus it is not surprising to find anoctamins broadly expressed in all cell types. However, we clearly need to distinguish between data from mouse and human tissue. So far, most expression data are from mouse [37, 42, 82, 90, 93, 94, 99, 100, 121] and rather little is known about expression in native human tissues [3, 7, 65]. While mouse native tissue and cultured human cells [10, 31, 32, 51, 76] express significant amounts of Ano 1, expression levels in native human tissues are currently not well determined. In preliminary RT-PCR analysis of Ano 1-expression in human nasal and colonic epithelium, we were unable to detected transcripts for Ano 1, while other anoctamins, such as Ano 6, were readily expressed (unpublished data). These results parallel earlier observations showing that Ca2+-dependent Cl− transport is small in human when compared to mouse airways [51] and that Ca2+-dependent Cl− secretion in adult human colon relies entirely on the presence of CFTR [53, 64]. A recent study by the Verkman team even questioned the role of Ano 1 a major component of CaCC in airway and intestinal epithelial cells [76]. Their conclusions are based on the fact that broad inhibitors of CaCC such as tannic acid and the arylaminothiophene CaCCinh-A01, fully inhibited CaCC current in human bronchial and intestinal cells, while an Ano 1—specific inhibitor had little effects on Ca2+-activated Cl− secretion in these cells [76]. In contrast, siRNA-knockdown of Ano 1 in HT29 colon epithelial cells and human airway epithelial cell lines inhibited CaCC [1, 32, 66]. At any rate, these results obtained from cultured cells may not necessarily reflect the true in vivo situation, particularly since expression of Ano 1 appears largely polarization/differentiation dependent (unpublished data).
Irrespective of the uncertainty of the role of Ano 1 in human tissues, the numbers of reports is continuously increasing, indicating an essential role of Ano 1 in various epithelial tissues, visceral and vascular smooth muscles, neuronal cells, and receptors (summarized in Table 1). Loss of expression of Ano 1 in knockout animals leads to multiple defects in epithelial organs such as airways, salivary glands, pancreatic glands, hepatobiliary tract, and large intestine [23, 33, 59, 82, 93, 94, 114, 121]. More recent studies show the importance of Ano 1 in Cajal pacemaker and smooth muscle cells of airways, [20, 36, 44, 65, 122]. Lack of Ano 1 in mouse Cajal cells leads to reduced slow wave activity in gastrointestinal muscles [44], while expression alternatively transcribed Ano 1 was found in human interstitial cells of Cajal of patients with diabetic gastroparesis [67]. Meanwhile, anoctamins have also been identified in neuronal tissues and in olfactory and taste receptors, the inner ear and the retina [11, 16, 46, 69, 84, 89, 106, 108, 117]. Defects in expression or function of these anoctamins lead to ataxia, panic disorder, defects in pain perception, and nonsyndromic hearing defects (Table 1). However, recent results obtained in a mouse loxP/cre knockout model for Ano 2 provided the rather surprising result that although Ano 2 clearly is the ciliary Ca2+-activated Cl− channel in the main olfactory epithelium that is virtually abolished in Ano 2−/− mice, it seems entirely dispensable for olfaction [8]. Moreover, anoctamins have been linked to muscular dystrophy, myopathy, gnathodiaphyseal dysplasia, hemolysis, and platelet adhesion, indicating a role of anoctamins in skeletal muscle, endothelium, erythrocytes, and cartilage/bone (Table 1).
Before Ano 1 was identified as Ca2+-activated Cl− channel, it was actually known as cancer-associated protein in gastrointestinal stromal tumors (GISTs) and head and neck squamous cell carcinomas [13, 14, 120]. Ano 1 was therefore called DOG1 (discovered on gastrointestinal stromal tumor 1), TAOS2 (tumor amplified and overexpressed sequence 2), and ORAOV2 (oral cancer overexpressed 2). Ano 1 may promote cancer development, a concept that is supported by the fact that it is embedded within the 11q13 amplicon, which is associated with a poor prognosis in squamous cell carcinoma of the head and neck [43]. It could work in concert with cancer-relevant proteins such as cyclin D1 or Fas-associated death domain. In preliminary experiments, however, we could not find a positive correlation between expression of Ano 1 and cell proliferation (unpublished data). A previous report pointed out that amplification of Ano 1 in head and neck squamous cell carcinoma (HNSC) cells stimulates attachment, spreading, detachment, and invasion, which could account for its effects on migration [4]. Amplification of ANO 1 may therefore be a marker for distant metastasis in HNSC, and overexpression of ANO 1 may affect cell properties linked to metastasis.
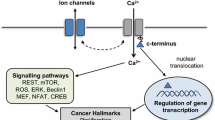
The ability of cancer cells to metastasize is linked to their ability to migrate. Migration of cells depends on their intracellular proton concentration and their ability to increase their volume at the leading edge with extension of the lamellipodium and to shrink at retract their rear part [101, 107] (Fig. 6b). A central role in this cell shrinkage has the charybdotoxin-inhibited and Ca2+-sensitive IK1 (or Gardos) K+ channels. It is suggested that activation of IK1 requires parallel activation of a Cl− exit pathway in order to maintain electroneutrality (Fig. 6b). Accordingly, anoctamins may serve as volume regulated Cl− channels (Fig. 6a). In fact, we found earlier that Ano 1 produces volume-regulated chloride currents that are reduced in mice lacking expression of Ano 1 [1]. We showed that Ano 1 together with other anoctamins are activated by cell swelling through an autocrine mechanism that involves ATP release and binding to purinergic P2Y2-receptors. Ano 1 channels are activated by ATP through increase in intracellular Ca2+ and by a Ca2+- independent mechanism engaging extracellular regulated protein kinases (Erk1/2) [1]. Moreover, in erythrocytes, Ca2+-activated Gardos channels are also essential for cell shrinkage, and we also found that in addition, Ano 1 is also important for volume regulation in mouse red blood cells, as cell shrinkage after exposure to bacterial hemolysin was significantly attenuated and lysis was enhanced in erythrocytes isolated from Ano 1 null mice compared with wild-type littermates [103]. Other anoctamins are also related to cancer. Thus, expression of Ano 7, also known as NGEP, is enhanced in prostate cancers [6, 19, 47]. Moreover, splicing variants of Ano 6 (TMEM16F) are associated with metastatic capability of mammary cancers in mouse and is also associated with poor prognosis of patients with breast cancer [22]. It will be very interesting to learn more about the role of these anoctamins in cancer development and metastasis. The fact that these proteins are related to malignancy provides another convincing example for the role of ion channels in cancer [49].
Role of Ano 1 in cancer: a Potential role of anoctamins and Gardos K+ channels in volume regulation and cell shrinkage after hypotonic cell swelling or increase in intracellular Ca2+. ATP release leads to activation of puringeric signaling along with activation of Erk1,2. b Anoctamin and Gardos-mediated cell shrinkage at the rear end of migrating cells
References
Almaca J, Tian Y, AlDehni F, Ousingsawat J, Kongsuphol P, Rock JR, Harfe BD, Schreiber R, Kunzelmann K (2009) TMEM16 proteins produce volume regulated chloride currents that are reduced in mice lacking TMEM16A. J Biol Chem 284:28571–28578
Anderson MP, Welsh MJ (1991) Calcium and cAMP activate different chloride channels in the apical membrane of normal and cystic fibrosis epithelia. Proc Natl Acad Sci USA 88:6003–6007
Ardeleanu C, Arsene D, Hinescu M, Andrei F, Gutu D, Luca L, Popescu LM (2009) Pancreatic expression of DOG1: a novel gastrointestinal stromal tumor (GIST) biomarker. Appl Immunohistochem Mol Morphol 17:413–418
Ayoub C, Wasylyk C, Li Y, Thomas E, Marisa L, Robe A, Roux M, Abecassis J, de Reynies A, Wasylyk B (2010) ANO 1 amplification and expression in HNSCC with a high propensity for future distant metastasis and its functions in HNSCC cell lines. Br J Cancer 103:715–726
Ball JM, Tian P, Zeng CQ, Morris AP, Estes MK (1996) Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science 272:101–104
Bera TK, Das S, Maeda H, Beers R, Wolfgang CD, Kumar V, Hahn Y, Lee B, Pastan I (2004) NGEP, a gene encoding a membrane protein detected only in prostate cancer and normal prostate. Proc Natl Acad Sci USA 101:3059–3064
Bergmann F, Andrulis M, Hartwig W, Penzel R, Gaida MM, Herpel E, Schirmacher P, and Mechtersheimer G (2011) Discovered on gastrointestinal stromal tumor 1 (DOG1) is expressed in pancreatic centroacinar cells and in solid-pseudopapillary neoplasms-novel evidence for a histogenetic relationship. Hum Pathol (in press)
Billig GM, Pál B, Fidzinski P, and Jentsch TJ (2011) Ca2+−activated Cl- currents are dispensable for olfaction. Nature Neurosc (in press)
Bolduc V, Marlow G, Boycott KM, Saleki K, Inoue H, Kroon J, Itakura M, Robitaille Y, Parent L, Baas F, Mizuta K, Kamata N, Richard I, Linssen WH, Mahjneh I, de Visser M, Bashir R, Brais B (2010) Recessive mutations in the putative calcium-activated chloride channel anoctamin 5 cause proximal LGMD2L and distal MMD3 muscular dystrophies. Am J Hum Genet 86:213–221
Bove PF, Grubb BR, Okada SF, Ribeiro CM, Rogers TD, Randell SH, O'Neal WK, Boucher RC (2010) Human alveolar type II cells secrete and absorb liquid in response to local nucleotide signaling. J Biol Chem 285:34939–34949
Brown DA, Passmore GM (2010) Some new insights into the molecular mechanisms of pain perception. J Clin Invest 120:1380–1383
Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ (2008) TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 322:590–594
Carles A, Millon R, Cromer A, Ganguli G, Lemaire F, Young J, Wasylyk C, Muller D, Schultz I, Rabouel Y, Dembele D, Zhao C, Marchal P, Ducray C, Bracco L, Abecassis J, Poch O, Wasylyk B (2006) Head and neck squamous cell carcinoma transcriptome analysis by comprehensive validated differential display. Oncogene 25:1821–1831
Carneiro A, Isinger A, Karlsson A, Johansson J, Jonsson G, Bendahl PO, Falkenback D, Halvarsson B, Nilbert M (2008) Prognostic impact of array-based genomic profiles in esophageal squamous cell cancer. BMC Cancer 8:98
Castoldi E, Collins PW, Williamson PL, Bevers EM (2011) Compound heterozygosity for 2 novel TMEM16F mutations in a patient with Scott syndrome. Blood 117:4399–4400
Chappe V, Hinkson DA, Zhu T, Chang XB, Riordan JR, Hanrahan JW (2003) Phosphorylation of protein kinase C sites in NBD1 and the R domain control CFTR channel activation by PKA. J Physiol 548:39–52
Chen Y, An H, Li T, Liu Y, Gao C, Guo P, Zhang H, and Zhan Y (2011) Direct or Indirect Regulation of Calcium-Activated Chloride Channel by Calcium. J Membr Biol (in press)
Clarke LL, Burns KA, Bayle JY, Boucher RC, Van Scott MR (1992) Sodium- and chloride-conductive pathways in cultured mouse tracheal epithelium. Am J Physiol 263:L519–L525
Das S, Hahn Y, Walker DA, Nagata S, Willingham MC, Peehl DM, Bera TK, Lee B, Pastan I (2008) Topology of NGEP, a prostate-specific cell:cell junction protein widely expressed in many cancers of different grade level. Cancer Res 68:6306–6312
Davis AJ, Forrest AS, Jepps TA, Valencik ML, Wiwchar M, Singer CA, Sones WR, Greenwood IA, Leblanc N (2010) Expression profile and protein translation of TMEM16A in murine smooth muscle. Am J Physiol Cell Physiol 299:C948–C959
Davis MJ, Hill MA (1999) Signaling mechanisms underlying the vascular myogenic response. Physiol Rev 79:387–423
Dutertre M, Lacroix-Triki M, Driouch K, de la Grange P, Gratadou L, Beck S, Millevoi S, Tazi J, Lidereau R, Vagner S, Auboeuf D (2010) Exon-based clustering of murine breast tumor transcriptomes reveals alternative exons whose expression is associated with metastasis. Cancer Res 70:896–905
Dutta AK, Khimji AK, Kresge C, Bugde A, Dougherty M, Esser V, Ueno Y, Glaser SS, Alpini G, Rockey DC, Feranchak AP (2010) Identification and functional characterization of TMEM16A, a Ca2+-activated Cl− channel activated by extracellular nucleotides, in biliary epithelium. J Biol Chem 286:766–776
Dutzler R, Campbell EB, Cadene M, Chait BT, MacKinnon R (2002) X-ray structure of a ClC chloride channel at 3.0 A reveals the molecular basis of anion selectivity. Nature 415:287–294
Eggermont J (2004) Calcium-activated chloride channels: (un)known, (un)loved? Proc Am Thorac Soc 1:22–27
Espinosa I, Lee CH, Kim MK, Rouse BT, Subramanian S, Montgomery K, Varma S, Corless CL, Heinrich MC, Smith KS, Wang Z, Rubin B, Nielsen TO, Seitz RS, Ross DT, West RB, Cleary ML, van de Rijn M (2008) A novel monoclonal antibody against DOG1 is a sensitive and specific marker for gastrointestinal stromal tumors. Am J Surg Pathol 32:210–218
Fahlke C, Knittle T, Gurnett CA, Campbell KP, George AL Jr (1997) Subunit stoichiometry of human muscle chloride channels. J Gen Physiol 109:93–104
Fallah G, Roemer T, Detro-Dassen S, Braam U, Markwardt F, Schmalzing G (2010) TMEM16A(a)/anoctamin-1 shares a homodimeric architecture with CLC chloride channels. Mol Cell Proteomics 79:649–661
Faria D, Schreiber R, Kunzelmann K (2009) CFTR is activated through stimulation of purinergic P2Y2 receptors. Pflügers Arch 457:1373–1380
Ferrera L, Caputo A, Galietta LJ (2010) TMEM16A protein: a new identity for Ca(2+)-dependent Cl channels. Physiology (Bethesda) 25:357–363
Ferrera L, Caputo A, Ubby I, Bussani E, Zegarra-Moran O, Ravazzolo R, Pagani F, Galietta LJ (2009) Regulation of TMEM16A chloride channel properties by alternative splicing. J Biol Chem 284:33360–33368
Ferrera L, Scudieri P, Sondo E, Pedemonte N, Caci E, Ubby I, Pagani F, and Galietta LJ (2011) Native calcium activated chloride channels and their association with TMEM16A protein expression. 8th ECFS Basic Science Conference, 30 March–2 April, Tirennia, Italy (Abstract)
Fischer H, Illek B, Sachs L, Finkbeiner WE, Widdicombe JH (2010) CFTR and Ca-activated Cl channels in primary cultures of human airway gland cells of serous or mucous phenotype. Am J Physiol Lung Cell Mol Physiol 299:L585–L594
Frings S, Reuter D, Kleene SJ (2000) Neuronal Ca2+-activated Cl− channels—homing in on an elusive channel species. Prog Neurobiol 60:247–289
Galietta LJ (2009) The TMEM16 protein family: a new class of chloride channels? Biophys J 97:3047–3053
Gomez-Pinilla PJ, Gibbons SJ, Bardsley MR, Lorincz A, Pozo MJ, Pasricha PJ, van de Rijn M, West RB, Sarr MG, Kendrick ML, Cima RR, Dozois EJ, Larson DW, Ordog T, Farrugia G (2009) Ano 1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol 296:G1370–G1381
Gritli-Linde A, Vaziri SF, Rock JR, Hallberg K, Iribarne D, Harfe BD, Linde A (2009) Expression patterns of the Tmem16 gene family during cephalic development in the mouse. Gene Expr Patterns 9:178–191
Hartzell HC, Putzier I, Arreola J (2005) Calcium-activated chloride channels. Annu Rev Physiol 67:719–758
He Q, Halm ST, Zhang J, Halm DR (2011) Activation of the basolateral membrane Cl conductance essential for electrogenic K secretion suppresses electrogenic Cl secretion. Exp Physiol 96:305–316
Hengl T, Kaneko H, Dauner K, Vocke K, Frings S, Mohrlen F (2010) Molecular components of signal amplification in olfactory sensory cilia. Proc Natl Acad Sci U S A 107:6052–6057
Hicks D, Sarkozy A, Muelas N, Koehler K, Huebner A, Hudson G, Chinnery PF, Barresi R, Eagle M, Polvikoski T, Bailey G, Miller J, Radunovic A, Hughes PJ, Roberts R, Krause S, Walter MC, Laval SH, Straub V, Lochmuller H, Bushby K (2011) A founder mutation in Anoctamin 5 is a major cause of limb-girdle muscular dystrophy. Brain 134:171–182
Huang F, Rock JR, Harfe BD, Cheng T, Huang X, Jan YN, Jan LY (2009) Studies on expression and function of the TMEM16A calcium-activated chloride channel. Proc Natl Acad Sci U S A 106:21413–21418
Huang X, Godfrey TE, Gooding WE, McCarty KS Jr, Gollin SM (2006) Comprehensive genome and transcriptome analysis of the 11q13 amplicon in human oral cancer and synteny to the 7F5 amplicon in murine oral carcinoma. Genes Chromosomes Cancer 45:1058–1069
Hwang SJ, Blair PJ, Britton FC, O'Driscoll KE, Hennig G, Bayguinov YR, Rock JR, Harfe BD, Sanders KM, Ward SM (2009) Expression of anoctamin 1/TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J Physiol 587:4887–4904
Ji J, Zheng PS (2010) Activation of mTOR signaling pathway contributes to survival of cervical cancer cells. Gynecol Oncol 117:103–108
Kalay E, Caylan R, Kiroglu AF, Yasar T, Collin RW, Heister JG, Oostrik J, Cremers CW, Brunner HG, Karaguzel A, Kremer H (2007) A novel locus for autosomal recessive nonsyndromic hearing impairment, DFNB63, maps to chromosome 11q13.2–q13.4. J Mol Med 85:397–404
Katoh M, Katoh M (2004) Characterization of human TMEM16G gene in silico. Int J Mol Med 14:759–764
Kidd JF, Thorn P (2000) Intracellular Ca2+ and Cl− channel activation in secretory cells. Annu Rev Physiol 62:493–513
Kunzelmann K (2005) Ion channels and cancer. J Membr Biol 205:159–173
Kunzelmann K, Allert N, Kubitz R, Breuer WV, Cabantchik ZI, Normann C, Schumann S, Leipziger J, Greger R (1994) Forskolin- and PMA-pretreatment alter the acute electrical response of HT29 cells to cAMP, ATP, neurotensin, ionomycin and hypotonic cell swelling. Pflügers Arch 428:76–83
Kunzelmann K, Kongsuphol P, AlDehni F, Tian Y, Ousingsawat J, Warth R, Schreiber R (2009) Bestrophin and TMEM16—Ca2+ activated Cl− channels with different functions. Cell Calcium 46:233–241
Kunzelmann K, Kongsuphol P, Chootip K, Toledo C, Martins JR, Almaca J, Tian Y, Witzgall R, Ousingsawat J, Schreiber R (2011) Role of the Ca(2+)-activated Cl(−) channels bestrophin and anoctamin in epithelial cells. Biol Chem 392:125–134
Kunzelmann K, Mall M (2002) Electrolyte transport in the colon: mechanisms and implications for disease. Physiol Rev 82:245–289
Kunzelmann K, Mall M, Briel M, Hipper A, Nitschke R, Ricken S, Greger R (1997) The cystic fibrosis transmembrane conductance regulator attenuates the endogenous Ca2+ activated Cl− conductance in Xenopus ooyctes. Pflügers Arch 434:178–181
Kunzelmann K, Milenkovic VM, Spitzner M, Barro Soria R, Schreiber R (2007) Calcium dependent chloride conductance in epithelia: is there a contribution by bestrophin? Pflügers Arch 454:879–889
Kunzelmann K, Schreiber R (1999) CFTR, a regulator of channels. J Membr Biol 168:1–8
Lalonde MR, Kelly ME, Barnes S (2008) Calcium-activated chloride channels in the retina. Channels (Austin) 2:252–260
Leblanc N, Ledoux J, Saleh S, Sanguinetti A, Angermann J, O'Driscoll KE, Britton F, Perrino BA, Greenwood IA (2005) Regulation of calcium-activated chloride channels in smooth muscle cells: a complex picture is emerging. Can J Physiol Pharmacol 83:541–556
Lee RJ, Foskett JK (2009) Mechanisms of Ca2+-stimulated fluid secretion by porcine bronchial submucosal gland serous acinar cells. Am J Physiol Lung Cell Mol Physiol 298:L210–L231
Liu B, Linley JE, Du X, Zhang X, Ooi L, Zhang H, Gamper N (2010) The acute nociceptive signals induced by bradykinin in rat sensory neurons are mediated by inhibition of M-type K+ channels and activation of Ca2+-activated Cl− channels. J Clin Invest 120:1240–1252
Lorrot M, Vasseur M (2007) How do the rotavirus NSP4 and bacterial enterotoxins lead differently to diarrhea? Virol J 4:31
Ludewig U, Pusch M, Jentsch TJ (1996) Two physically distinct pores in the dimeric ClC-0 chloride channel. Nature 383:340–343
Mahjneh I, Jaiswal J, Lamminen A, Somer M, Marlow G, Kiuru-Enari S, Bashir R (2010) A new distal myopathy with mutation in anoctamin 5. Neuromuscul Disord 20:791–795
Mall M, Bleich M, Greger R, Schürlein M, Kühr J, Seydewitz HH, Brandis M, Kunzelmann K (1998) Cholinergic ion secretion in human colon requires co-activation by cAMP. Am J Physiol 275:G1274–G1281
Manoury B, Tamuleviciute A, Tammaro P (2010) TMEM16A/Anoctamin1 protein mediates calcium-activated chloride currents in pulmonary arterial smooth muscle cells. J Physiol 588:2305–2314
Martins JR, Kongsuphol P, Sammels E, AlDehni F, Clarke L, Schreiber R, De Smedt H, Amaral MD, Kunzelmann K (2011) F508del-CFTR increases intracellular Ca2+ signaling that causes enhanced calcium-dependent Cl− conductance in cystic fibrosis. Acta physiol Scand (abtract) 201S682:P271
Mazzone A, Bernard CE, Strege PR, Beyder A, Galietta LJ, Pasricha PJ, Rae JL, Parkman HP, Linden DR, Szurszewski JH, Ordog T, Gibbons SJ, Farrugia G (2011) Altered expression of ANO 1 variants in human diabetic gastroparesis. J Biol Chem 286:13393–13403
Melvin JE, Yule D, Shuttleworth T, Begenisich T (2005) Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol 67:445–469
Mercer AJ, Rabl K, Riccardi GE, Brecha NC, Stella SL Jr, Thoreson WB (2010) Location of release sites and calcium-activated chloride channels relative to calcium channels at the photoreceptor ribbon synapse. J Neurophysiol 105:321–335
Meyerholz DK, Stoltz DA, Namati E, Ramachandran S, Pezzulo AA, Smith AR, Rector MV, Suter MJ, Kao S, McLennan G, Tearney GJ, Zabner J, McCray PB Jr, Welsh MJ (2010) Loss of CFTR function produces abnormalities in tracheal development in neonatal pigs and young children. AmJ Respir Crit Care Med 182:1251–1261
Middleton RE, Pheasant DJ, Miller C (1996) Homodimeric architecture of a ClC-type chloride ion channel. Nature 383:337–340
Milenkovic VM, Brockmann M, Stohr H, Weber BH, Strauss O (2010) Evolution and functional divergence of the anoctamin family of membrane proteins. BMC Evol Biol 10:319–324
Mizuta K, Tsutsumi S, Inoue H, Sakamoto Y, Miyatake K, Miyawaki K, Noji S, Kamata N, Itakura M (2007) Molecular characterization of GDD1/TMEM16E, the gene product responsible for autosomal dominant gnathodiaphyseal dysplasia. Biochem Biophys Res Commun 357:126–132
Morris AP, Scott JK, Ball JM, Zeng CQ, O'Neal WK, Estes MK (1999) NSP4 elicits age-dependent diarrhea and Ca2+ mediated I- influx into intestinal crypts of CF mice. Am J Physiol 277:G431–G444
Moyer BD, Hevezi P, Gao N, Lu M, Kalabat D, Soto H, Echeverri F, Laita B, Yeh SA, Zoller M, Zlotnik A (2009) Expression of genes encoding multi-transmembrane proteins in specific primate taste cell populations. PLoS ONE 4:e7682
Namkung W, Phuan PW, Verkman AS (2011) TMEM16A inhibitors reveal TMEM16A as a minor component of CaCC conductance in airway and intestinal epithelial cells. J Biol Chem 286:2365–2374
Namkung W, Thiagarajah JR, Phuan PW, Verkman AS (2010) Inhibition of Ca2+-activated Cl− channels by gallotannins as a possible molecular basis for health benefits of red wine and green tea. FASEB J 24:4178–4186
Nilius B, Droogmans G (2001) Ion channels and their functional role in vascular endothelium. Physiol Rev 81:1415–1459
Olver RE, Walters DV, Wilson M (2004) Developmental regulation of lung liquid transport. Annu Rev Physiol 66:77–101
Otowa T, Yoshida E, Sugaya N, Yasuda S, Nishimura Y, Inoue K, Tochigi M, Umekage T, Miyagawa T, Nishida N, Tokunaga K, Tanii H, Sasaki T, Kaiya H, Okazaki Y (2009) Genome-wide association study of panic disorder in the Japanese population. J Hum Genet 54:122–126
Ousingsawat J, Martins JR, Kongsuphol P, Schreiber R, Rock JR, Harfe BD, and Kunzelmann K (2009) Defective Ca2+ dependent chloride secretion in TMEM16A −/− pups. 11th International Symposium on Exocrine Secretion, Tokushima, Japan, 23–26 July 2009
Ousingsawat J, Martins JR, Schreiber R, Rock JR, Harfe BD, Kunzelmann K (2009) Loss of TMEM16A causes a defect in epithelial Ca2+ dependent chloride transport. J Biol Chem 284:28698–28703
Ousingsawat J, Tian Y, AlDehni F, Roussa E, Schreiber R, Mirza M, Cook DI, Kunzelmann K (2011) Rotavirus toxin NSP4 activates the calcium dependent chloride channel TMEM16A and inhibits absorptive Na+ transport. Pflügers Arch 461:579–589
Pedemonte N, Tomati V, Sondo E, Galietta LJ (2010) Influence of cell background on pharmacological rescue of mutant CFTR. Am J Physiol Cell Physiol 298:C866–C874
Perez-Cornejo P, Arreola J (2004) Regulation of Ca(2+)-activated chloride channels by cAMP and CFTR in parotid acinar cells. Biochem Biophys Res Commun 316:612–617
Pifferi S, Dibattista M, Menini A (2009) TMEM16B induces chloride currents activated by calcium in mammalian cells. Pflugers Arch 458:1023–1038
Pifferi S, Pascarella G, Boccaccio A, Mazzatenta A, Gustincich S, Menini A, Zucchelli S (2006) Bestrophin-2 is a candidate calcium-activated chloride channel involved in olfactory transduction. Proc Natl Acad Sci U S A 103:12929–12934
Qu Z, Hartzell HC (2000) Anion permeation in Ca(2+)-activated Cl(−) channels. J Gen Physiol 116:825–844
Rasche S, Toetter B, Adler J, Tschapek A, Doerner JF, Kurtenbach S, Hatt H, Meyer H, Warscheid B, Neuhaus EM (2010) Tmem16b is specifically expressed in the cilia of olfactory sensory neurons. Chem Senses 35:239–245
Rock JR, Futtner CR, Harfe BD (2008) The transmembrane protein TMEM16A is required for normal development of the murine trachea. Dev Biol 321:141–149
Rock JR, Harfe BD (2008) Expression of TMEM16 paralogs during murine embryogenesis. Dev Dyn 237:2566–2574
Rock JR, Lopez MC, Baker HV, Harfe BD (2007) Identification of genes expressed in the mouse limb using a novel ZPA microarray approach. Gene Expr Patterns 8:19–26
Rock JR, O'Neal WK, Gabriel SE, Randell SH, Harfe BD, Boucher RC, Grubb BR (2009) Transmembrane protein 16A (TMEM16A) is a Ca2+ regulated Cl−—secretory channel in mouse airways. J Biol Chem 284:14875–14880
Romanenko VG, Catalan MA, Brown DA, Putzier I, Hartzell HC, Marmorstein AD, Gonzalez-Begne M, Rock JR, Harfe BD, Melvin JE (2010) Tmem16A encodes the Ca2+-activated Cl− channel in mouse submandibular salivary gland acinar cells. J Biol Chem 285:12990–13001
Runft LL, Watras J, Jaffe LA (1999) Calcium release at fertilization of Xenopus eggs requires type I IP(3) receptors, but not SH2 domain-mediated activation of PLCgamma or G(q)-mediated activation of PLCbeta. Dev Biol 214:399–411
Sagheddu C, Boccaccio A, Dibattista M, Montani G, Tirindelli R, Menini A (2010) Calcium concentration jumps reveal dynamic ion selectivity of calcium-activated chloride currents in mouse olfactory sensory neurons and TMEM16b-transfected HEK 293T cells. J Physiol 588:4189–4204
Schittny JC, Miserocchi G, Sparrow MP (2000) Spontaneous peristaltic airway contractions propel lung liquid through the bronchial tree of intact and fetal lung explants. Am J Respir Cell Mol Biol 23:11–18
Schneppenheim R, Castaman G, Federici AB, Kreuz W, Marschalek R, Oldenburg J, Oyen F, Budde U (2007) A common 253-kb deletion involving VWF and TMEM16B in German and Italian patients with severe von Willebrand disease type 3. J Thromb Haemost 5:722–728
Schreiber R, Uliyakina I, Kongsuphol P, Warth R, Mirza M, Martins JR, Kunzelmann K (2010) Expression and function of epithelial anoctamins. J Biol Chem 285:7838–7845
Schroeder BC, Cheng T, Jan YN, Jan LY (2008) Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 134:1019–1029
Schwab A (2001) Ion channels and transporters on the move. News Physiol Sci 16:29–33, 16: 29–33
Sheridan JT, Worthington EN, Yu K, Gabriel SE, Hartzell HC, Tarran R (2010) Characterization of the oligomeric structure of the Ca2+-activated Cl− channel Ano 1/TMEM16A. J Biol Chem 286:1381–1388
Skals M, Jensen UB, Ousingsawat J, Kunzelmann K, Leipziger J, Praetorius HA (2010) E. coli alpha-hemolysin triggers shrinkage of erythrocytes via KCa3.1 and TMEM16A channels with subsequent phosphatidyl serine exposure. J Biol Chem 285:15557–15565
Skals M, Jorgensen NR, Leipziger J, Praetorius HA (2009) Alpha-hemolysin from Escherichia coli uses endogenous amplification through P2X receptor activation to induce hemolysis. Proc Natl Acad Sci U S A 106:4030–4035
Sones WR, Davis AJ, Leblanc N, Greenwood IA (2010) Cholesterol depletion alters amplitude and pharmacology of vascular calcium-activated chloride channels. Cardiovasc Res 87:476–484
Stephan AB, Shum EY, Hirsh S, Cygnar KD, Reisert J, Zhao H (2009) ANO 2 is the cilial calcium-activated chloride channel that may mediate olfactory amplification. Proc Natl Acad Sci U S A 106:11776–11781
Stock C, Schwab A (2009) Protons make tumor cells move like clockwork. Pflugers Arch 458:981–992
Stohr H, Heisig JB, Benz PM, Schoberl S, Milenkovic VM, Strauss O, Aartsen WM, Wijnholds J, Weber BH, Schulz HL (2009) TMEM16B, a novel protein with calcium-dependent chloride channel activity. associates with a presynaptic protein complex in photoreceptor terminals. J Neurosci 29:6809–6818
Strauss O (2005) The retinal pigment epithelium in visual function. Physiol Rev 85:845–881
Suzuki J, Umeda M, Sims PJ, Nagata S (2010) Calcium-dependent phospholipid scrambling by TMEM16F. Nature 468:834–838
Taylor R, Roper S (1994) Ca(2+)-dependent Cl− conductance in taste cells from Necturus. J Neurophysiol 72:475–478
Thevenod F (2002) Ion channels in secretory granules of the pancreas and their role in exocytosis and release of secretory proteins. Am J Physiol Cell Physiol 283:C651–C672
Tian Y, Kongsuphol P, Hug MJ, Ousingsawat J, Witzgall R, Schreiber R, Kunzelmann K (2011) Calmodulin-dependent activation of the epithelial calcium-dependent chloride channel TMEM16A. FASEB J 25:1058–1068
Tradtrantip L, Namkung W, Verkman AS (2009) Crofelemer, an antisecretory antidiarrheal proanthocyanidin oligomer extracted from Croton lechleri, targets two distinct intestinal chloride channels. Mol Pharmacol 77:69–78
Tsutsumi S, Kamata N, Vokes TJ, Maruoka Y, Nakakuki K, Enomoto S, Omura K, Amagasa T, Nagayama M, Saito-Ohara F, Inazawa J, Moritani M, Yamaoka T, Inoue H, Itakura M (2004) The novel gene encoding a putative transmembrane protein is mutated in gnathodiaphyseal dysplasia (GDD). Am J Hum Genet 74:1255–1261
Vennekens R, Trouet D, Vankeerberghen A, Voets T, Cuppens H, Eggermont J, Cassiman JJ, Droogmans G, Nilius B (1999) Inhibition of volume-regulated anion channels by expression of the cystic fibrosis transmembrane conductance regulator. J Physiol Lond 515:75–85
Vermeer S, Hoischen A, Meijer RP, Gilissen C, Neveling K, Wieskamp N, de Brouwer A, Koenig M, Anheim M, Assoum M, Drouot N, Todorovic S, Milic-Rasic V, Lochmuller H, Stevanin G, Goizet C, David A, Durr A, Brice A, Kremer B, van de Warrenburg BP, Schijvenaars MM, Heister A, Kwint M, Arts P, van der Wijst J, Veltman J, Kamsteeg EJ, Scheffer H, Knoers N (2010) Targeted next-generation sequencing of a 12.5-Mb homozygous region reveals ANO 10 mutations in patients with autosomal-recessive cerebellar ataxia. Am J Hum Genet 87:813–819
Wei L, Vankeerberghen A, Cuppens H, Cassiman JJ, Droogmans G, Nilius B (2001) The C-terminal part of the R-domain, but not the PDZ binding motif, of CFTR is involved in interaction with Ca2+-activated Cl− channels. Pflügers Arch 442:280–285
Wei L, Vankeerberghen A, Cuppens H, Eggermont J, Cassiman JJ, Droogmans G, Nilius B (1999) Interaction between calcium-activated chloride channels and the cystic fibrosis transmembrane conductance regulator. Pflugers Arch 438:635–641
West RB, Corless CL, Chen X, Rubin BP, Subramanian S, Montgomery K, Zhu S, Ball CA, Nielsen TO, Patel R, Goldblum JR, Brown PO, Heinrich MC, van de Rijn M (2004) The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am J Pathol 165:107–113
Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U (2008) TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455:1210–1215
Yermolaieva O, Leonard AS, Schnizler MK, Abboud FM, Welsh MJ (2004) Extracellular acidosis increases neuronal cell calcium by activating acid-sensing ion channel 1a. Proc Natl Acad Sci U S A 101:6752–6757
Zygmunt AC, Gibbons WR (1992) Properties of the calcium-activated chloride current in heart. J Gen Physiol 99:391–414
Acknowledgments
Supported by DFG SFB699/A7, TargetScreen2 (EU-FP6-2005-LH-037365), Deutsche Krebshilfe (Projekt-Nr.:7207561), and Mukoviszidose e.V. (Projekt-Nr.:S02/10). We thank Mrs. Brigitte Wild and Ms. Julia Redekopf for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kunzelmann, K., Tian, Y., Martins, J.R. et al. Anoctamins. Pflugers Arch - Eur J Physiol 462, 195–208 (2011). https://doi.org/10.1007/s00424-011-0975-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-011-0975-9